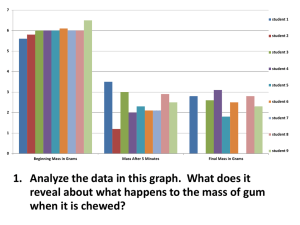
New synthetic methodologies for natural products by William Gerard Bornmann
advertisement

New synthetic methodologies for natural products by William Gerard Bornmann A thesis submitted in partial fulfillment of the requirements for the degree of MASTER OF SCIENCE in Chemistry Montana State University © Copyright by William Gerard Bornmann (1978) Abstract: The orbital symmetry allowed [3.3] sigmatropic rearrangement of enamines derived from 2-acetyl-6-methyl-3,4-dihydro-2H-pyran has allowed for facile entry into highly functionalized cyclohexanone derivatives. This new synthetic methodology offers promise for entry into sesquiterpene natural products. A by-product of this research has led to a symple synthesis of brevicomin, the aggregating sex pheromone of the pine bark beetle, Dendroctonus brevicomis. / t' ' STATEMENT OF PERMISSION TO COPY In p r e s e n tin g t h i s t h e s is in p a r t i a l f u l f i l l m e n t o f th e require m en ts ■for an advanced degree a t Montana S ta te U n i v e r s i t y , L i b r a r y s h a ll make i t I agree t h a t th e f r e e l y a v a i l a b l e f o r in s p e c t io n . I f u r t h e r agree t h a t p e rm is s io n f o r e x te n s iv e copying o f t h i s t h e s is f o r s c h o l a r l y purposes may be g ra n te d by my m a jo r p r o f e s s o r , o r , in h is absence, by th e D i r e c t o r o f L ib ra rie s . I t i s understood t h a t any copying o r p u b l i c a t i o n o f t h i s t h e s i s f o r f i n a n c i a l g a in s h a l l n o t be a llo w e d w i t h o u t my w r i t t e n p e rm is s io n . S ig n a tu re Date / <77F To my w i f e . D aria NEW SYNTHETIC METHODOLOGIES FOR NATURAL PRODUCTS by . WILLIAM GERARD BORNMANN A t h e s is s u b m itte d in p a r t i a l f u l f i l l m e n t o f the re q u ire m e n ts f o r the degree • of MASTER OF SCIENCE in Chem istry Approved: XVvvi on, Graduate Committee Chairperson Head, M ajor Department Graduate Dean MONTANA STATE UNIVERSITY Bozeman, Montana December, 1978 -V • iv ACKNOWLEDGMENTS Several people have had a m a jo r r o l e in. making t h i s research stu d y p o s s ib le and I would l i k e t o ta ke t h i s o p p o r t u n it y to thank them. I would e s p e c i a l l y l i k e to thank my pare n ts w i t h o u t whose help the c o m p le tio n o f t h i s t h e s is would n o t have been made p o s s ib le . I would l i k e t o thank Mr. C h ris Evans f o r the e x p e r t g la ss b lo w in g , and M rs. K je s t in e Carey o f th e L i b r a r y f o r always g e t t i n g a much needed book. S p ecial thanks a re a ls o extended to Mr. Gordon W illia m s o n o f Mechanical E n g in e e rin g f o r le n d in g me p re c io u s equipment and g iv in g h e l p f u l a d v ic e . I would a ls o l i k e t o thank my f e l l o w re s e a r c h e r s . My s p e c ia l thanks t o Dr. B ra d fo rd P. Mundy f o r h is guidance and unending p a tie n c e w i t h my many ta n g e n ts . And, most im p o r t a n t l y , I would l i k e thank my w i f e , D a r i a , f o r her p a t ie n c e , u n d e rs ta n d in g and u n s e l f i s h s a c r i f i c e s which have enable me to a c h ie ve . TABLE OF CONTENTS. Page DEDICATION...................... ............................................................................................... VITA................................... ..................... '............................................................ ■ . . . i iii ACKNOWLEDGMENTS.................................. iv TABLE OF CONTENTS................................... ................................................... : • v LIST OF TABLES..................................................................■......................................... vi LIST OF FIGURES ......................................................................................................... V ii ABSTRACT. . ........................ x PART I A NEW SYNTHETIC APPROACH TO SESQUITERPENE INTERMEDIATES Chapter 1. HISTORICAL.................................................................................. ..................... 2. RESULTS AND DISCUSSION. . . ......................................................... .... 3. APPLICATION OF THE ENAMINE COPE REARRANGEMENT .......................... 33 ■ 4. FUTURE RESEARCH ...................................................................... 36 5. EXPERIMENTAL. . ...........................■ ....................................38 REFERENCES. , .......................... .............................. . I . . . . . . . . . . . . H 64 PART I I A NEW SYNTHESIS OF BREVICOMIN 1. HISTORICAL........................................... 70 2. RESULTS AND DISCUSSION...................... ..............................' ....................... 84 3. EXPERIMENTAL........................................... .... ....................................... 90 REFERENCES. . .............................................................................................................. 102 . vi LIST OF TABLES Table 1. 2. 3. 4. Page P r i n c i p l e S p e c tro s c o p ic C h a r a c t e r i s t i c s o f Reactant and Products .......................................................................... 13 Temperature Study o f th e P y r o ly s is C o n d itio n s o f The Methyl Imine o f 2 - A c e t y l- 6 - M e t h y l- 3 ,4 -D ih y d ro -2 H -P y ra n . . 14 P r i n c i p l e S p e c tro s c o p ic C h a r a c t e r i s t i c s o f Reactants and P r o d u c ts .......................................................................... 244 Temperature Study o f . t h e P y r o ly s is C o n d itio n s o f the P y r r o l i d i n e Enamine o f 2 - A c e t y l- 6 - M e t h y l3 ,4 -D i h yd ro -2 H -P yra n .............................................................................. 26 vi i LIST OF FIGURES Fi gure 1. ' Page [ 3 , 3 ] S ig m a tro p ic Rearrangement o f 2 - f o r m y l- 2 , 5 d i h y d r o - 4 H - p y r a n . . ................................................................................... 2 2. Biichi S y n th e sis o f S u b s t it u t e d 4 - A c e ty l cyclohexenes . . . . 3 3. L ip k o w it z S y n th e sis o f 2 - A c e t y l- 6 - M e t h y l- 3 , 4 - D ih y d r o 2H -Thipyran ....................................... 4 Proposed S yn th e sis o f 1 , 2 - D im e t h y l- 6 A c e t y l - P i p e r i d i n e ....................................... . 4 4. . . ........................... 5. [ 3 , 3 ] S ig m a tro p ic Rearrangement o f O - a lT y lh e xa n o la c tim . 5. Amino [ 3 , 3 ] S ig m a tro p ic Rearrangement o f H i l l 7. Thermal S ig m a tro p ic Rearrangement o f 4 - A l l y l IsopyrazoTe t o a P y r a z o I . . .......................................... 7 8. Eschenmoser1s S y n th e s is o f y ,s -U n s a tu ra te d Amides .................. 3 9. H i l l and Gilman S y n th e sis o f N -M e th y l- 2 ,2 -D im e th y l P e n t - 4 - E n a m in e . ....................................... 9 10. 11. 12. . . 5 and Newkom. . 6 [ 3 , 3 ] S ig m a tro p ic Rearrangement o f the P y r r o T id in o Enamine o f A c e ty l Furan t o O-Amino Phenol . . . . . . . . 10 P r e p a ra tio n o f 4 - A c e ty l Cyclohexene and 3 - A c e ty l Cyclohexanone By th e Enamine Cope Rearrangement . . . . . 12 Thermal I s o m e r iz a tio n o f the Imine t o the Corresponding Enamin e . ................................... .... . 16 13. Proposed [ 1 , 3 ] S ig m a tro p ic Hydrogen S h i f t ............................... . 16 14. Klopman's Proposed Keto-Enol T a u to m e r iz a tio n ............................... 17 15. Proposed [ 3 , 3 ] S ig m a tro p ic M i g r a t i o n ...............................' . . . . 18 16. Boat T r a n s i t i o n S ta te Geometry....................................... 19 17. Geometry o f S - A c e t y l - I - ( I - M e t h y l ) Amino Cyclohexene . . . . 20 18. Proposed Hydrogen Bonding . . . . '............................. 21 . . . v iii F ig u re Page 19. Proposed S upraannular E f f e c t ...................................................................... 20. P r e p a ra tio n o f 5 - A c e t y l - I - ( I - P y r r o l i d i n y l ) Cyclohexene by the Enamine Cope Rearrangement Proceeded by E th e r A l k y l a t i o n o r A cid H y d r o l y s i s .......................... .... 21 . . 25 Proposed [ 3 , 3 ] S ig m a tro p ic Rearrangement o f th e P y r r o l i d i n o Enamine o f 2 - A c e t y l- 6 - M e th y l- 3 ,4 - D ih y d r o - 2 H - P y r a n . . . . 28 22. Geometry o f 5 - A c e t y l - I - ( I - P y r r o l i d i n y l ) C y c l o h e x e n e ................. 29 23. Proposed S y n th e sis o f 3 -A c e ty l cyclohexanone by D i e l s - A ld e r Reaction and th e Form ation o f th e Corresponding P y r r o l i d i n o Enamines....................................... 31 21. 24. Proposed Cope Rearrangement o f the A c e ty l Enol o f 2 -A c e ty l -6 -M e th y l - 3 ,4 -D i h y d ro -2 H -P y ra n ............................................ 32 25. Proposed S y n th e s is o f a -C yperone......................................................... 34 26. Proposed S y n th e sis o f 7g,10g S e lin a - 4 , 1 1 - D ie n e ........................... 35 27. General S u b s t i t u t i o n P a tte r n o f Reactants and Products in th e Enamine Cope Rearrangement................................... 37 28. Vacuum P y r o ly s is A p p a ra tu s ...................................................................... 40 29. S i l v e r s t e i n ' s B revicom in S y n th e s is .................................................... 73 30. S i l v e r s t e i n 1s A l t e r n a t e Scheme.................................................... .... . 74 31. Wasserman S y n th e sis o f B revicom in .................................................... 75 32. Improved S i l v e r s t e i n S yn th e sis o f B re v ic o m in ............................... 76 33. K o cienski S y n th e sis o f B revicom in .................................................... 77 34. Coke S y n th e sis o f B re v ic o m in .............................. 78 35. Mundy S y n th e sis o f B revicom in ............................................................. 80 36. Kassanye S y n th e s is o f B r e v ic o m in .......................................................... 81 37. S harpless S y n th e s is o f C y c lic E t h e r s ................................................ 82 IX F i gure - Page 38. N ic o la o u S y n th e sis o f B i c y c l i c Ethers ................................... 39. S y n th e sis o f 2 - P r o p io n y l- 5 - M e th y l- 3 , 4 - D ih y d r o - 2 H - P y r a n . . . 85 40. Novel S y n th e tic Approach to B r e v i c o m i n . - ....................................... 87 41. Reaction Mechanism f o r the Form ation o f 5 - M e th y l- 7 E t h y l - 6 , 8 - D io x a b ic y c l o [ 3 . 2 . 1 ] o c t - 3 - e n e ....................................... 88 R e action Mechanism f o r th e Formation o f 5 - M e th y l- 7 E th yl - 6 ,8 -D i oxabi c y c l o [ 3 .2.1 ] o c t - 4 - o n e ....................................... 89 42. 83 X ABSTRACT The o r b i t a l symmetry a llo w e d [ 3 . 3 ] s ig m a tr o p ic rearrangem ent o f enamines d e r iv e d from 2 - a c e 't y l- 6 - m e t h y l- 3 ,4 -d ih y d ro -2 H -p y ra n has a llo w e d f o r f a c i l e e n t r y i n t o h i g h l y f u n c t i o n a l i z e d cyclohexanone d e riv a tiv e s . This new s y n t h e t i c methodology o f f e r s promise, f o r e n t r y i n t o s e s q u ite rp e n e n a t u r a l p r o d u c ts . A b y - p r o d u c t o f t h i s research has le d t o a symple s y n th e s is o f b r e v ic o m in , the a g g re g a tin g sex pheromone o f the p in e bark b e e t l e , Dendrootonus brevioomis. xi E xperim ental ideas are o f t e n born by chance, w i t h the help o f some casual o b s e r v a tio n . N othing i s more common; and t h i s i s r e a l l y the s im p le s t way o f b e g in n in g a piece o f s c i e n t i f i c work. We take a w a lk , so t o speak, i n the realm o f s c ie n c e , and we pursue what happens to p re s e n t i t s e l f to o u r eyes. " S e r e n d ip it y " Claude Bernard 1865 P a rt I : A NEW SYNTHETIC APPROACH TO SESQUITERPENE • INTERMEDIATES CHAPTER I HISTORICAL In r e c e n t y e a r s , two concepts have emerged in s y n t h e t i c method­ o lo g y ; t h a t o f l a t e n t f u n c t i o n a l i t y and th e use o f h e t e r o c y c l ic conI pounds in o r g a n ic s y n t h e s i s . D. L e d n ic e r in 1972 q u i t e a p t l y v e r b a l i z ­ ed the concept o f l a t e n t f u n c t i o n a l i t y as: "One c a r r i e s some necessary f u n c t io n th ro u g h one o r more steps o f a s y n th e s is in a p re c u r s o r fo rm ; and a t the p ro p e r stage th e p r e c u r s o r i s conve rted to th e needed g r o u p ." One c o u ld say t h a t t h i s concept i s a more s o p h is t ic a t e d use o f p r o t e c t ­ in g g ro u p s . The second and th e most im p o r ta n t concept was discussed by A. I . Meyers, s y n t h e s is . and in v o lv e s th e use o f h e t e r o c y c l ic compounds in o r g a n ic By d i r e c t l y a p p ly in g t h e . p r i n c i p l e o f l a t e n t f u n c t i o n a l i t y in a h e t e r o c y c l i c s y n t h e s i s , one c o u ld , i n e f f e c t , design a h e te r o c y c le which c o u ld a llo w f o r the i n t r o d u c t i o n o f a v a r i e t y o f f u n c t io n a l groups o r carbon s k e le to n s . Thus in s h o r t , Meyers' concept was t h a t o f using a h e te r o c y c le as a p r e c u r s o r o r v e h i c le f o r the s y n th e s is o f a more complex m o le c u le . These concepts were r e f l e c t e d in the s y n t h e t ic approach t o sub­ s t i t u t e d cyclohexenes taken by B u c h i3 ' 4 which was based on the p re v io u s work by J. D. R o berts5 and L u tz t h a t d e u te r iu m - la b e le d 2 - f o r m y l- 2 , 5 - d i ­ methyl - 2 , 3 - d ih y d ro -4 H -p y ra n [ 1 ] c o u ld t h e r m a lly is o m e riz e by means o f a [ 3 , 3 ] s ig m a tr o p ic rearrangem ent. A s e r ie s o f w e ll- p la n n e d NMR s tu d ie s c o n c l u s i v e ly demonstrated t h a t th e a ld e h y d ic d e u te riu m CDO la b e l e x- 2 changed w i t h H-6. I t was a ls o noted t h a t the o p t i c a l a c t i v i t y due to the asymmetric c e n te r ( * ) was n o t l o s t . H 3 2 F igure I . d i hydro-4H-Pyran [ 3 , 3 ] S ig m a tro p ic Rearrangement o f 2 - f o r m y l - 2 , 5 - By d i m e r iz in g a ,B -u n s a tu r a te d compounds [ 4 ] , Biichi was a b le to o b t a in acyl d ih y d ro p y ra n s [ 5 ] w h ic h , when fo llo w e d by a W i t t i g condensa­ t i o n , gave 3 , 4 - d ih y d r o - 2 H - p y r a n e t h y lenes [ 5 ] , essence, a l l y ! These compounds were, in v i n y l e th e rs whose thermal oxy-Cope rearrangem ent had been p r e v io u s ly w e ll documented in the l i t e r a t u r e . J u s t as had been e xpe cted , these s u b s t it u t e d 3 ,4 -d ih y d ro - 2 H -p y r a n e t h y l enes underwent oxy-Cope rearrangem ents to y i e l d s u b s t it u t e d cyclohexene [ 7 ] compounds. Such s u b s t i t u t e d acyl cyclohexenes are n o t r e a d i l y o b t a in a b le from mixed D i e l s - A ld e r r e a c t io n s and, in f a c t , are o n ly m inor p ro d u cts from these r e a c tio n s . 3 R Il 4 0 5 0 6 R 0 7 F ig u re 2. Biichi S yn th e sis o f S u b s titu te d 4 - A c e ty l cyclohexenes Now, in a c o n t in u in g e f f o r t in t h i s la b o r a t o r y to r e a l i z e the fu ll i m p l i c a t i o n s o f t h i s w o rk, i t was conceived t o s u b s t i t u t e d i f f e r e n t heteroatoms f o r the carbon yl oxygen and re a rra n g e them i n t o the r i n g . Thus K. L i p k o w it z 7 ' 8 f i r s t made th e th io c a r b o n y l by r e f l u x i n g 2 - a c e t y l 6 - m e th y l- 3 , 4 -d ih y d ro -2 H -p y ra n in p y r id i n e and phosphorous p e n t a s u lf id e . Sealed tube p y r o l y s i s o f the t h i o a c e t y l ra n y l system [ 1 0 ] . pyran [ 9 ] y ie ld e d the t h ia p y - 4 F ig u re 3. L ip k o w it z S y n th e sis o f 2- A c e t y l- 6 - M e t h y l- 3 , 4 D ihydro-Z H -T hiapyran Thus, my o b j e c t i v e was to a tte m p t the n e xt heteroatom , n itr o g e n The methyl im ine o f 2 - a c e t y l - 6 - m e t h y l - 3 , 4 -d ih y d ro -2 H -p y ra n [ 1 1 ] was pre pared and was s u b je c te d to c o n d it io n s o f the Cope rearran gem ent. The expected p ro d u c t from the p y r o l y s i s was the s u b s t i t u t e d I , 2 -d im e th y -6 a c e ty l-p ip e rid in e [1 2 ]. O N Il F ig u re 4. O ^ 12 Proposed S y n th e sis o f 1 , 2 - D im e t h y l- 6 - A c e t y l- P i p e r ­ i d in e However, t h i s was n o t observed, and c a r e f u l a n a ly s is o f the s p e c tr a l data from the p ro d u c t le d to our assignment f o r the s t r u c t u r e o f the 5 observed compound as 5 - a c e t y l - I - ( I - m e t h y l) amino cyclohexene [ 1 3 ] . A f t e r c o n s id e r a t io n o f a mechanism f o r the r e a c t i o n , i t became app arent t h a t an im ine-enam ine t a u t o m e r iz a t io n had f i r s t taken p la c e , fo llo w e d by a [ 3 , 3 ] s ig m a tr o p ic s h i f t o r Cope rearrangem ent. This would account f o r the carbon s k e le to n o f the r i n g . Thermal is o m e r iz a t io n o f im ine to enamines have been th o ro u g h ly discussed i n the l i t e r a t u r e 9 f o r many y e a r s . However, the a p p l i c a t i o n o f such is o m e r iz a t io n p r i o r to a [ 3 , 3 ] s ig m a tr o p ic s h i f t has re c e iv e d ve ry l i t t l e however. a tte n tio n . Some i n t e r e s t i n g examples can be p o in te d t o , P y r o ly s is o f 0 - a l I y l h exa nola c t im 10 [ 1 4 ] gave the 3 - a l l y l - hexanolactam [ 1 6 ] in b e t t e r than 60% y i e l d . F ig u re 5. [ 3 , 3 ] S ig m a tro p ic Rearrangement o f 0 - a ll y lh e x a n o la c t im 6 R a t i o n a l i z a t i o n o f t h i s r e a c t io n sequence was t h a t th e im ine [ 1 4 ] had r e a d i l y iso m e rize d to the enamine [ 1 5 ] . T h is in te r m e d ia te was then s e t up f o r a t h e r m a lly - a llo w e d [ 3 , 3 ] s ig m a tr o p ic s h i f t . H ill and Newkom11 demonstrated t h a t when q u a te rn a ry ammonium s a l t s o f i mines w i t h an a l k y l group in the 2 p o s i t i o n were conve rted to the anhydro base ( e n a m in e )[T 8 ],th e y co u ld be then t h e r m a lly rearranged to [ 1 9 ] , presumably by a [ 3 , 3 ] s ig m a tr o p ic s h i f t . F ig u re 6. Amino [ 3 , 3 ] S ig m a tro p ic Rearrangement o f H i l l and Newkom Based on t h i s w o r k , 12 Bramley and G rigg demonstrated t h a t 4 , 4 - d i a l I y l 3 , 5 - d im e t h y lp y r a z o le f i r s t is o m e rize d to the enamine [ 2 1 ] and then r e ­ arranged by way o f a [ 3 , 3 ] s ig m a tr o p ic s h i f t to [ 2 2 ] . 7 N -N 20 F ig u re 7. z o le t o a Pyrazol N -N H 21 N -N H 22 Thermal S ig m a tro p ic Rearrangement o f 4 - A l l y l Isopyra- Another system in v e s t i g a t e d by the same a u th o rs was t h a t o f 3 - a l l y l i n doIe m ime ketemine [ 2 3 ] which f i r s t t h e r m a lly iso m e rize d to the enamine [ 2 4 ] and then rearran ged to [ 2 5 ] by means o f a [ 3 , 3 ] s ig m a tr o p ic s h i f t . To s u b s t a n t ia t e t h i s c o n c lu s io n , th e enamine [ 2 6 ] was prepared (CH3I / NaOH), and therm al rearrangem ent gave the expected p ro d u c t [ 2 7 ] . 8 27 Eschenmoser has employed the method o f t r a n s v i n y l e t h e r i f i c a t i o n o f 1d im e th yla m in o -1 -m e th o xy-e th e n e and the d im e th yl a c e ta l o f N ,I V d im e t h y lacetamide as a v i n y l a t i n g a ge nt. Again note the enamine in te r m e d ia te [ 3 1 ] which then p a r t i c i p a t e s in the a c tu a l [ 3 , 3 ] s ig m a tr o p ic s h i f t . (OCH3)2 OCH3 + \ 28 N(CH3)2 31 F ig u re 8. CH3- C CH2— ( / / \ N(CH3 )2 N(CH3)2 N(CH3)2 32 Eschenmoser' s S y n th e sis o f Y ,6 -U n s a tu ra te d Amides 9 H i l l and Gilman have demonstrated t h a t the enamine [ 3 3 ] prepared from N-methyl a l ly l a m i n e and a - d i s u b s t i t u t e d aldehydes p y ro ly z e d q u a n t i t a ­ t i v e l y to [ 3 4 ] v ia a [ 3 , 3 ] s ig m a tr o p ic s h i f t a t 250° C. M ild h y d r o ly s is o f [ 3 4 ] gave the aldehyde [ 3 5 ] . F ig u re 9. Pent-4-Enamine H ill and Gilman S y n th e sis o f N -M e th y l- 2 ,2 Dimethyl We now c o u ld view o u r enamine s ig m a tr o p ic rearrangem ent in terms o f E. J . C o re y 's 15 r e t r o - s y n t h e t i c a n a ly s is as: From such a n a ly s is o f th e s y n t h e t ic t r e e , i t amine tr a n s fo r m i s the key in te r m e d ia t e . seems obvious t h a t the en­ Thus, th e d i r e c t p r e p a ra tio n and rearrangem ent o f th e enamine was u nd ertake n. The p y r r o l i d i n e en- 10 amine o f 2 - a c e t y l - 6 - m e t h y l - 3 , 4 - d ih y d r o pyran rearranged q u i t e e a s i l y under the p y r o l y s i s c o n d it io n s as b e fo r e . R e tr o s y n t h e t ic a n a ly s is y i e l d s th e obvious c o n c lu s io n t h a t the new enamine [ 4 1 ] i s a ls o q u i t e a u s e fu l s y n t h e t ic in t e r m e d ia t e : 40 41 The o n ly l i t e r a t u r e analogy to our system was re p o r te d by B i r k o f f e r 16 and Daum in 1962. Here the p y r r o l i d i n e enamine o f a c e ty l fu ra n [ 4 3 ] was prepared and Cope rearran ged to the co rre s p o n d in g o-amino phenol [ 4 4 ] . F ig u re 10. [ 3 , 3 ] S ig m a tro p ic Rearrangement o f the P y r r o l i d i n e Enamine o f A c e ty l Furan to o -Amino Phenol CHAPTER 2 RESULTS AND DISCUSSION The N-methyl im ine o f 2 - a c e t y l- 6 - m e t h y l- 3 , 4 - d ih y d r o - 2 H - p y r a n was prepared by passing methyl amine i n t o a c o ld s o l u t i o n o f e t h e r c o n t a i n ­ in g 2 - a c e t y l - 6 - m e t h y l - 3 , 4 -d ih y d ro -2 H -p y ra n and m o le c u la r s ie v e s . F o l­ lo w in g i s o l a t i o n and p u r i f i c a t i o n , th e methyl im ine [ 1 1 ] was vacuum p y ro ly z e d a t 250° C to y i e l d [ 1 5 ] in 66% y i e l d a f t e r d i s t i l l a t i o n . The p ro d u c t o f th e p y r o l y s i s was assigned th e s t r u c t u r e [ 1 3 ] based on the f o l l o w i n g s p e c tr o s c o p ic evidence (T able 1 ) : -I the im ine C=N s t r e t c h 1667 cm fo r [1 1 ]; The i n f r a r e d s p e c tra shows -I and th e e t h e r C-O-C s t r e t c h 105.3 cm however, th e p ro d u c t does n o t e x h i b i t a C-O-C s t r e t c h nor a C=N s t r e t c h ; “I however an N-H s t r e t c h 3413 cm was observed as w e ll as a C=C s t r e t c h 1645 cm~^ which i s a ls o c h a r a c t e r i s t i c o f a c a r b o n y l. ondary enamines C=C s t r e t c h a t 1695 cm~^ was observed. a b s o r p tio n s p e c tra shows the d i s t i n c t n [1 1 ]; tt * Sec­ The u l t r a v i o l e t band 231 nm f o r the im in e however, th e p y r o l y s i s p ro d u c t has an a b s o rp tio n a t 220 nm as w e l l . a s 284 nm. T h is i s i n d i c a t i v e o f both a secondary enamine and a carbon yl group p re s e n t as w e l l . NMR sp ectroscopy c o n firm e d the s t r u c ­ t u r e as t h a t o f [ 1 3 ] by th e o b s e rv a tio n o f the v i n y l hydrogen as a t r i p l e t a t 4.486 which i s in e x c e l l e n t agreement w i t h th e observed chemical s h i f t o f the v i n y l h y d r o l y s is o f 1 - N - p ip e r id in o c y c lo h e x - l- e n e a t 4 .5 6 s , which i s a ls o observed as a t r i p l e t . a d ja c e n t to the carbon yl was observed a t 2.166. A lso th e methyl group F u r th e r s t r u c t u r a l 12 a n a ly s is was done by chemical d e g ra d a tio n as f o l l o w s . Simple c a t a l y t i c h yd ro g e n a tio n o f [ 1 3 ] w it h Pd/C produces the s a tu r a te d amine [ 4 5 ] in 82% y ie ld . The q u a te rn a ry ammonium s a l t o f [ 4 5 ] was prepared in e th e r and excess methyl i o d id e , and t h i s , when f o llo w e d by a Hoffman e l i m i n a t i o n w it h potassium t e r t - b u t o x i d e 17 gave 4 - a c e t y l cyclohexene [ 4 7 ] in 62% y ie ld . A cid h y d r o l y s is o f [ 1 3 ] gave 3 - a c e t y l cyclohexanone [ 3 6 ] in 72% y ie ld . A tem p era ture versus p ro d u c t stu d y (Table 2) was then c a r r i e d o u t w i t h the r e s u l t s t h a t 250° C was the optimum p y r o l y s i s te m p e ra tu re . Temperatures any h ig h e r u s u a ll y gave e x te n s iv e p o ly m e r iz a t io n and a marked decrease in p ro d u c t y i e l d . F ig u re 11. P r e p a ra tio n o f 4 - A c e ty l Cyclohexene and 3 -A c e ty l Cyclohexanone By th e Enamine Cope Rearrangement P r i n c i p l e S p e c tro s c o p ic C h a r a c t e r i s t i c s o f R eactant and Products NMR Spectroscopy I n f r a r e d S pectroscopy 1053 cm ^ C-O-C s tre tc h o f th e e t h e r rn 3.076 \ C=N s t r e t c h o f im ine 13 O Il 1667 cm"^ 11 N-H s t r e t c h o f secondary enamine 3413 cm '1 C=C s t r e t c h o f secondary enamine 1645 cm 1 C=O s t r e t c h 1695 cm 1 E oJ 13 11 Z Table I . CH- C xN 4.486 CH3-NH 2.686 CH3 i " 0 2.166 14 Table 2. Temperature Study o f The P y r o ly s is C o n d itio n s o f The Methyl !mine o f 2 - A c e t y l- 6 - M e t h y l- 3 , 4-D ihyd ro -2 H -P yra n 11 T ( 0C) % 11* % 13* 5 .0 grams 150° C 100% ------ 5 .0 grams 175° C 100% 5 .0 grams 200° C 81 .6% ,18.4% 5 .0 grams 225° C 29.0% 71.0% 5 .0 grams 250° C 5 .0 grams 275° C — 73.0%** 5 .0 grams 300° C ------ 48.2%** 100% * R e l a t iv e g lc peak areas o f m a te r ia l i s o l a t e d , a n a ly s is was done on 10% SE-30 Chromosorb W a t 160° C and peak areas were measured by t r i a n g u la t io n . * * E x t e n s iv e p o ly m e r iz a t io n was observed in th e p y r o l y s i s tu b e . 15 In c o n s id e r in g the r e a c t io n mechanism f o r o u r plan i t was o f i n ­ t e r e s t to e s t a b l i s h the c o n f i g u r a t i o n o f the N-methyl im in e as syn o r a n ti. C a re fu l e v a lu a t io n o f the NMR18 data e s t a b lis h e d the C-methyl group was observed a t a h ig h e r f i e l d syn. f o r the a n t i isomer than f o r the T his i s a ls o observed f o r the N-methyl group f o r the a n t i as w e l l ; however, th e C-H p ro to n i s observed a t a lo w e r f i e l d f o r th e a n t i isomer than f o r th e s y n . The c o u p lin g c o n s ta n t f o r the C-methyl group and the N-methyl group i s s m a lle r (J = 8 .9 H e rtz ) f o r the a n t i isomer than f o r the S^m isomer (J = 1.5 H e r t z ) . The c o u p lin g c o n s ta n t between CH and N-methyl i s l a r g e r f o r the a n t i (J = 8 .9 H e rtz ) than f o r the Sjm (J = 0 .5 H e r t z ) . We can conclude t h a t our N-methyl im ine i s 92% a n t i and 8% s y n . The f i r s t c r i t i c a l step in th e rearrangem ent i s the thermal i s o ­ m e r i z a t io n 19 o f the N-methyl k e tim in e [ 1 1 ] to the N-methyl enamine [ I I A ] . 16 F ig u re 12. ponding Enamine Thermal I s o m e r iz a tio n o f the Imine to the C o rre s ­ The mechanism f o r t h i s is o m e r iz a t io n can be p o s tu la te d to be a [1 ,3 ] s ig m a tr o p ic hydrogen s h i f t . For such a s h i f t to be th e r m a lI y a llo w e d an a n t a r a f a c i a l m i g r a t i o n 203-0 o f the hydrogen on C-j, would have to be accom plished ( F ig u re 13). F ig u re 13. Proposed [ 1 , 3 ] S ig m a tro p ic Hydrogen S h i f t From the o r b i t a l to p o lo g y diagram , we can see t h a t as the n i - tro g e n atom r e h y b r id iz e s from sp p to sp O th e re i s a sim ultaneous re h y - 17 b r i d i z a t i o n o f C-I from an sp o 2 c o n f i g u r a t i o n to sp . Thus as the h y d ro ­ gen i s being t r a n s f e r r e d from carbon to the n itr o g e n th e re i s a syn­ chronous is o m e r iz a tio n o f th e double bond from C^=N to Thus th e non-bonding p a i r o f e le c t r o n s on the n i tr o g e n now becomes a v a i l a b l e f o r o v e r la p w i t h the n-system o f the double bond, and c o u ld be the d r i v i n g f o r c e f o r the r e a c t i o n . Klopman20f has suggested t h a t k e to -e n o l tautom - erism co u ld p o s s ib ly be a [ 1 , 3 ] s ig m a tr o p ic s h i f t system in v o lv i n g a heteroatom (oxygen) possessing an occupied p - o r b i t a l p e r p e n d ic u la r to the p -ir -p la n e . F ig u re 14. Klopman's Proposed Keto-Enol T a u to m e riz a tio n 18 One can note t h a t i n the geometry o f IA severe s t e r i c i n t e r a c ­ t io n s between the enamine and the methyl group are a t a minimum and t h a t c o p l a n a r i t y has been m a in ta in e d between the n itr o g e n and the carbon o f the double bond. The second ste p o f the rearrangem ent i s a co n c e rte d [ 3 , 3 ] sigmat r o p i c m i g r a t i o n . 2 0 ' 22 For t h i s rearrangem ent to be t h e r m a lly a llo w e d , th e r e must be a s u p r a f a c i a l - s u p r a f a c i a l o v e r la p o f the p - o r b i t a l lobes ( F ig u re 1 5 ). c lic An o r b i t a l to p o lo g y diagram c l e a r l y shows t h i s in the c y ­ six-membered t r a n s i t i o n s t a t e [ I I B ] . Il-A F ig u re 15. We w i l l Il-B Proposed [ 3 , 3 ] S ig m a tro p ic M ig r a t io n a ls o note t h a t in both [ I I A ] and [ I I B ] t h a t the o v e r la p between the u n p a ire d e le c t r o n s on the n itr o g e n and th e ir-system o f the double bond are a t a maximum due to the c o p l a n a r i t y o f the N and carbon atoms. Thus in a co n c e rte d f a s h io n , as the carbons 3 and 3' are re h y - 19 b r i d i z i n g t o form the a-bond, carbon I ' from sp o to sp O which w i l l i n t e r a c t i o n o f the c e n t r a l and are a ls o r e h y b r i d i z i n g form th e ir-system between I ' and 2 1. The p -lo b e s o f C -2 1 and oxygen s l i g h t l y d e s t a b i- I i z e the system and c o n t r i b u t e to the extreme p y r o l y s i s c o n d it io n s . F ig u re 16. 22 Boat T r a n s i t i o n S ta te Geometry Even though the c h a i r - l i k e t r a n s i t i o n s t a t e geometry i s lo w er in energy due to the q u a s i- p la n a r arrangement o f the s i x p - lo b e s , i t a ls o s t e r i c a l I y im p o s s ib le to be a t t a i n e d . 22,3 is Thus th e o n ly a c c e s s i­ b le t r a n s i t i o n s t a t e geometry i s t h a t o f the boa t. 20 F ig u re 17. Geometry o f 5 - A c e t y l - I - ( I - M e t h y l) Amino Cyclohexene Again in the p r o d u c t, 5 - a c e t y l - I - ( I - m e t h y l) amino cyclohexene c o p l a n a r i ­ t y has been m a in ta in e d between the n it r o g e n atom and the carbon s, hence, the o v e r la p between the u np aired e le c tr o n s o f the n i tr o g e n and the system o f the double atom i s a t a maximum. tt- 23 One would e xpe ct t h a t the p ro d u c t [ 1 3 ] would is o m e riz e again from th e enamine to the im ine [ 4 8 ] . However, NMR data does not show [ 4 8 ] to be p re s e n t in a p p re c ia b le amounts ( le s s than 1%). One may be a b le to r a t i o n a l i z e t h i s by the f a c t t h a t in c e r t a i n systems hydrogen bonding may s t a b i l i z e the enamine ta u to m e r24 thro ugh i n t e r - 21 m o le c u la r c h e la t e fo r m a tio n between the carbon yl oxygen and the NH p r o ­ ton ( F ig u re 1 8 ). T his co u ld a ls o account f o r the high b o i l i n g p o i n t o f the rearran ged p ro d u c t [ 4 8 ] . F ig u re 18. Proposed Hydrogen Bonding An a l t e r n a t i v e e x p la n a tio n c o u ld be a t t r i b u t e d to the supraannuI a r e f f e c t . 25 In o u r system, the w - e le c tr o n s o f the enamine double bond co u ld i n t e r a c t w it h th e carbon yl group. Thus we would n o t see the r e ­ v e r s io n back to the methyl im ine b u t r a t h e r the s t a b i l i z e d enamine form . 3 F ig u re 19. Proposed S upraannular E f f e c t 22 To t e s t the v a l i d i t y o f t h i s rearran gem ent, o t h e r examples were examined. The e t h y l [5 1 A ] , pro p yl [5 1 B ] , and b u ty l [51C] i mines were prepared in the manner p r e v io u s ly d e s c rib e d and p y ro ly z e d under the same c o n d it io n s to y i e l d the c o rre s p o n d in g a l k y l amin o - 5 - a c e t y lcyclohexene in every case. A cid h y d r o l y s is a ls o y ie ld e d the 5 - a c e t y I cyclohexanone. The severe disadvan tag e o f s y n t h e t i c a l l y u sin g these compounds would be th a t a ll the a l k y l amin o - 5 - a c e t y lcyclohexenes are e x tre m e ly a i r s e n s i t i v e and have to be s to re d under n i t r o g e n . Based on the data a lre a d y d is c u s s e d , the s p e c i f i c i t y o f p y r o l y ­ s is co u ld be f u r t h e r e d by the d i r e c t p r e p a r a tio n o f th e enamine, thus e l i m i n a t i n g the i n i t i a l im ine-enam ine t a u t o m e r iz a t io n . A ls o , i t would unambiguously e s t a b l i s h t h a t the fo r m a tio n o f the enamine i s the key step in th e thermal rearran gem ent. The p y r r o l i d i n e enamine o f 2 - a c e t y l - 6 - m e t h y l- 3 , 4 -deh ydro -2H -p yra n [ 5 3 ] was formed by g e n t ly warming a m ix ­ t u r e o f the pyran and p y r r o l i d i n e o ver m o le c u la r s ie v e s . The vacuum p y r r o l y s i s o f the r e s u l t i n g enamines [ 5 3 ] under the same c o n d it io n s p r e ­ v i o u s l y d e s c rib e d p ro v id e d an e x c e l l e n t y i e l d o f the d e s ire d p ro d u ct 23 [4 1 ]. In s tru m e n ta l a n a ly s is aga in v e r i f i e d the s t r u c t u r e (Table 3 ) . The i n f r a r e d s p e c tra o f th e i n i t i a l enamine [ 8 ] e x h i b i t e d a C=C s t r e t c h o f enamine a t 1672 cnf^ and an e t h e r C-O-C s t r e t c h a t 1070 cm \ The re a rra n g e d p ro d u c t [ 4 1 ] d id n o t e x h i b i t the e t h e r C-O-C s t r e t c h , however th e C=C s t r e t c h o f enamine was observed a t 1639 cm" 1 as w e ll as the C=O s t r e t c h o f carbon yl a t 1698 cm"1 . The u l t r a v i o l e t a b s o rp tio n s p e c tra showed an a b s o r p tio n a t 228 nm f o r [ 8] , however th e rearranged p ro d u c t [ 4 1 ] g ive s tw o: 227 nm ( c h a r a c t e r i s t i c o f enamine) and 281 nm ( c h a r a c t e r i s t i c o f th e t r a n s i t i o n o f a c a r b o n y l) . The n u c le a r magnetic resonance spectrum co n firm e d th e s t r e t c h as [ 4 1 ] by th e o b s e rv a tio n o f th e methyl group a d ja c e n t t o th e carbon yl a t 2.146 and a ls o the v in y l hydrogen as a t r i p l e t a t 4 .2 8 6 . Simple a c id h y d r o l y s is o f [ 4 1 ] gave a c e t y l c y c l ohexanene [ 3 6 ] i n 73% y i e l d . A l k y l a t i o n o f [ 4 1 ] w it h methyl - io d id e f o llo w e d by a c id h y d r o l y s is y i e l d 2 - m e th y l-5 -a c e ty lc y c lo h e x a n o n e [ 4 0 ] i n 76% y i e l d . Note: *B oth isomers were observed. Table 3. P r i n c i p l e S p e c tro s c o p ic C h a r a c t e r i s t i c s o f Reactants and Products NMR Spectroscopy I n f r a r e d Spectroscopy 41 53 1070 cm' 1 C-O-C s t r e t c h o f the e th e r 1672 cm" 1 C=C s t r e t c h o f the enamine C=O s t r e t c h o f the carbon yl 53 3.645 41 CH2— CXN_ 1639 cm" 1 XcH=cvI 4.285 1598 cm' 1 CHo— C— 2.145 3 Ii 25 F ig u re 20. P r e p a ra tio n o f 5 - A c e t y l - I - ( I - P y r r o l i d i n y l )Cyc1ohexene by the Enamine Cope Rearrangement Proceeded by E th e r A l k y l a t i o n o r A cid H y d ro ly s is A g a in , a tem p era ture versus p ro d u c t study (Table 4) was u nd er­ taken w it h the r e s u l t s t h a t 250° C was the optimum p y r o l y s i s tem p era ture which concurs w it h t h a t o f the im ine rearrangem ent. Temperatures any h ig h e r gave e x te n s iv e p o ly m e r iz a t io n and a marked decrease in p rodu ct j u s t as we observed in the p re v io u s imine p y r o l y s i s The i n i t i a l (Table I , f o rm a tio n o f the p y r r o d id in o enamine c o u ld , in t h e o r y , y i e l d two isomers [53A] and [ 5 3 B ] ; however, o n ly [53A] was observed. ,N * 8 O p. 13). 53-A 5 3 -B 26 Table 4. Temperature Study o f th e P y r o ly s is C o n d itio n s o f the P y r r o l i d i n e Enamine o f 2 - A c e t y l - 6- M e th y l-3 ,4 -D ih y d ro -2 H -P y ra n 53 T ( 0C) % 53* % 41* 5 .0 grams 150° C 100% ' ' ---- 5 .0 grams 175° C 100% — 5 .0 grams 200° C 79.0% 21. 0% 5 .0 grams 225° C 31.0% 69.0% 5 .0 grams 250° C — 5 .0 grams 275° C — 70.0%** 5 .0 grams 300° C — 55.0%** . 100% * R e l a t iv e g l c peak areas o f m a te r ia l i s o l a t e d ; a n a ly s is was done on 10% SE-30 Chromosorb W a t 160° C peak areas were measured by tria n g u la tio n . * * E x t e n s iv e p o ly m e r iz a t io n was observed in th e p y r o l y s i s tu b e . 27 T his c o u ld be r a t i o n a l i z e d by the severe s t e r i c i n t e r a c t i o n s between the methylene groups ( * ) a d ja c e n t to the n i tr o g e n atom and the c o p la n a r pyran r i n g in [ 5 3 B ] . Such i n t e r a c t i o n s are m inim ized in [53A ] w h ile o v e r la p o f the e l e c t r o n p a i r on the n it r o g e n and the m -e le c tro n s o f the double bond are maximized by the r e q u ire d c o p l a n a r i t y o f the n itr o g e n o f the n i tr o g e n and carbon atom s. 23,28 Thus, severe d e s t a b i l i z i n g non- bonded r e p u ls io n a r is e s from the more s u b s t it u t e d iso m er. T h is can be f u r t h e r s u b s t a n t ia te d by the re p o rte d p r e p a r a tio n o f the p y r r o l i d i n e enamine o f a c e t y l cyclo p e n ta n e [ 5 3 ] [ 5 4 ] e x c l u s i v e l y in the le s s s u b s t i ­ tu te d isomer [55A] . 29 The mechanism f o r the thermal rearrangem ent o f th e p y r r o l i d i n e enamine o f 2 - a c e t y l - 6- m e t h y l- 3 , 4- d ih y d ro -2 H -p y ra n to 5 - a c e t y l - I - ( I p y r r o l i d i n y l ) cyclohexene was aga in e n v is io n e d as a co n c e rte d [ 3 , 3 ] s i g m a tro p ic s h i f t o f oxy-Cope re a rra n g e m e n t. 20' 22 For such a rearrangement to be t h e r m a lly a llo w e d , th e r e must be a s u p r a f a c i a l - s u p r e f a c i a l o v e rla p o f the i n t e r a c t i n g p - o r b i t a l to p o lo g y o f [ 5 3 C ] . lobes which can be seen from the o r b i t a l 28 F ig u re 21. Proposed [ 3 , 3 ] S ig m a tro p ic Rearrangement o f the P y r r o l i d i n o Enamine o f 2 - A c e t y l - 6- M e t h y l- 3 ,4-D ih yd ro -2 H -P yra n A g a in , even though the c h a i r - l i k e t r a n s i t i o n s t a t e geomet r y 2 2 ' 3 a ' b i s c l e a r l y fa v o re d o ver th e b o a t, i t i s a ls o c l e a r once again t h a t the c h a i r t r a n s i t i o n s t a t e i s s t e r i c a l l y im p o s s ib le to be a t t a in e d in [ 5 3 C ] , hence the boat t r a n s i t i o n s t a t e re p re s e n ts th e o n ly a c c e s s ib le pathway. One must note t h a t in the rearran ged p ro d u c t [ 4 1 ] , the o v e r la p between the e l e c t r o n p a i r on the n it r o g e n and the e le c t r o n s o f the irsystem are a t a maximum due to the c o p l a n a r i t y o f th e n itr o g e n and the 2 3 , 28Cyclohexene carbon s. S t e r ic h indrance between the methylene h y d ro - 29 gens a d ja c e n t to the n itr o g e n ( * ) and the 4 a c e ty l cyclohexene r i n g are a t a minimum, which s i g n i f i c a n t l y c o n t r ib u t e s to the s t a b i l i t y o f the m olecu le. 41 F ig u re 22. Geometry o f S - A c e t y l - I - ( I - P y r r o l i d i n y l ) C y c l o h e x e n e In c o n c lu s io n , o f the two systems in v e s t i g a t e d , the im ine and enamine; the 5- a c e t y l - I - ( I - p y r r o l i d i n y l jcyclohe xene i s e a s ie r to work w i t h , a i r s t a b le f o r s h o r t p e rio d s o f tim e and the y i e l d s are much b e tte r. In s y n t h e t ic s t r a t e g i e s t h i s would be the in te r m e d ia te compound o f c h o ic e . Another p l a u s i b l e s y n t h e t ic s t r a t e g y would be the D i e l s - A ld e r r e a c t io n between 2-methoxy b u ta d ie n e -5 ,6 and m e th y l-3 -b u te n e -2 -o n e [ 5 7 ] ; however, th e r e would be two pro d u cts from t h i s r e a c t io n [58A] and [58B] and w hether o r n o t one co u ld se pa ra te these i s unknown. However, i f one could sepa ra te these two components, m ild a c id h y d r o l y s is o f [58A] would g iv e the 5 - a c e t y l cyclohexanone [ 5 9 ] . Now the p r e p a r a t io n o f the p y r r o l - id in o enamine o f 5- a c e t y l cyclohexanone w i l l y i e l d f i v e compounds 30 [OOA9B ] , o f which o n ly one w i l l be the t a r g e t compound [6 0 A ]. T h e re fo re one can conclude t h a t th e enamine-Cope rearrangem ent would be the method o f ch o ic e f o r p re p a rin g compounds o f the n a tu re because o f the f a c t t h a t th e enamine f u n c t i o n a l i t y w i l l f o r a d d i t i o n a l work". be in th e c o r r e c t c o n f i g u r a t i o n I t has been a ls o suggested t h a t th e a c e ty l enol [ 6 1 ] d e r i v a t i v e co u ld a ls o p o s s ib ly be Cope rearran ged t o g iv e [ 6 2 ] , which then co u ld be a l k y l a t e d in th e 2 p o s i t i o n . The shortcom ings to t h i s co u ld be th e f a c t t h a t upon a c id h y d r o ly s is to th e ketone [ 6 3 ] , enamine fo r m a tio n w i l l be i n d i s c r i m i n a t e and y i e l d 6 compounds [6 4 A -F ]. 31 F ig u re 23. Proposed S y n th e sis o f 3 -A c e ty l cyclohexanone By D i e l s - A ld e r R eaction And the Formation o f the Corresponding P y r r o l i d i n e Enamines 32 F ig u re 24. Proposed Cope Rearrangement o f the A c e ty l Enol o f 2 - A c e t y l - 6- M e th y l-3 ,4 -D ih y d ro -2 H -P y ra n CHAPTER 3 APPLICATION OF THE ENAMINE COPE REARRANGEMENT R e tr o s y n t h e t ic a n a l y s i s 15 o f the b i c y c l i c s e s q u ite rp e n e s s k e le t o n s 30a,b o f the eudesmanes [ 6 5 ] , erem ophilanes [ 66] , and valera nes [ 6 7 ] re ve a l one key in te r m e d ia te [ 68] which m ig h t serve as a common p r e c u r s o r . For eudesmanes (R = CH3 ) , and f o r erem ophilane (R = H ), Robinson a n n e la tio n o f [ 68] w i t h e th y l v i n y l ketone w i l l a llo w f o r the c o n s t r u c ­ t i o n o f the b a sic o c t a l ene s k e le to n e s s e n t ia l f o r th e t o t a l s y n t h e s is 31 o f compounds r e la t e d to the abovementioned carbon s k e le to n s . Thus, a p p l i c a t i o n o f the enamine Cope rearrangem ent can b e s t be demonstrated 34 by the proposed s y n th e s is o f a-cyperone [ 7 4 ] . Robinson a n n e la t i o n 32a,b o f the r e s u l t i n g enamine [ 7 1 ] would g iv e [ 7 2 ] . S e le c t iv e k e t a l i z a t i o n o f oc,0-u n s a tu r a te d ketones such as [ 7 2 ] w it h o t h e r ketones p re s e n t i s w e ll-k n o w n . Thus the p ro d u c t [ 7 3 ] fo llo w e d by a W i t t i g condensation would c o n v e rt the a c e t y l carbon yl to a methylene group. H y d ro ly s is o f the k e ta l would once again re g e n e ra te our a ,B - u n s a tu r a te d ketone and thus complete the t o t a l s y n th e s is o f a-cyperone [ 7 4 ] . Note t h a t the s y n th e s is i s accom plished in f i v e h i g h - y i e l d steps as compared to the s y n th e s is developed by P ie rs which was accomplished in e i g h t s te p s . Another s y n th e s is o f a-cyperone by J. K. Roy34 c o n s is te d o f the Robinson a n n exation o f dehydrocarvone which gave a r e p o rte d 3% y i e l d . 74 F ig u re 25. Proposed S y n th e sis o f a-Cyperone 35 Another d e m o n s tra tio n o f the enamine Cope rearrangem ent as a u s e fu l s y n t h e t i c t o o l co u ld be in the proposed s y n th e s is o f 7 b , 106 s e l i n a - 4 , 1 1- d ie n e . Again Robinson a n n e la t i o n 32 o f [ 7 1 ] w i t h e th y l v i n y l ketone would g iv e [ 7 2 ] . P re fe re n tia l k e t a l i z a t i o n w i t h e t h a n e d i t h i o l 33 o f the a ,B -u n s a tu r a te d ketone in [ 7 2 ] , fo llo w e d by Raney n ic k e l d e s u l­ f u r i z a t i o n , would y i e l d th e u n s a tu ra te d compound [ 7 5 ] . Subsequent W i t t i g cond ensatio n o f [ 7 6 ] would g iv e [ 7 7 ] . F ig u re 26. Proposed S y n th e sis o f 7 b , IO b S e li n a - 4 , 1 1-Diene CHAPTER 4 FUTURE RESEARCH F uture research in the area o f the enamine-oxy-Cope r e a rra n g e ­ ment w i l l have to depend on the s y n th e s is o f v a rio u s s u b s t it u t e d 2- a c e t y l - 3 , 4 - d ih y d r o - 2 H - p y r a n s . 36-39 We can e a s i l y see t h a t a s p e c i f i ­ c a l l y s u b s t it u t e d pyran p r e c u r s o r w i l l g iv e a s p e c i f i c a l l y s u b s t it u t e d 5 - a c e t y l - I - ( I - p y r r o l i d i n y l )cyclo h e xe n e . Thus we co u ld g e n e r a liz e the s u b s t i t u t i o n p a t t e r n in both s t a r t i n g pyran [ 8 1 ] and rearran ged enamine [ 8 3 ] as: 37 Ra 81 F igure 27. General S u b s t i t u t i o n P a tte rn o f Reactants and Products in the Enamine Cope Rearrangement T h e re fo r e , one co u ld s y n th e s iz e a s p e c i f i c a l l y s u b s t it u t e d pyran which would in t u r n g iv e a s p e c i f i c enamine [ 8 5 ] . Several e x c e l l e n t re v ie w s 36,37 have been p u b lis h e d which deal w it h the D i e l s - A ld e r r e a c t io n s o f a , g - u n s a tu r a te d carbon yl compound g i v ­ in g 3 , 4 - d i h y d r o - 2H-pyrans and v a r io u s o t h e r f u n c t io n a l groups in the 2p o s i t i o n as a c e t y l o r carbom ethoxy. One must keep in mind t h a t an a c e ty l group (methyl ketone) in the 2- p o s i t i o n i s a p r e r e q u i s i t e f o r p y r r o l i d i n o enamine fo r m a tio n and subsequent rearran ged p ro d u c t. we can see, the f u l l As p o t e n t i a l o f t h i s s y n t h e t ic te c h n iq u e can o n ly be r e a li z e d by the pro p e r development o f the i n i t i a l pyran r i n g p r e c u r s o r . CHAPTER 5 EXPERIMENTAL Proton n u c le a r m agnetic resonance s p e c tra were recorded on V a rian A-60 o r V a rian T-60 n u c le a r magnetic resonance S pectrom eters. Peak p o s i t i o n s are g ive n in p a r t s per m i l l i o n d o w n fie ld from t e t r a m e th y ls il a n e as an i n t e r n a l s ta n d a rd . Mass s p e c tra f o r both l i q u i d and s o l i d compounds were o b ta in e d from a V a rian Model CH-5 mass spec­ t r o m e te r . Glc analyses were c a r r i e d o u t u sin g a V a rian Aerograph s e r ie s 2700 in s tr u m e n t and a l l work was done u s in g a 10' x 3 / 8 ' copper column packed w i t h 1.5% 0V-101 on Chromosorb G. m l/s e c . Gas f lo w was m a in ta in e d a t 18 I n f r a r e d s p e c tra were o b ta in e d w it h Beckman IR 5A o r IR20 i n ­ f r a r e d sp e c tro p h o to m e te rs . UV s p e c tra were recorded on a Cary 14 r e ­ c o r d in g sp e c tro p h o to m e te r o r on a V a ria n s e r ie s 634 U V - v i s ib le s p e c t r o ­ photom eter. M e lt in g p o in t s were performed on a Fishe r-Jo h n s h o t stage m e lt ­ in g p o i n t apparatus and are u n c o rr e c te d . and re p o rte d in mm o f mercury ( b . p . p o i n t was 75° C a t 10 mm o f m e rc u ry ). B o i l i n g p o in t s are u n c o rre c te d 75° C in d ic a t e s th e b o i l i n g S o lve n ts were g e n e r a lly removed under reduced p re ssu re v ia Buchi r o t a t o r y e v a p o ra to r. General Procedure f o r th e P y r o ly s is o f !mines and Enamines The vacuum p y r o l y s i s c o n d it io n s f o r e i t h e r th e im ines o r enamines were th e same. A 15 ml round bottom f l a s k was charged w it h the 39 i mine o r examine to be pyr o ly z e d and was placed on the Pyrex p y r o l y s i s : 1 '■ *- 1 tube ( Pyrex h e l ic e s ) and th e e n t i r e system was evacuated ( 0 .3 mm o f m e rc u ry ). The h e a tin g element (18 f e e t o f r e s i s t a n t mi chrome w ir e ) f o r I the p y r o l y s i s tube was c o n t r o l l e d by a V a r ia b le P ow erstat T ra n s fo rm e r. The i n t e r n a l te m p e ra tu re o f th e column was m onitored w i t h an ir o n - c o n s ta n ta n therm ocouple fused i n t o th e w a ll o f th e column and the tempera­ t u r e was recorded w i t h an Omega Type 2809 D i g i t a l Thermometer. When th e i n t e r n a l tem p era ture reached 250° C the h e a tin g m antle was a c t iv a t e d and th e im ine o r enamine was a llo w e d to r e f l u x . The crude p y ro ly z e d p ro d u c t was c o l l e c t e d in a pyrex U-tube f i l l e d w i t h pyrex h e lic e s and e x t e r n a l l y cooled in a Dewar f l a s k con­ t a i n i n g e i t h e r is o p ro p y l a l c o h o l / d r y ic e o r i c e / s a l t m ix t u r e s . When e i t h e r the im in e o r enamine was no lo n g e r p re s e n t in th e 15 ml round bottom f l a s k , both tra n s fo r m e rs were s h u t o f f and th e system was a llo w e d to cool down to room te m p e ra tu re . A t t h i s p o i n t the vacuum was broken , and the c o n te n ts o f th e U-tube were removed by f l u s h i n g w i t h d ry hexane. Removal o f t h i s s o lv e n t under reduced p ressure y i e l d s th e p ro d u c t. (Re­ f e r to diagram on f o l l o w i n g p a g e .) Pr e p a r a t io n o f 2 - A c e t y l - 6- M e t h y l- 3 , 4-D ihyd ro -2 H -P yra n [ 8 ] As d e s c rib e d in th e l i t e r a t u r e , 40 m e th y l-3 -b u te n e -2 -o n e 100 grams (1 .4 2 moles) was placed in a s t e e l pressure vessel and d im e rize d a t 185° C f o r f o u r h ou rs. vacuum d i s t i l l e d A f t e r c o o l i n g , the c o n te n ts were removed and (w a te r a s p i r a t o r ) t o g iv e a c o l o r l e s s l i q u i d , b .p . 40 HOSE TO V A C C U U M G LASS WOOL W ITH A LU M IN U M b F O IL W R A P P IN G IUND G L A S S JO IN T TO V A R IA B L E POWER TRANSFORM ER 2 c m D IA M E T E R P Y R E X T U B IN G TEM PERTURE BATH N IC RO M E W IRE PYREX H E L IC E S A S B E S T O S TA P E W R A P P IN G ' DEW AR 2 cm . X 3 0 cm . P YR EX T U B IN G FLASK PYREX H E LIC E S TO D IG IT A L THERM OM ETER TO V A R IA B LE POWER TR A N S FO R M E R P O IN T E D STO PPER G R O U N D G LA S S JO IN T ' TO V A R IA B L E PO W ER TRANSFORM ER F ig u re 28. Vacuum P y r o l y s is Apparatus 15 m l. ROUND B O TTO M F L A S K H E A TIN G M AN TLE 41 74° C, 4 2.5 grams (85% y i e l d ) . nmr data 8 ' Il O Chemical S h i f t ( 6) 4.17 I broad t r i p l e t 2 .0 3 -1 .5 6 4 m u ltip le t 2 3,4 Nature o f Peaks Number o f Protons 5 4.48 1(7 c p s ) m u lt i pi e t 7 1 .73 3 s in g le t 8 2.17 3 s in g le t I . R. ( f i l m ) cm 2874 (C-H s t r e t c h ) ; 1712 (C=O s t r e t c h ) ; 1385; 1232 (C-O-C s t r e t c h ) ; 1351; s tre tc h ); 1279; 9 1 7 .4 ; 8 9 4 .5 ; U. V. (hexane) 208 nm (e3.32) 281 ( e l .59) 1675 (C=C s t r e t c h ) ; 1163; 758.7 (CH==CH s t r e t c h ) . 1103; 1429; 1070 ( C-O-C 42 Mass Spectrum m/e ( r e l a t i v e i n t e n s i t y 140 (37%); 97 (100%); 43 (2 .5 % ); 41 (15.1% ). ■ 79 (5.3% ); 69 (9.0 % ); General P re p a ra tio n o f N -A lk y l 55 (7 .5 % ); 53 (4.5% ); !mines o f 2 - A c e t y l - 6- M e t h y l- 3 , 4-D ih yd ro Pyrans For th e sake o f b r e v i t y we w i l l o n ly c o n s id e r in d e t a i l the p r e p a r a t io n o f th e N-methyl im ine o f 2 - a c e t y l - 6- m e t h y l- 3 ,4-dehydro pyran [ 1 1 ] . A l l o t h e r i mines were prepared in the same manner w i t h the id e n tic a l r e s u lts . Only t h e i r s p e c t r a l c h a r a c t e r i s t i c s w i l l be g iv e n . A s o l u t i o n o f 2 - a c e t y l - 6- m e t h y l- 3 , 4 - d ih y d r o pyran (19 grams, 0.071 mole) and 100 ml o f d i e t h y l e t h e r c o n t a in in g 15 grams o f Linde 4 A m o le c u la r 41 sie ve s was cooled t o O0 C in an i c e / s a l t b a th . I n to t h i s s o l u t i o n was bubbled 2.48 grams (8.0 85 moles) o f m ethyl amine (a 20% e x c e s s ). The r e s u l t i n g s o l u t i o n was a llo w e d to come to room tem p era ture and s t i r o v e r n i g h t , a t th e end o f which tim e the m o le c u la r sie ves were f i l t e r e d from th e r e a c t io n m ix tu re and washed tw ic e w i t h 25 ml p o r t io n s o f anhydrous d i e t h y l e t h e r . The s o lv e n t was then removed from the com­ bined e t h e r f r a c t i o n s under reduced p re s s u re and the rem a ining re s id u e was vacuum d i s t i l l e d 97% b .p . component. (w a te r a s p i r a t e d ) t o g iv e 9.72 grams (0.068 mole) 25) 37° C, a 97% y i e l d , A n a ly s is by g lc in d ic a t e d o n ly one 43 4 Nature o f Peaks Number o f Protons Chemical S h ift ( 6) 2 4 .18 I m u lt i p i e t 3,4 1 .4 4 -2 .2 3 4 muI t i p l e t 5 4.42 I muI t i p l e t 7 I .74 3 s in g le t 8 1 .87 3 s in g le t 9 3.07 3 s in g le t ( f i l m ) cm" 1 1667 (C=N s t r e t c h ) ; s tre tc h ); 1163; 1429; 1379; 1053 ( C-O-C s t r e t c h ) ; 1299; 1282; 917.4; 873.4; 1235 ( C-O-C 754.7 U.V. (hexane) 231 nm ( e3.70) Amax. Mass Spectrum m/e ( r e l a t i v e i n t e n s i t y ) 153 (28.2%); 124 (13.6%); 97 (100%); 70 (16.1%); 56 (32.1%); 29 44 (12.4%) N -E thyl !mine o f 5 - M e t h y l - 2 - A c e t y l- 3 , 4 -D ihydro-2H -P yran [51 A] 9 Chemical S h ift ( 6) 2 3,4 4.16 I .2 9 -2 .2 6 Number o f Protons Nature o f Peaks I m u ltip le t 4 m u lt i p i e t 5 4.37 I m u ltip le t 7 1 .70 3 s in g le t 8 2.15 3 s in g le t 9 3.09 2 q u a rte t 10 0.91 3 trip le t I . R. ( f i l m ) cm 2902; 1678 (C=N s t r e t c h ) ; 1660 (C=C s t r e t c h ) ; 1440; 1386; 1238 45 1170 ( C-O-C s t r e t c h ) ; 1072 ( C-O-C s t r e t c h ) ; 923; 754 U.V. (hexane) 230 nm (e 3 .4 7 ) Xmas. Mass Spectrum m/e ( r e l a t i v e i n t e n s i t y ) 167 (29.3% ); N-Propyl 124 (16.1% ); 97 (100%); 70 (56.1% ); 43 (16.2%) !mine o f 5 - M e t h . y l - 2 - A c e t y l - 3 , 4-D ihyd ro -2 H -P yra n [5 1 B] 4 IO 9 46 . Chemical S h ift ( 6) Number o f Protons Nature o f Peaks 4.14 I m u l t i pi e t I .3 2 -2 .2 4 6 m u l t i pi e t 5 4.39 I m u lt i p i e t 7 1 .71 3 s in g le t 8 2.14 3 s in g le t 9 2.15 3 broad t r i p l e t 11 0.93 3 trip le t 2 3 ,4 ,1 0 I.R . ( f i l m ) cm 2899; 1675 (C=N s t r e t c h ) ; 1168 ( C-O-C s t r e t c h ) ; U.V. 1664 (C=C s t r e t c h ) ; 1070 ( C-O-C s t r e t c h ) ; 1440; 9 2 0 .8 ; 1383; 753.0 (hexane) 232 nm (e 3 .4 4 ) Xmax. Mass Spectrum m/e ( r e l a t i v e i n t e n s i t y ) 181 (31.1% ); N -Butyl 124 (14.6% ); 97 (100%); 84 (40.1% ); 57 (18.8%) !mine o f 2- A c e t y l - 6- M e t h y l- 3 , 4-Dih.ydro-2H-P,yran [51C] b - p * (0 .2 5 ) 67° C 1236; 47 4 7 11 9 12 Chemical S h ift ( 6) 4.12 2 3 ,4 ,1 0 ,1 1 I .3 0 -2 .2 5 Nature o f Peaks Number o f Protons I m u lt i p i e t 8 muI t i p l e t 5 4.36 I m u lt i p i e t 7 1 .76 3 s in g le t 8 2.18 3 s i n g le t 9 3.19 2 trip le t 12 0.96 3 trip le t ( f i l m ) cm ^ 2892; 1674 (C-N s t r e t c h ) ; 1167 ( C-O-C s t r e t c h ) ; U.V. (hexane) 236 ntn (e 3 .4 0 ) Xmax. 1665 (C=C s t r e t c h ) ; 1070 ( C-O-C s t r e t c h ) ; 1446; 921; 756 1380; 1236 48 Mass Spectrum m/e ( r e l a t i v e i n t e n s i t y ) 195 (28.6% ); 98 (51.2% ); 97 (100%); 71 (26.1%) General P re p a ra tio n o f S - A c e t y I - I - ( I - A l k y I ) A m i n o Cyclohexene Again we w i l l o n ly d e s c r ib e in d e t a i l the p r e p a r a t io n o f 5a c e t y l - I - ( I -m ethyl Jamino cyclohexene [ 1 3 ] . a ls o in v e s t i g a t e d ( e t h y l [ 5 2 8 ] , pro p yl The o t h e r im ines which were [5 2 8 ] and b u ty l [5 2 C ]) were a c ­ com plished in e x a c t ly the same manner, o n ly the s p e c t r a l data w i l l be g ive n f o r these compounds. Using the general p y r o l y s i s p ro ce d u re , 5.0 grams (0.035 mole) o f [ 11] were placed i n th e 15 ml round bottom f l a s k and f o l l o w i n g the c o n d it io n s p r e v io u s ly d e s c r ib e d , p y r o l y s i s was i n i t i a t e d . A f t e r th re e h o u rs, th e apparatus was cooled and the crude p y r o l y s i s p ro d u c t was removed, y i e l d i n g 4.7 grams o f an a i r - s e n s i t i v e compound which had to be s to re d under n i t r o g e n . p r e s e n t. A n a ly s is by g lc suggested o n ly one compound D i s t i l l a t i o n under high vacuum gave 3.3 grams (9.023 mole) o f [ 1 3 ], b .p . (Q g) 125° C, 66% y i e l d . re s id u e remained in the d i s t i l l a t i o n 9 (N ote: fla s k .) O 1.4 grams o f polym e rized 49 0.84-2.41 3 , 4 , 5 ,6 Nature o f Peaks Number o f Protons Chemical S h ift ( 6) 7 u n reso lved m u lt i p i e t 2 4.48 I trip le t 7 2.16 3 s in g le t 8 3.40 I broad m u l t i pi e t 9 2.68 3 d o u b le t I . R. ( f i l m ) cm 3413 (NH s t r e t c h ) ; (C=C-H s t r e t c h ) ; 2857; 3268; 1645 (C=C s t r e t c h ) ; 1695 (C=O s t r e t c h ) ; 1429; 1342; 1563; 8 4 0 .3 ; 3086 751.9 (C=C) U.V. (hexane) 220 nm (e3.79) 284 nm (el .77) Xmax. Mass Spectrum m/e ( r e l a t i v e i n t e n s i t y ) 153 (28.6%); (11.8%); 123 (16.3%); 110 (100%); 83 (12.6%); 43 (26.8%) 5- A c e t y l - I - ( I - E t h y l ) Amino Cyclohexene [52A] b -p- ( 0 . 8) 132° C 80 (98.2%); 70 50 3 =If T B ^ 8 NH 6 O , o > Chemical S h i f t ( 6) 3 , 4 , 5 ,6 Nature o f Peaks Number o f Protons I .0 2 -2 .5 0 7 mu I t i p l e t 2 4.44 I trip le t 7 2.14 3 s in g le t 8 3.44 I mult i p l e t 9 2.74 2 m u lt i pi e t 10 0.87 3 trip le t I . R. ( f i l m ) cm 3421 (N-H s t r e t c h ) ; s t r e t c h o f ena m ine); 3254; 1558; 2850; 1428; U.V. (hexane) 222 nm (c3.80) 283 nm ( e l . 71) Xmax. 1697 (C=0 s t r e t c h ) ; 134; 8 4 4 .6 ; 750.1 1637 (C=C 51 Mass Spectrum m/e ( r e l a t i v e i n t e n s i t y ) 167 (41.6% ); 123 (27%); 80 (100%); 70 (21.7% ); 44 (33.4%) 5 - A c e t y l - I - ( I - P r o p y l ) Ami no Cyclohexene [52B] b‘ p* (0.8) 140° C 3 I4 2 If 8 ^ 8 NH 6 Il O IO Chemical S h ift ( 6) 3 ,4 ,5 ,6 ,1 0 I .R . I .0 8 -2 .5 6 Nature o f Peaks Number o f Protons 9 m u lt i p i e t 9 2.70 2 m u ltip le t 2 4.45 I mu I t i p l e t 7 2.16 3 s in g le t 8 3.38 I mult i p l e t 11 0.91 3 tr ip le t ( f i l m ) cm 3405 (N-H s t r e t c h ) ; 3259; 3094 (=Cx ^ s t r e t c h ) ; 2890; 1704 (C=0 52 s tre tc h ); U.V. 1643 (enamine C=C); 1408; 1351; 986; 892; 740.9 (hexene) 223 nm ( e 3 . 7 7 ) ; 2.81 nm ( e l . 6 8 ) , Xmax. Mass Spectrum m/e ( r e l a t i v e i n t e n s i t y ) 181 (30.6% ); (21.2% ); 138 (172%); 123 (22%); 80 (100%); 43 (39.1%) 5- A c e t y l - I - ( I - B u t y l ) Ami no Cyclohexene [52C] b-p -( 0. 8) 146° C 12 IO 70 (15%); 58 53 Chemical S h i f t (S) 3 ,4 , 5 ,6 ,1 0 ,1 1 I.R . Number o f Protons Nature o f Peaks 11 mul t i pi e t 1 .1-2 .51 2 4.42 I m u ltip le t 7 2.15 3 s in g le t 8 3.31 I m u ltip le t 9 2.67 2 m u ltip le t 12 0.92 3 trip le t ( f i l m ) cm 3400 (NH s t r e t c h ) ; enam ine); 1562; 3261; 1423; 3089 (=Cs ^ s t r e t c h ) ; 1353; 852; 1649 (C=C s t r e t c h o f 746.1 U.V. (hexane) 229 nm ( e3 .7 6 ) ; 282 nm ( e l . 8 8 ) , Xmax. Mass Spectrum m/e ( r e l a t i v e i n t e n s i t y ) 195 (36.4% ); (14.2% ); 152 (16.9% ); 123 (21.3% ); 80 (100%); 72 (20.6% ); 70 43 (33.2%) H y d ro q e n o lysls o f S - A c e t y l - I - ( I - M e t h y l) A m in o Cyclohexene [ 4 5 ] 3 .0 grams (0.021 mole) o f 5 - a c e t y l - I - ( I -m eth yl)a m in o cyclohexene were d is s o lv e d in a 50 ml o f d r y e t h a n o l , to which was added '1 .5 grams o f 10% Pd/C. T h is e n t i r e m ix tu r e was placed in a P a rr C a t a l y t i c Hydro­ g e n a tio n apparatus under a hydrogen atmosphere o f 25 I b s . w i t h shaking a t room te m p e ra tu re . A t th e end o f 24 h o u rs , the c o n te n ts were removed. 54 and the Pd/C was f i l t e r e d and washed tw ic e w it h 25 ml p o r t io n s o f d ry e t h a n o l. The ethanol f r a c t i o n s were combined and the s o lv e n t removed under reduced p re s s u re . The rem a ining re s id u e was vacuum d i s t i l l e d (w a te r a s p i r a t o r ) to g iv e 2.46 grams (0.017 m o le ), b . p . (82% y i e l d ) ; ^ 23 C g lc a n a ly s is on 10% SE-30 150° C in d ic a t e d o n ly one component. 3 Chemical S h i f t (6) 7 1 , 2 ,3 ,4 ,5 ,6 9 LR. 2.16 3 0 .8 4 -2 .1 4 10 2.42 3 s in g le t u n re so lve d m u l t i p l e t s in g le t ( f i l m ) cm™1 3333 (NB s t r e t c h ) ; U.V. Nature o f Peaks Number o f Protons 2857; 1701 (C=0 s t r e t c h ) ; (hexane) 284 nm ( e l . 9 2 ) ; 201 nm ( e 3 . 8 ) , xmax. 1445; 1346 55 Mass Spectrum m/e ( r e l a t i v e i n t e n s i t y ) 155 (22.4% ); (13.3% ); 140 (12.3% ); 125 (9 .6 % ); 112 (100%); 97 (14%); 82 43 (26.1%) P re p a ra tio n o f 4 - A c e ty l cyclohexene [ 4 7 ] 2 .0 grams (0.0 13 mole) o f [ 4 5 ] was added to a s o l u t i o n o f 50 ml o f anhydrous d i e t h y l e t h e r and 2 .3 grams (0.016 mole) o f 25% excess methyl io d id e . The s o l u t i o n was a llo w e d to s t i r under N2 atmosphere f o r f o u r hours a t room te m p e ra tu re , a t the end o f which tim e the q u a te rn a ry ammonia s a l t was c o l l e c t e d by vacuum f i l t r a t i o n w it h 25 ml p o r t io n s o f anhydrous e t h e r . and washed th re e tim es The s a l t s were then added to a s o l u t i o n o f 1.45 grams (0.013 mole) o f potassium t e r t - b u t o x i d e in 50 ml o f t e r t - b u t y l a lc o h o l a t 80° C w i t h s t i r r i n g . A f t e r f i v e h ou rs, the s o l u t i o n was cooled and e x t r a c te d w i t h 50 ml p o r t io n s o f anhydrous e t h e r , d r ie d o ver anhydrous magnesium s u l f a t e , and the s o lv e n t removed under reduced p r e s s u re . The rem a ining re s id u e was vacuum d i s t i l l e d (w a te r a s p i r a t o r ) to g iv e a c l e a r l i q u i d a y i e l d o f 62%. 1.0 grams, b .p . 75° C, A n a ly s is by g lc in d ic a t e d o n ly one compound p re s e n t. 3 O 56 Number o f Protons Chemical S h ift (6) Nature o f Peaks I .1 2 -2 .8 4 7 u n re so lve d m u lt i p i e t L2 5.64 2 broadened s i n g l e t 8 2.14 3 s in g le t 3 , 4 , 5 ;6 s tro n g peaks a t 2.19 and 2 .0 6 . I.R . 2857; U.V. ( f i l m ) cm~^ 1724; 1429; 1351; 1220; 7 1 6 .3 ; 651.6 (hexane) 180 nm ( e4 . 0 3 ) ; 284 nm ( e3.380) , Xmax. Mass Spectrum m/e ( r e l a t i v e i n t e n s i t y ) 124 (22.6% ); 81 (59.3% ); 70 (33.8% ); 54 (29.2% ); 43 (100%) General P re p a ra tio n o f 3 - A c e ty l cyclohexanone [ 3 6 ] Since a l l o f th e 5- a c e t y l - I - ( I - a l k y l ) ami no cyclohexenes were a c id h yd ro ly z e d to th e same p r o d u c t, 3 - a c e t y l cyclohexanone [ 3 6 ] , o n ly the a c id h y d r o l y s is o f 5 - a c e t y l - I - ( I - m e t h y l) ami no cyclohexene w i l l d e s c rib e d in f u l l be d e ta il. The p r e p a r a t io n o f 3 - a c e t y l cyclohexanone was achieved in the f o l l o w i n g manner. 3 .0 grams (0.0 19 mole) o f f r e s h l y d i s t i l l e d were d is s o lv e d in 50 ml o f a 5% aqueous s u l f u r i c a c i d . [1 3 ] This e n t i r e s o l u t i o n was r e f l u x e d under a n it r o g e n atmosphere f o r 24 hours and th e r e s u l t i n g o ra n g e -c o lo re d s o l u t i o n was cooled and th e benzene la y e r 57 se p a ra te d . o f benzene. The aqueous la y e r was e x t r a c te d tw ic e w it h 25 ml p o r t io n s The combined benzene p o r t io n s were washed th re e times w i t h 50 ml p o r t io n s o f s a tu r a te d sodium b ic a rb o n a te and d r ie d o ver anhydrous magnesium s u l f a t e . Removal o f the s o lv e n t under reduced pressure l e f t a re s id u e which was vacuum d i s t i l l e d (w a te r a s p i r a t o r ) , b .p . 124° C, to y i e l d 1.97 grams o f a c o l o r l e s s l i q u i d , a y i e l d o f 72%. A n a ly s is by g lc showed o n ly one compound p r e s e n t. 5 6 4 6 O O Chemical S h i f t (6) 2.16 3 s in g le t 1 .16-2.48 9 m u lt i p i e t 8 2 ,3 ,4 ,5 ,6 Nature o f Peaks Number o f Protons I . R. ( f i l m ) cm 2857; 1701 (CK) s t r e t c h ) ; LI. V. (hexane) 288 nm (e3.70) 1721; 1429; 1351; 1255; 1220; 1163 58 Mass Spectrum m/e ( r e l a t i v e i n t e n s i t y ) 140 (10%); 97 (61.25% ); (71.25% ); 69 (37.5% ); 55 (40%); 43 (100%); 41 39 (33.5%) General P re p a ra tio n o f P y r r o l i d i n o Enamine o f 2 - A c e t y l- 6 - M e t h y l- 3 , 4 D ihydro-^H -P yran [ 5 3 ] 10 grams (0.071 mole) o f 2 - a c e t y l - 6 - m e t h y l - 3 ,4 -d ih y d ro - 2 H pyran were added to 5.5 grams (0.077 mole) o f p y r r o l i d i n e and 15 grams o f Linde 4A m o le c u la r s ie v e s . This m ix tu r e was then warmed to 35° C and m a in ta in e d a t t h i s te m p e ra tu re , w h ile s t i r r i n g under a n itr o g e n atmosphere f o r 24 h ou rs. A f t e r t h i s tim e the r e a c t io n m ix tu re was c o o le d , the m o le c u la r sie ve s were f i l t e r e d from th e m ix t u r e and were washed tw ic e w it h 25 ml p o r t io n s o f anhydrous e t h e r . combined w i t h th e f i l t r a t e The e th e r was and removed under reduced p re s s u re . re s id u e was vacuum d i s t i l l e d The to g iv e a c o l o r l e s s l i q u i d , b .p . 83° C, 12.45 grams, a y i e l d o f 91%. Glc a n a ly s is suggested t h a t o n ly one compound was p r e s e n t. nmr data 8 59 Chemical S h i f t (<5) 6 Number o f Protons 1 .82 Nature o f Peaks 3 s in g le t I .4 2 -2 .2 6 8 u n reso lved m u lt i p i e t 4 4.49 I mu I t i p l e t I 4.24 I m u lt i pi e t 8 2.64 2 d o u b le t 9,12 2.94 4 u n re so lve d mult i p l e t 2 ,3 ,1 0 ,1 1 I.R . ( f i l m ) cm~^ 3078 ((X ^ s t r e t c h ) ; 2857; 1250; 1070 ( C-O-C s t r e t c h ) ; U.V. 1235; 1166; 1672 (C=C s t r e t c h o f enam ine); 9 5 0 .6 ; 1613; 1287; 754.7 (C=C s t r e t c h ) (hexane) 228 n'm (e 3 .7 8 ) Amax. Mass Spectrum m/e ( r e l a t i v e i n t e n s i t y ) 193 (3 2 .3 % );. 123 (22.1% ); 122 (32%); 97 (100%); 96 (51.2% ); 70 (23%) A n a l: C a lc 'd f o r C-j9H-|gON; C 7 4 .5 6 ; H 9 .9 0 . Found: C 7 6 .9 0 ; H 10.26. P r e p a ra tio n o f S - A c e t y l - I - ( I - P y r r o l i d i n y l ) C y c l o h e x e n e [ 4 1 ] Using th e general p y r o l y s i s p ro c e d u re , 5 .0 grams (0.025 mole) o f p y r r o l i d i n e enamine was placed in th e 15 ml round bottom f l a s k and f o l ­ lo w in g th e c o n d it io n s p r e v io u s ly d e s c r ib e d , p y r o l y s i s was i n i t i a t e d . 60 A f t e r th r e e hours the apparatus was cooled and the crude p y r o ly s i s p ro d u c t was removed, y i e l d i n g 5 grams o f an a i r s e n s i t i v e produ ct which had to be s to re d under n i t r o g e n . Glc a n a ly s is in d ic a t e d a homogeneous compound, and high vacuum d i s t i l l a t i o n c l e a r y e llo w l i q u i d , b .p . tio n . (N ote: tilla tio n ^ gave 4 .3 grams o f a 135° C, a y i e l d o f 86% a f t e r d i s t i l l a ­ 0.61 grams o f polym e rized re s id u e s remained in the d i s ­ fla s k .) 3 3 ,4 ,5 ,6 ,9 ,1 0 Chemical S h i f t (6) Number o f Protons Nature o f Peaks I .0 8 -2 .4 6 11 m u ltip le t 2 4.28 I mu I t i p l e t 7 2.14 3 s in g le t 8,11 2.89 4 mu I t i p l e t I . R. ( f i l m ) cm 2890; 1698 (C O s t r e t c h ) ; 1639 (D O s t r e t c h ) ; 1 395; 1351; 1163; 61 7 5 4 .7 ; . ; ' U.V. 2082 C==Cxu s t r e t c h ) 11 . ’ ■ ^ (hexane) 227 nm (e3.81); 281 nm (el .62), Xmax. Mass Spectrum m/e ( r e l a t i v e i n t e n s i t y ) 193 (21%); (8.9 % ); A n a l: 1 7 8 (8 .2 % ); 70 (44%); 1 5 0 (1 0 0 % ); 1 0 8 (6 .3 % ); 123 (42.6% ); 80 43 (57.6%) C a lc 'd f o r CpH^gNO; C 7 4 .5 8 ; H, 9 .9 0 . Found: C 7 4 .6 8 ; H5 9.6 8 P r e p a ra tio n o f 3 - A c e ty l cyclohexanone [3 6 ] 4 .0 grams (0.020 mole) o f f r e s h l y - d i s t i l l e d 5 - a c e t y l - I - p y r r o l i d in e -rl-c y c lo h e x e n e were d is s o lv e d in 50 ml o f d ry benzene. The r e s u l t ­ in g s o l u t i o n was placed under a n it r o g e n atmosphere to which was added 10 ml o f a 5% aqueous s u l f u r i c a c id and r e f lu x e d f o r a p e rio d o f 24 h ou rs, a f t e r which th e s o l u t i o n was cooled and e x t r a c te d th re e times w it h 100 ml p o r t io n s o f e t h e r . The combined e t h e r e x t r a c t s were washed th re e tim es w i t h 100 ml p o r t io n s o f s a tu r a te d sodium b ic a rb o n a te , d r ie d o ver anhydrous magnesium s u l f a t e and the s o lv e n t removed under reduced p re s s u re . The re m a in in g re s id u e was vacuum d i s t i l l e d (w a te r a s p i r a t o r ) to g iv e a c o l o r l e s s l i q u i d , b .p . 124° C, 2.11 grams, a y i e l d o f 73%. Spectra was i d e n t i c a l t o t h a t p r e v io u s ly d iscu sse d . 62 P r e p a ra tio n o f 2 - M e th y l-5 -A c e ty lc y c lo h e x a n o n e [4 0 ] 3 .0 grams (0.015 mole) o f 5 - a c e t y l - I - p y r r o l id i n e - 1 - c y c lo h e x e n e were d is s o lv e d in 50 ml o f d r y benzene, and the e n t i r e s o l u t i o n was placed under a n i tr o g e n atmosphere. W hile s t i r r i n g , 2.12 grams (0.015 mole) o f methyl io d id e were i n je c t e d o v e r a p e rio d o f 1/2 hour. The r e s u l t i n g m ix t u r e was then a llo w e d to r e f l u x f o r 24 h o u rs , a t the end o f which tim e 10 ml o f aqueous 5% s u l f u r i c a c id was added. The r e s u l t i n g s o l u t i o n was f u r t h e r r e f l u x e d f o r an a d d i t i o n a l f i v e hours to a f f e c t h y d r o l y s i s . The r e s u l t i n g o ra n g e -c o lo re d s o l u t i o n was cooled and e x t r a c te d th r e e tim es w i t h 100 ml p o r t io n s o f e t h e r . The combined e t h e r e x t r a c t s were washed th re e tim es w i t h 100 ml p o r t io n s o f s a tu r a te d sodium b ic a r b o n a t e , d r ie d o v e r anhydrous magnesium s u l f a t e and the e t h e r removed under reduced p re s s u re . was vacuum d i s t i l l e d The r e s u l t i n g re s id u e to y i e l d a c o l o r l e s s l i q u i d , b .p . C, 1.81 grams (76%). ^ 131-132° Glc a n a ly s is i n d ic a t e s o n ly one compound p re s e n t. n ^ U \ / 6 5\ 7 Il O / 8 \ 63 Number . o f Protons Chemical S h i f t (6) Nature o f Peaks 8 2.18 3 s in g le t 9 0.92 3 m u lt i pi e t (both isomers p re s e n t) 8 m u lt i pi e t 3 ,4 ,5 ,6 ,2 1 .1 2 -2 .5 5 ( f i l m ) , cm""^ 2924; 1712-1689 (C=O s t r e t c h ) ; 1456; 1429; 1359; 1266; . 1176; 965.3 U.V. (hexane) 290 nm ( s 3 .7 2 ) Xtnax. Mass Spectrum m/e ( r e l a t i v e i n t e n s i t y ) 43 (100%); 55 (72%); 96 (65.6% ); 111 (57.8% ); 139 (15.6% ); 154 (19.6%) A n a l: C a lc 'd f o r CgH-j^Og; N C, 7 0 .1 0 ; H, 9 .1 4 . Found: C, 69.-82; H, 8.53 REFERENCES REFERENCES K • D. L e d n ic e r , " L a t e n t F u n c t i o n a l i t y in Organic S y n t h e s i s Advances in O rganic C h e m is try , V o l . 8 , E. C. T a y lo r ( E d . ) , W iley I n t e r s c ie n c e , New York (1 9 7 2 ), p. 179. 2. A. I . Meyers, H e te ro c y c le s in O rganic S y n th e s is , J. W ile y , 1974. 3. G. Buchi and J. E. P o w e ll, J r . , 4. G. Buchi and J. E. P o w e ll, J r . , J^ Am. Chem. Soc. , 9 2 , 3126 (1970). 5. R. P. L u tz and J . D. R o b e rts , J^ Am. Chem. Soc. , 8 3 , 2198 (1961 ) . 6. D. L. D a lrym p le , I . L. Kruger and W. N. W hite, The Chem istry o f th e E th e r L in k a g e , S. P atai ( E d . ) , W ile y I n t e r s c i e n c e , New York (1 9 6 7 ), p. 644. 7. K. B. L i p k o w it z , Ph.D. T h e s is , Montana S ta te U n i v e r s i t y , 1975. 8. K. B. L ip k o w it z and B. P. Mundy, T e t . L e t t . , in p re s s . 9. a. R. W. L a y e r, "The C hem istry o f !m in e s ," Chem. Rev. , 6 3 , 489 (1963 ). b. J. P. A n s lerne. The Chem istry o f th e C a rbo n-N itrogen Double Bond, S. P a tai ( E d . ) , W ile y I n t e r s c i e n c e , New York (1969 ). c. R. A. C la rk and D. C. P a rk e r, J. Amer. Chem. Soc. , 9 3 , 7257 (1971 ). d. H. A h lb r e c h t and S. F is c h e r , T e t . , e. H. A h lb r e c h t and G. Papke, T e t ., L e t t . , l[. Am. Chem. Soc. , 8 9 , 4559 (1967 ). 29, 659 (1973 ). 4443 (1972 ). f . H. A h lb r e c h t and S. F is c h e r , T e i x , 2&_, 2836 (1 9 7 0 ). g. H. A h lb r e c h t and S. F is c h e r , T e t . , 3 0 , 2571 (1974) h. H. Qust and A. H e u b le in , Chem. B e r . , 108, 2574 (1974). 10. D. S. Black and A. M. Wade, Chem. Comm., 871 (1970 ). 11. R. K. H i l l and G. R. Newhom, T e t . L e t t . , 5059 (1968) 12. R. K. Bramley and G. G rig g , Chem. Comm. , 99 (1969 ). 66 13. D. F e l i x , K. Gschwend-Steen, A. E. Wick and A. Eschenmeser, Helv. Chim. A c t a , 52^, 1030 (1969 ). 14. R. K. H i l l 15. a. E. J. Corey, R. D. Cramer and W. Howl, J . Amer. Chem. S o c., 94, 440 (1972 ). b. E. J. Corey, R. D. Cramer and W. Howl, J. Amer. Chem. S o c., 94, 431 (1972 ). and N. W. Gilm an, l e t . L e t t . , 1421 (1967 ). 16. L. B i r k o f f e r and D. Daum, Chem. B e r . , 9 5 , 183 (1962 ). 17. D. E. Pearson and C. A. B u e h le r , "Potassium T e r t - B u to x id e in S y n th e s is , " Chem. Rev. , 7 4 , 45 (1974). 18. D. Y. C u r t i n , E. J. Grubbs, C. G. McCarty, 88, 2775 (1966 ). 19. W. B. Jenninqs and D. R. Boyd, J . Amer. Chem. S o c ., 94, 7187 (1971 ). 20. a. R. B. Woodward and R. Hoffman, The C o nservation o f O r b it a l Symmetry, Academic P ress, New York (1971). b. T. L. G i l c h r i s t and R. C. S t o r , Organic Reactions and O r b i t a l Symmetry, Cambridge U n i v e r s i t y Press, New York (1972). c. I. Amer. Chem. Soc. , Flem ing, F r o n t i e r O r b i t a l s and O rganic Chemical R e a c tio n s , John W ile y , New York (1976 ). d. K. F u k i , Theory o f O r i e n t a t i o n and S t e r e o - S e l e c t io n , S p r in g e r V e r la g , New York (1975 ). e. J. E. Baldwin and R. H. F lem ing, J_. Amer. Chem. Soc. , 94, 2140 (1972). f . G. K. Klopman ( E d . ) , Chemical R e a c t i v i t y and R e action P a th s, John W ile y and Sons, New York (1974 ), p. 129. 21. W. J. LeNoble , H i g h l ig h t s o f O rganic C h e m is try , Marcel Dekker, New York (1 9 7 4 ). 22. a. S. J. Rhoads and N. R. Raul i n s , O rg. R e a c tio n s , 22_, I (1975 ). 67 22. b. M. J. S. Dewar and L. E. Wade, J r . , J. Amer. Chem. S o c ., 95, 290 (1973 ). •c. ■ M. J. S. Dewar and L. E. Wade, J r . , J. Amer. Chem. S o c ., 99, 4417 (1977 ). 23. W. D. G urow itz and M. A. Joseph, J_. O rg. Chem. , 32, 3289 (1967). 24. a. G. 0. Dudek and R. H. Holm, (1963). b. G. 0. Dudek and R. H. Holm, J_. Amer. Chem. Soc. , 8 3 , 2914 (1963 ). a. Y. A. Ovchinnkov and G. P. Kugatwa-Shemyakina, J e t . , 23, 697 (1962 ). b. G. M. N ik o la e v and G. P. Kugatwa-Shemyakina, l e t . , 2 3 , 2987 ' (1967 ). c. G. M. N ik o la e v and G. P. Kugatwa-Shemyakina, T e t . , 23^, 2721 ■ (1967). 25. Amer. Chem. Soc. , 8 3 , 2099 26. A. DeSavignac and A. L a t , Comptes Rendus, S e rie s C, 277, 1367 (1973 ). 27. E. F. Mooney ( E d . ) , Annual Review o f MMR S p e c tro s c o p y , V o l . I , Academic Press, New York (1970 ). 28. a. A. G. Cook, Enamines: S y n th e s is , S t r u c tu r e and R e a c tio n s , Marcel D ekker, New York (1969 ). b. S. F. Dyke, The C hem istry o f Enamines, Cambridge U n iv e r s it y Press, New York (1973). 29. D. J. Dunham and R. G. Lawton, J_. Amer. Chem. Soc. , 9 3 , 2074 (1971 ). 30. a. T. K. Devon and A. I . S c o t t , Handbook o f N a t u r a l l y O ccu rrin g Compounds, V o l . I I , Academic Press, New York (1972 ). b. K o ji Nakanishi and T i s h io Goto, N a tural Products C h e m is try , . V o l . I , Academic P ress, New York (1974). 31. J. ApSimon, The T o ta l S yn th e sis o f N a tu ra l P r o d u c ts , V o l . I I , W ile y I n t e r s c i e n c e , New York (1973 ). 68 32. R. M. Coates and J. E. Shaw, J_. O rq. Chem., 35, 2597 (1970). 33. E. P ie rs and K. F. Cheng, Can. 34. J. K. Roy, Chem. and I n d . , 1393 (1954 ). 35. K. K le in and W. Rojahn, l e t . L e t t . , 279 (1970 ). 36. G. Desemoni and G. T a c c o n i, "H e te ro d ie n e S yn th e sis w i t h a ,B U n satu ra ted Compounds, " Chem. Rev. , 75, 651 (1 9 7 5 ). 37. "a,B-Unsaturated Carbonyl Compounds as D ie n e s ," J. Colonge and G. Descotes, 1 , 4 - C y c lo a d d it io n R e a c tio n s , Jan. Hamer ( E d . ) , Academic Press, New York (1 9 6 7 ). 38. B. P. Mundy, K. B. L ip k o w it z and G. W. D i r k s , H e te r o c y c le s , 6_, 51 (1977). 39. B. P. Mundy, K. B. L ip k o w it z and G. W. D ir k s , " C y c lo a d d it io n R eactions Leading to Pyran D e r i v a t i v e s , " ( s u b m it te d ) . 40. K. A l d e r , H. Offermanns and E. Ruder, Chem. B e r . , 74^, 905 (1941 ) . 41. K. Taguchi and F. H. W estheimer, J_. O rg. Chem. , 36, 1570 (1971 ) . Chem., 46, 377 (1968 ). A MEW SYNTHESIS OF BREVICOMIN C hapter I HISTORICAL The western pine bark b e e t l e , D endvoaotonus b v e v ia o m is , has been r e s p o n s ib le f o r mass d e s t r u c t i o n o f the ponderosa p in e in both the U n ited S ta te s and Canada. The in v a s io n and even tua l d e s t r u c t i o n o f the t r e e by D. b v e v ia o m is occurs in two phases. in itia l In th e f i r s t , an a t t a c k i s made by a few b e e tle s which bore i n t o the t r e e and c o n s t r u c t a n u p t i a l chamber. f r a s s , a m ix tu re o f f e c a l In t h i s p e rio d o f a c t i v i t y , they expel p e l l e t s and wood fra g m e n ts . The fr a s s con­ t a in s a t h i r d component, a sex pheromone^ which in t u r n t r i g g e r s the secondary massive in v a s io n which u nd oubtedly k i l l s the t r e e . In 1968, S i l v e r s t e i n 2 and coworkers i s o l a t e d 2 mg o f the agg re ­ g a tin g sex pheromone o f D. b v e v ia o m is from 1.6 kg. o f f r a s s and re p o rte d the s t r u c t u r e as e x o - 7 - e t h y l - 5 - m e t h y l- 6 , 8 - d i o x a b i c y c l o [ 3 . 2.1 ) octane ( I ) o r e x o -b re v ic o m in . 71 Because one co u ld a c t u a l l y use e x o - b r e v ic o m in f o r the mani­ p u l a t io n o f the m ating h a b its o f D. b v e v io o m is one co u ld p o t e n t i a l l y p r o v id e the F o re s t S e rv ic e w ith an e c o l o g i c a l l y advantageous means o f p o p u la tio n c o n t r o l f o r t h is s p e c ific in s e c t. I t is i n t e r e s t i n g to note t h a t th e re are no e f f e c t i v e means o f c o n t r o l l i n g D. b re v io o m is up to t h i s p o i n t . Thus, a t t e n t i o n was then turn e d toward the t o t a l s y n th e s is o f b re vico m in because o f the obvious i s o l a t i o n problem s, e s p e c i a l l y in la r g e q u a n t i t i e s . As Mundy 3 has p o in te d o u t in a re c e n t r e v ie w , r e t r o - s y n t h e t i c a n a ly s is o f the 7 - e t h y l - 5 - m e t h y l - 6 , 8 - d i o x a b i c y c l o [ 3 . 2 . 1 ] octane r in g s k e le to n re v e a ls two p o s s ib le ro u te s f o r the c o n s t r u c t i o n o f the k e ta l m oiety o f t h i s m o le cu le . In ro u te [ a ] , the s y n t h e t ic s t r a t e g y demands the c o n s t r u c t io n 72 i o f a m olecule w i t h ketone and g ly c o l f u n c t i o n a l i t y , w h il e ro u te [ b ] c o n s is ts o f th e c o n s t r u c t i o n o f a d ih y d ro p y ra n c a r b i n o l . In 1969, S i l v e r s t e i n 4 p u b lis h e d the f i r s t s y n th e s is o f b r e v i - comin in which the s y n t h e t i c s t r a t e g y c o n s is te d o f the p r e p a ra tio n Of the e th y le n e k e ta l (3J o f 6^bromohexane-2-one ( 2 ) , f o llo w e d by t r e a t ­ ment w i t h t r i p h e n y l phosphine t o y i e l d the phosphomiurn s a l t ( 4 ) . Con­ d e n s a tio n o f (4) w i t h p r o p i nonaldehyde y ie ld e d a m ix tu re o f c is and tra n s non-6 -en e-2 -on e e th y le n e k e ta l (5^), which upon t re a tm e n t w it h m -c h lo ro p e rb e n z o ic a c id gave the c i s and tra n s epoxides ( 6 ) separated by g l c . H y d ro ly s is gave th e in te r m e d ia te d i o l clo sed t o the b i c y c l i c k e t a l . which were ( 7) which 73 ^ o CH3-C-(CH2)4-Br H° r-\ r~ \Q ° H' H% CH3-C -(C H 2)4-B r CH3-C ^(C H 2)4- P +O3 r n o -----------> CH03-C-(CH 2)3-CH-CH-CH 2-C h 3 m-C'C6H4C0?- ^ CH3-C-(CH 2)3CH-CH-CH2-C h 3 2 .) C H 3 - C H 2 -C H O 3 ^ 3 H 2 SO4 Z H 2O ----------------> CH3-C -(C H 2) 3-C H -C H -C H 2- C h 3 A c e to n e L I OH F ig u re 29. » 1 S i l v e r s t e i n ' s B revicom in S y n th e s is . An a l t e r n a t i v e approach taken by S i l v e r s t e i n in v o lv e d the a c id h y d r o ly s is o f 2 - a c e t y l b u t y r o la c to n e ( 8 ) , which when f o llo w e d by k e t a l i z a t i o n gave 5-brom o pentan-2-one, e th y le n e k e ta l (9) in 60% y i e l d . Treatment o f 9 w i t h NaZNH3 and 1 -b u tyn e gave the n o n -6 -yn -2 -o n e e t h y l ­ ene k e ta l in 16.7% y i e l d . T his was reduced w i t h n i c k e l t e t r a ­ a c e ta te and sodium b o ro h y d rid e to g iv e c is -n o n -6 -e n e -2 -o n e e th y le n e k e ta l (2) in 80% y i e l d . 74 o \\ x o y ^o 8 o H , CH3-C -C H 2-C H 2-C H 2-Br 1. ) H Br1 H2O 2. ) H ( T l) H , H+ No, NH3 CH3CH2 C=CH^ 9 ^ r\ O H2l Ni-(OAc)4 °x ^ CH3-C -(C H 2)3-C = C -C H 2-C H 3 — ------> CH3 -C -(C H 2)3-C H =CH-C h 2-CH 3 Ox IO F ig u re 30. S i l v e r s t e i n ' s A l t e r n a t e Scheme. 5 Working along the same l i n e s , Wasserman and coworkers a l k y l ­ ated a c e t o a c e tic e s t e r w it h c i s - 1 -bromo-3-hexene (TJJ w h ic h , f o llo w e d by s a p o n i f i c a t i o n and d e c a r b o x y la t io n , gave c is -n o n -6 -e n e -2 -o n e (1 2 ). The ketone ( I j H was t r e a t e d w i t h m -c h lo ro p e rb e n z o ic a c id to g iv e the c is - e p o x id e ( U ) which upon sealed tube p y r o ly s i s a t 210°C gave b r e v i comin in a 95% y i e l d : w i t h an exo/endo r a t i o o f 90:10. + CH3- ^ - C H 2- H - O C H 2 CH3 Br Il 75 -> ( I ) 9 0 % Exo I 0 % Endo F ig u re 31. Wasserman S yn th e sis o f B r e v icomin. S i l v e r s t e i n 6 and c o w o rk e rs , in 1971, were a b le to g r e a t l y improve t h e i r o r i g i n a l (14) to i t s s y n th e s is by the con ve rsio n o f c i s - 3 - h e x e n e - l- o l t o s y l a t e e s t e r ( 1 5 J , which was used to a l k y l a t e e th y l a c e to a c e ta te . T h is , f o llo w e d by a c id h y d r o ly s is and d e c a rb o x y la tio n y ie ld e d ( 1 2 ) , c i s - 6 - n o n e n e - 2 - o l , in 37% y i e l d . Treatment o f 12 w i t h m -c h lo ro p e rb e n z o ic a c id in benzene a t 15°C gave c i s-6 ,7 -o xe d o n o n a n -2 one, which in t u r n was r e f l u x e d in benzene to g iv e I a 55% y i e l d o f which was exo and 5% e n d o ). (95% 76 Ch 3-C H 2 -CH = C H -(C H 2)2-OH T tC I CH3-C H 2-CH=CH-(CH 2)2 OTs 80% 15 14 O Il C H 3 - C - C H 2- C - O E l IN NoOH 37% 12 OH, re flu x F ig u re 32. Improved S i l v e r s t e i n S yn the sis o f B revicom in. In 1976, P. L. K o cienski and R. W. Ostrow7 p u b lis h e d y e t a n o th e r s y n th e s is o f b re v ic o m in based on the ketone d i o l synthon developed by S i ! v e rs te in . y ie ld e d H k The Eschenmoser fra g m e n ta tio n o f the epoxy ketone (15) K e ta ly z a t io n o f 1_5 gave the S i l v e r s t e i n in t e r m e d ia t e , 6-nonyn-2-one e th y le n e k e t a l , ( 1 0 ) . Reduction o f the a c e t y le n ic k e ta l (10) by BH3 -Me2S f o llo w e d by p r o t o n a t io n gave the S i l v e r s t e i n i n t e r ­ m edia te , c is - n o n - 6 - e n e - 2 - o n e , ( 5 ) . S t e r e o s p e c if ic a c id cleavage o f the c is epoxide (6) w i t h co n co m ita n t h y d r o l y s is o f the k e t a l gave the keto d io l in t e r m e d i a t e , (7 ). 77 O Ii :o P-Ts-NHNH2 0 ----------------- > CH3-C(CH2) 3-C iC -C H 2 -CH 3 CH2CI2 , HO-Ac 15 F ig u re 33. 16 K o cienski S y n th e sis o f B r e v icomin. The S i l v e r s t e i n in te r m e d ia te was s t i l l to be used in an o th e r 78 s y n th e s is o f b re vico m in in 1977 by Coke^, which c o n s is te d o f the a l k y l a ­ t i o n o f 1 ,3 -c y c lo h e x a n e d io n e w it h e t h y l io d id e to g iv e 2 - e t h y l - 1 ,3 - cyclo h e xa n e d io n e , which when fo llo w e d by tre a tm e n t w i t h phosphorous t r i c h l o r i d e y ie ld e d th e c h l o r i d e ( 1 7 ) . Treatment o f YJ_ w i t h m e th y l- l i t h i u m , fo llo w e d by p y r o l y s i s , g ives 16. This a c e t y le n ic ketone was reduced t o the c i s o l e f i n w i t h 10% p a lla d iu m on barium s u l f a t e . T re a t­ ment o f 12 w i t h m -ch lo ro p e rb e n z o ic a c id y ie ld e d the S i l v e r s t e i n i n t e r ­ m edia te , ( 1 3 ) ; w h ic h , when heated a t 21O0C gave both exo and endo b re vico m in in a 9:1 r a t i o . O Il CH %- C - (CHg)^-CEC -CHg-C H^ O _Ha______ CH3 -H -(C H g )3-C H = C H -C H g -C H 3 Pd(BaSO4) 12 16 Fn-ClC6H4CO3H (I ) CH2CI2 F ig u re 34. Coke S yn th e sis o f B revicom in. 79 Now t u r n in g o u r a t t e n t i o n t o the second r e t r o s y n t h e t i c r o u t e , b, H. C. Brown^ p ro v id e d the i n i t i a l s y n t h e t ic impetus in a paper pub­ li s h e d in 1970, in which c y c l i c e th e rs were c o n s tr u c te d from unsatu­ ra te d a lc o h o ls v ia o r g m e r c u r a tio n / d e m e r c u r a tio n . 1. ) Hg(OAc)2 > 2 . ) OH- ZNoBH4 : oh The mechanism f o r t h i s r e a c t io n was th o u g h t to proceed along the l i n e s o f f i r s t e l e c t r o p h i l i c a t t a c k by the m e rc u ria c e ta te c a t io n to form the in te r m e d ia te c y c l i c m ercu riniu m io n which then undergoes subsequent i n t r a m o le c u la r n u c l e o p h i l i c a t t a c k o f the hyd ro xyl group. Demercuration and d e p ro to n a tio n gave a f i ve-membered c y c l i c e t h e r . In a s e r ie s o f papers f i r s t p u b lis h e d in 1971 M undy^ demon­ s t r a t e d a t h r e e - s t e p s y n th e s is o f b re v ic o m in . The s y n t h e t i c s t r a t e g y developed from the -n j* s + Trg2 c y c lo a d d i t i o n o f methyl v i n y l ketone and a c r o l e i n to y i e l d 2 - f o r m y l- 6 - m e t h y l- 2 , 3 - d ih y d r o - 2 H - p y r a n , ( 1 9 ) . The G rignard r e a c t io n o f e th y lm a g n e s iurn bromide w it h lj) gave both isomers o f 2 - ( 2 - h y d r o x y e t h y l) - 6 - m e t h y l- 2 , 3 - d ih y d r o - 2 H - p y r a n , ( 2 0 j . C y c l iz a t i o n o f the p y ra n y l c a r b i n o l , ( 2 0 ) , v ia the o x y m e rc u ra tio n /d e m e rc u ra tio n 80 method o f Brown gave exo/endo b re vico m in in a 1:1 r a t i o . EtMgBr I . IHg(OAc) 2.) HO- ZNa BH F ig u re 35. Mundy S y n th e sis o f B revicom in. Mundy has a ls o demonstrated t h a t tre a tm e n t o f 20 w i t h p - to lu e n e s u l f o n i c a c id in benzene g iv e s b re v ic o m in in 87% y i e l d w i t h a 64:36 r a t i o o f exo to endo isom ers. I t i s a ls o w o r th w h ile to mention t h a t u n l ik e any o f the b e fo r e mentioned syn th e s e s , Kossanye11 re p o r te d a q u i t e novel s y n th e s is o f b re vico m in in 1977. Here the s e l e c t i v e i r r a d i a t i o n o f 2 - p r o p i o n y l- 6 - methyl - 2 , 3 - d ih y d r o - 4 H - p y r a n , (21_), by s i n g l e t s e n s i t i z a t i o n and t r i p l e t quenching by I - m e th y ln a p th a !ene gave the s t e r e o s e l e c t i v e exo-isom ers o f I - m e t h y l - 6 - e x o - e t h y l- 7 , 8 - d i o x a b i c y c l i c [ 3 . 2 . I ] o c t - 2 - e n e , ( 2 2 ) , in 23% y ie ld . y ie ld . C a t a l y t i c hydro g e n a tio n o f 22 gave exo b r e v i c o m i n , ( I j , in 95% 81 X> 3 0 0 mm vycor filter H2 > Pd/C Pentane • P * - 22 F ig u re 36. I Kassanye S y n th e sis o f B r e v icomin. Thus, in a c o n t in u in g e f f o r t in our la b o r a t o r y to improve the o rig in a l Mundy s y n th e s is o f b r e v ic o m in , a search f o r a b e t t e r e l e c t r o ­ p h i l i c reage nt was begun. C a reful i n v e s t i g a t i o n o f the l i t e r a t u r e re ve a le d such an e l e c t r o p h i l e in p h e n y ls e le n y l c h l o r i d e . Sharpless In 1974, K. B. re p o r te d a new ro u te f o r th e s y n th e s is o f a l l y l i c the e l e c t r o p h i l i c a d d i t i o n o f v a r io u s *Sex reagents (where x = o r oAc) t o a lk e n e s . e th e rs by c l , Br These reagents always undergo tra n s -1 ,2 a d d it io n s to g iv e the a d d u c t, ( 2 3 ) , which i s q u i t e s t a b le when x = oAc, b u t is both t h e r m a lly and s o l v o l y t i c a l I y u n s ta b le when x = c l o r Br. A d d it io n o f an a lc o h o l gave the p h e n y ls e le n o e th e r , ( 2 4 ) , which when o x id iz e d w i t h 30% H2O2 in THF a t room tem p era ture gave 25. n a tio n v ia 26 to g iv e the a l l y l i c e th e r, This underwent syn e l i m i ­ ( 2 7 ) , in 93% y i e l d . 82 F ig u re 37. Sharpless S y n th e s is o f C y c lic E th e rs . Based on t h i s work o f V e m u ra ^ , N i c o l a o u ^ re p o r te d in 1977 t h a t tre a tm e n t o f u n s a tu ra te d a lc o h o ls such as 28 w i t h p h e n y lse le n yI c h l o r i d e in methylene c h l o r i d e a t -78°C a f fo r d e d the p h e n y lseleno e th e rs , (30). e th e r, O x id a tio n o f 30 w i t h 30% H2O9ZTHF gave the a l l y l i c ( 3 2 ) , in 87% y i e l d . Raney n i c k e l Treatment o f the phe nylse le n o e t h e r w it h in THF a t 25°C r e d u c t i v e l y removed the p he nylse leno group to g iv e the b i c y c l i c e t h e r , (31_), in 95% y i e l d . 53 F ig u re 38. N icolaou S y n th e sis o f B i c y c l i c E th e rs . The mechanism f o r t h i s r e a c t io n was again p o s t u la te d to proceed v ia an i n i t i a l a t t a c k o f the h i g h l y e l e c t r o p h i l i c <j>Secl on the double bond fo llo w e d by the in t r a m o le c u la r n u c l e o p h i l i c a t t a c k by the h ydroxyl group, v ia 29. R e a liz in g the im portance o f these e x p e rim e n ta l f i n d i n g s , the q u e s tio n we addressed o u rs e lv e s to was, can the a d d i t i o n o f <f>Secl to d ih y d ro p y ra n c a r b in o ls produce a b i c y c l i c k e t a l , and, i f so, can we in f a c t s y n th e s iz e b re v ic o m in . C hapter 2 RESULTS AND DISCUSSION Ic The D i e l s - A ld e r r e a c t io n 0 o f I -b u te n e -3 -o n e gave 2 - a c e t y l - 6 m e th y l- 3 ,4 - d ih y d r o - 2 H - p y r a n in 85% y i e l d . p r o p i o n y l- 6 - m e t h y l- 3 ,4 -d ih y d ro - 2 H -p y r a n w it h a p p ro x im a te ly equal e f f i c i e n c y . and Doud 17 21 The a c tu a l p r e p a r a t io n o f 2was accom plished in two ways The f i r s t method was t h a t o f S to rk which c o n s is te d o f th e fo r m a tio n o f the c y c lo h e x y li mine o f 2 3 , f o llo w e d by the a d d i t i o n o f e t h y l magnesium bromide which y i e l d s the enamine, ( 23a) . A l k y l a t i o n o f 23a w i t h methyl io d id e a t 0°C, fo llo w e d by h y d r o l y s is w i t h one e q u iv a le n t o f 5% aqueous a c e t ic a c id gave 21_ in 81% y i e l d . The second method in v o lv e d the d i r e c t a l k y l a t i o n o f the p y r r o l i d i n e enamine, ( 2 4 J , a t O0C w i t h methyl i o d id e , aga in fo llo w e d by c a r e f u l h y d r o l y s is w i t h one e q u iv a le n t o f 5% aqueous a c e t i c a c id t o g iv e 21_ in 88% y i e l d . One must be c a r e f u l in the a c id h y d r o l y s i s , o th e rw is e Ig the 2 - p r o p i o n y l - 6 - m e t h y l- 3 , 4 - d ih y d r o -2 H - p y ra n , (21_), r i n g w i l l open. 85 / F ig u re 39. S yn th e sis o f 2-P ro p io n y l- 5 - M e t h y l- 3 , 4 -D ih yd ro -2 H - P yran. The n o n - s t e r e o s p e c if ic r e d u c t io n d ih y d ro -2 H -p y ra n , 19 o f 2 - p r o p i o n y l- 6 - m e t h y l- 3 , 4 - ( 2 1 J , by sodium b o ro h y d rid e in is o p ro p y l a lc o h o l a t O0C gave both isomers 2- ( 2- h y d r o x y e t h y l) - 6 - m e t h y l- 3 , 4 - d ih y d r o - 2 H - p y r a n , ( 2 0 ) , in a 1:1 r a t i o ; determ ined by g lc on 15% 0V-101 a t I l O 0C. ment o f 20 w i t h I e q u iv a le n t o f p h e n y ls e le n y l c h l o r i d e a t -78°C in T re a t­ 86 methylene c h l o r i d e c o n t a in in g two e q u iv a le n t s o f potassium carbonate gave 4 - p h e n y ls e le n o - 5 - m e th y l- 7 - e t h y l - 6 , 8 - d i o x a b i c y c l o [ 3 . 2 . 1 ]octa ne ( 3 8 ) in q u a n t i t a t i v e y i e l d . K e t a l i z a t i o n was evidenced by th e appearance o f f o u r i n f r a r e d a b s o rp tio n s in the 1200-1050 cm ^ r e g i o n s , c h a r a c t e r i s t i c o f c y c lic k e ta ls . Both n u c le a r m agnetic resonance and mass s p e c tr a l data su pp orted t h i s c o n c lu s io n . Treatm ent o f 38^ w i t h a c t iv a t e d Raney N icke l in THF a t room tem p era ture gave both endo and exo isomers o f 7 - e t h y l - 5 - m e t h y l - 6 , 8 - d i o x a b i c y c l o [ 3 . 2 . 1]o c ta n e , ( Tj , in 92% y i e l d . The exo and endo isomer r a t i o was determ ined to be in I :1 by g l c a n a ly s is on 5% SE-30. O x id a tio n o f 38 w i t h 30% hydrogen p e ro x id e in THF a t room tem p era ture w i t h p e r i o d i c c o o lin g gave th e expected p r o d u c t, 5-methyl 7 - e t h y l - 6 , 8 - d i o x a b i c y c l o [ 3 . 2 . 1] o c t - 3 - e n e , ( 2 2 ) , in o n ly 29% y i e l d . The m ajor p r o d u c t . o f th e r e a c t io n was 5 - m e t h y l - 7 - e t h y l - 6 , 8 - d io x a b i c y c lo [3 .2 .1 ]o c ta n e -4 -o n e , ( 3 9 ) , in 71% y i e l d . C a t a l y t i c h y d ro g e n a tio n o f .22 w it h 10% Pd/C in e t h y l a c e ta te gave a 91% y i e l d o f I w i t h a 1:1 isomer ra tio . 87 F ig u re 40. Navel S y n th e tic Approach to B r e v icomin. O x id a tio n o f 38 w i t h m -c h lo ro p e rb e n z o ic a c id in methylene c h l o r i d e a t 25°C gave 2^2 in 86% y i e l d w it h an exo/endo isomer r a t i o o f I :I . In the o x i d a t io n r e a c t io n o f 4 - p h e n y ls e l e n o -5 -m e th y l- 7 - e t h y l 6 , 8 - d io x a b ic y c l o [ 3 . 2 . 1 ] o c t a n e w it h 30% hydrogen p e r o x id e , we can r a t i o n ­ a l i z e the fo rm a tio n o f 5 - m e t h y l - 7 - e t h y l - 6 , 3 - d i o x a b i c y c l o [ 3 . 2 . 1 ] o c t- 3 - e n e by th e syn e l i m i n a t i o n o f the s e le n o x id e , ( 4 0 ) , in the same fa s h io n as those re p o r te d by Reich a c id . w ith c o n co m ita n t fo rm a tio n o f p h e n y ls e le n ic 88 ■> / + 0SeOH 0 Tl n F ig u re 41. Reaction Mechanism f o r the Formation o f 5 - M e th y l- 7 E th y l-6 ,3 -D io x a b ic y c lo [3 .2 .1 ]o c t-3 -e n e . The fo rm a tio n o f the m ajor p ro d u c t o f the r e a c t io n 39^ was 20 p o s tu la te d to be d e r iv e d from a Pummer-Iike ' rearrangem ent. P rotona­ t i o n o f the s e le n o x id e g iv in g 41 which upon e l i m i n a t i o n o f w a ter gives the Pummer in te r m e d ia te ( 4 3 ) . H y d r o ly s is o f t h i s in te r m e d ia te , f o llo w e d by a hydrogen t r a n s f e r to the p h e n y ls e le n id e g ives a species which e v e n t u a ll y leads to H 20 39 OH o Ii - H 2O 4l7| n n F ig u re 42. Reaction Mechanism f o r the Form ation o f 5 -M e th y l- 7 E th y l- 6 ,8 -D io x a b ic y c lo [3 .2 .I ]o c t-4 -o n e . The r o l e o f the w a te r h y d r o l y s is in the Rummer rearrangem ent was confirm ed w i t h the o x i d a t i o n o f 38 w i t h one e q u iv a le n t o f m -c h lo ro p e rbenzoic a c id which o n ly gave one p r o d u c t, 2 2 , in 36% y i e l d . C hapter 3 EXPERIMENTAL ■ - ‘ ■ P re p a ra tio n o f 2 - p r o p i o n y l- 5 - m e t h y l- 3 ,4 -d i.hydro-2H -pyran T h is compound was prepared by two methods o u t l i n e d below. Method A 15.0 grams (0.0 77 mole) o f the p y r r o l i d i n e enamine o f 2 - a c e t y l 6 - m e th y l- 3 ,4 -d ih y d ro - 2 H -p y r a n was placed in d ry benzene and cooled to 0°C. W hile the m ix tu r e was s t i r r i n g under a n itr o g e n atmosphere, 11.00 grams (0.0 77 mole) o f methyl io d id e were added o v e r a p e r io d o f t h i r t y m in u te s. A f t e r s t i r r i n g f o r one hour a t O0C, the m ix t u r e was r e f lu x e d f o r t w e n t y - f o u r h o u rs ; a t the end o f which tim e th e s o l u t i o n was cooled to O0C and h y d ro ly z e d by one e q u iv a le n t o f a 5% aqueous s o l u t i o n o f a c e t ic a c id (4 .6 2 grams o f a c e t ic a c id in 87.9 ml o f w a t e r . ) This m ix tu re was then a llo w e d to s t i r f o r 45 m inutes a t th e end o f which tim e the s o l u t i o n was e x t r a c t e d w it h f o u r 100 ml p o r t io n s o f e t h e r which was washed w i t h two 15 ml p o r t io n s o f sodium b ic a r b o n a te , d r ie d over anhy­ drous magnesium s u l f a t e , f i l t e r e d and the e t h e r removed under vacuum v ia a r o t a t o r y e v a p o r a to r. The re s id u e was vacuum d i s t i l l e d to g iv e 10.44 grams o f a c o l o r l e s s l i q u i d , b .p . 84°C, 88% y i e l d . > Method B 20 grams (0.1 42 mole) o f 2 - a c e t y l- 6 - m e t h y l- 3 , 4 r d ih y d r o - 2 H - p y r a n and 14.1 grams (0.1 42 mole) o f c y c lo h e x y l amine was placed in 200 ml o f 91 d ry benzene and r e f lu x e d w it h a Dean-Stark w a ter s e p a ra to r f o r tw e n ty f o u r hours a t the end o f which tim e the benzene was removed under reduced pressure v ia r o t a t o r y e v a p o r a to r and the r e s id u e was vacuum d i s ­ tille d to y i e l d 29.2 g o f a c l e a r l i q u i d , b . p . ^ 95-96°C. 14 grams (0.0 63 mole) o f the c y c lo h e x y li mine o f 2 - a c e t y l - 6 m e th y l- 3 ,4 - d ih y d r o - 2 H - p y r a n were added to a s o l u t i o n o f 150 ml o f d ry THF and 9.23 grams (0.069 moles) o f ethylmagnesium brom ide. This s o l u ­ t i o n was r e f l u x e d f o r f i v e h o u rs , a t the end o f which tim e the s o l u t i o n was cooled to O0C and 8.94 grams (0.0 63 m oles) o f methyl io d id e were added o v e r a p e rio d o f one hour. This m ix tu re was then a llo w e d to s t i r a t room tem p era ture f o r t w e n t y - f o u r h o u rs . The m ix tu re was then cooled to 0°C and h y d ro ly z e d w i t h I e q u iv a le n t o f 5% aqueous a c e t i c a c id (3 .7 8 grams, in 71.87 m l) f o r t h i r t y m in u te s . The m ix tu re was e x t r a c te d w i t h f o u r 100 ml p o r t io n s o f e t h e r , washed tw ic e w it h 50 ml p o r t io n s o f 5% sodium b ic a rb o n a te d r ie d o v e r magnesium s u l f a t e f i l t e r and the e t h e r was removed under vacuum v ia r o t a t o r y e v a p o r a to r. d is tille d The re s id u e was vacuum to y i e l d 81% o f a c o l o r l e s s l i q u i d , b .p . 84°C. Products from both r e a c t io n s were i d e n t i f i e d by IR , NMR, UV and mass s p e c tra d a ta . A n a ly s is by g lc on 15% SE-30 a t I 30°C showed the compounds to be i d e n t i c a l . NMR Data , 92 Chemical S h i f t (<s) Number o f Protons Nature o f Peaks 10 1 .7 3 s in g le t 4 4.44 I m u lt i p i e t 2,,3 1.92 4 m u lt i pi e t I 4.16 I m u ltip le t 8 2.5 2 q u a r t e t (J=7.2 H2 ) 9 1 .03 3 t r i p l e t (J=7.2 H2 ) ' L R . . ( t h i n f i l m ) cm ^ 2882, 1712, 1678; 1449; 1385; 1355; 1299; 1241; 1190; 1167; 1111; 1071; 929. 4; 3 9 9 .3 ; 8 1 5 .7 ; 751 .9., U.V. Data (cyclohe xane) 208(=2100) 281(=38) 1 5 5 (4 .2 ) ;; 1 5 4 (2 8 .1 ); 112 ( 3 . 4 ) ; 1 1 1 ( 2 8 .6 ) ; 9 8 ( 1 1 . 1 ) ; 9 7 (1 0 0 ); 9 5 ( 1 8 . 2 ) ; 9 3 ( 1 0 . 1 ) ; 8 3 ( 1 1 .4 ) ; 81 ( 1 3 . 4 ) ; 7 9 ( 1 6 . 2 ) ; 7 1 ( 1 1 . 1 ) ; 7 0 ( 1 6 . 0 ) ; 69 ( 3 2 . 1 ) ; 6 7 ( 1 4 . 6 ) ; 5 7 ( 1 6 .6 ) ; 55 ( 3 6 . 2 ) ; 5 3 ( 1 9 .1 ) ; 4 3 ( 4 2 . 6 ) ; 4 1 ( 2 9 .3 ) . C a lc u la t e d : Found: C 70.10%; C 70.07%; H 9.14% H 9.35% P re p a ra tio n o f 2 - ( 2 - h y d r o x y e th y l) - 6 - m e th y 1 - 3 ,4 - d ih y d r o - 2 H - p y r a n ( 20) To a s t i r r e d s o l u t i o n o f 100 ml o f is o p ro p y l a lc o h o l and 14.0 grams (0.0 90 mole) o f , 2 - p r o p io n y l- 5 - m e t h y l- 3 ,4 -d ih y d ro -2 H -p .y ra n , which was e x t e r n a l l y cooled to 0°C, was added 3.45 grams (0.0 90 mole) o f 93 sodium b o ro h y d rid e o v e r a p e rio d o f one hour. The r e a c t io n m ix tu re was a llo w e d to s t i r f o r two hours a t O0C a t the end o f which tim e 15 ml o f 20% aqueous sodium h y d ro x id e were added. a d d itio n a l S t i r r i n g was c o n tin u e d f o r an two hours a f t e r which the s o l u t i o n was poured i n t o 200 ml o f d i s t i l l e d w a te r and e x t r a c t e d w it h f o u r 100 ml p o r t io n s o f e t h e r , which were combined, d r ie d o ver anhydrous magnesium s u l f a t e and the s o lv e n t removed under reduced p ressure to y i e l d a re s id u e which was vacuum d i s ­ tille d to y i e l d 12.19 grams (86%) o f a c l e a r c o l o r l e s s l i q u i d , b .p . 84°C. Glc a n a ly s is on 15% 0V-101 a t I l O 0C showed the two a lc o h o l i s o ­ mers in a r a t i o o f 1 :1 . NMR Data M Cr 6 9 i OH IO 11 4 2,3,8 1,7 Nature o f Peaks Chemical S h i f t (6) Number o f Protons 1 .7 3 s in g le t 4.40 I mul t i p l e t I .1-2.2 6 m u ltip le t 3.18-3.71 2 mul t i p l e t 9 I .02 3 tr ip le t 10 2.98 I broad s i n g l e t 94 I . R . Data ( t h i n f i l m ) cm~ 3367; 2890; 1678; 1449; 1385; 1292; 1241; 1171; 1964; 9 8 0 .4 ; 943.3; 8 7 7 .2 ; 758.7 Mass Spectrum m/e ( r e l a t i v e i n t e n s i t y ) 1 5 6 ( 1 8 .3 ) ; 1 4 1 ( 1 . 6 ) ; 1 2 7 ( 2 .1 ) ; 1 2 3 ( 1 . 2 ) ; 114 ( T l . 3 ) ; 1 0 9 ( 2 . 3 ) ; 9 9 ( 3 1 . 2 ) ; 9 8 ( 9 1 . 2 ) ; 9 7 (1 0 0 ); 95 (3 3 ); 8 6 (2 1 .3 ); 8 5 (4 2 .6 ); 8 4 (5 2 .3 );.8 3 (1 0 .4 ); 8 1 (1 3 .3 ); 7 9 (6 .3 ); 7 2 (1 2 .3 ); 7 1 (3 4 .3 ); 7 0 (7 .3 ); 6 9 (4 1 .3 ); 6 7 (8 .6 ); 5 7 ( 1 3 .9 ) ; 5 5 ( 1 0 .3 3 ) ; 4 3 ( 6 1 . 4 ) ; 4 1 (1 2 .9 ) C a lc u l a t e d : Found: C 69.2%; C 69.01%; H 10.31% H 10.61% P re p a ra tio n o f 4 - p h e n y ls e le n o - 5 - m e th y l- 7 - s t h y l - 6 , 8 - d i o x a b i c y c l o [ 3 . 2 . 1 ] octane (38) A s o l u t i o n o f 100 ml o f d ry methylene c h l o r i d e and 8 .2 grams (0.052 mole) o f 2 - ( 2 - h y d r o x y e t h y l) - 6 - m e t h y l- 3 , 4 - d ih y d r o - 2 H - p y r a n , ( 2 0 ) , and 20 grams o f anhydrous potassium carbonate was prepared and placed in a 200 m l, th re e -n e c k e d ,ro u n d -b o tto m f l a s k equipped w i t h an i n j e c t i o n septum, mechanical s t i r r e r and r e f l u x condenser. The e n t i r e apparatus was evacuated and placed under a n it r o g e n atmosphere surrounded by a tem p era ture bath o f acetone and d ry ic e (-7 8 ° C ). W hile s t i r r i n g , a s o l u t i o n o f 10 ml o f d ry methylene c h l o r i d e and 10 grams (0.052 mole) o f p h e n y ls e le n y l c h l o r i d e was i n j e c t e d . The r e s u l t i n g o ra n g e -c o lo re d s o l u t i o n was a llo w e d to s t i r a t -78°C f o r f o u r hours and then a t room 95 tem p era ture f o r 24 hours a t the end o f which tim e the r e a c t io n m ix tu re was removed and f i l t e r e d . The methylene c h l o r i d e was removed under reduced p ressure v ia the r o t a t o r y e v a p o ra to r and then column chromato­ graphed on a 2.5 cm x 48 cm s i l i c a w i t h methylene c h l o r i d e . gel column which had been prepacked The column was e lu te d w i t h methylene c h l o r i d e . C o ll e c t i o n o f the compound and removal o f the methylene c h l o r i d e under reduced p ressure v ia r o t a t o r y e v a p o ra to r l e f t a red o i l . chromatography on s i l i c a gel R^r 0 .712. Thin la y e r IB/CHgClg showed o n ly one compound p r e s e n t, The y i e l d was q u a n t i t a t i v e . NMR Data ,, „ Chemical S h i f t (6) Number o f Peaks Mature o f Peaks I .1-2.01 6 m u ltip le t I 3.99 I mu I t i p l e t 7 3.80 I m u ltip le t 10 0.88 3 trip le t 11 1.35 3 s in g le t 12 7.48 2 mul t i p l e t 13 7.19 3 m u ltip le t 4 3.26 I m u ltip le t 2 ,3 ,9 96 Mass Spectrum m/e ( r e l a t i v e i n t e n s i t y ) 311(12.3% ); 269(6.3% ); 268(3.2% ); 253(16.3% ); 156(55.2% ); 155(12.3%); 126(6.7% ); 113(26%); 112(19.3% ); 97(32%); 34(19.3% ); 83(42.3% ); 66 (36.2% ); 43(100%); 41(17.4%) O x id a tio n o f 4 - p h e n y ls e le n o - 5 - m e th y l- 7 - e t h y l - 6 , 8 - d i o x a b i c y c l o [ 3 . 2 . 1 ] octane w i t h 30% Hydrogen Peroxide . In a 12 5 ml Erlenmeyer f l a s k equipped w i t h a m agnetic s t i r r e r , was placed 75 ml o f d ry THF and 4 .0 grams (0.012 moles) o f 4-phenyl s e le n o - 5 - m e th y l- 7 - e t h y l - 6 , 8 - d i o x a b i c y c l o [ 3 . 2 . I ]o c ta n e . added o ver a p e r io d o f 20 m inutes 1.36 ml 30% HgOg. To t h i s was (0.408 grams, 0.012 mole) o f E x te rn a l c o o lin g became necessary d u r in g th e r e a c t io n to m a in ta in 25°C. A f t e r f o u r hours o f s t i r r i n g , the m ix tu r e was added to 250 ml o f d i s t i l l e d w a te r and potassium carbonate was added u n t i l a b a s ic pH was m a in ta in e d . The r e s u l t i n g s o l u t i o n was then e x t r a c te d w i t h th re e 150 ml p o r t io n s o f e t h e r , d r ie d o ver anhydrous magnesium s u l f a t e , f i l t e r e d and e t h e r removed under reduced p re s s u re v ia r o t a t o r y e v a p o r a to r. The r e s id u e was vacuum d i s t i l l e d to y i e l d two f r a c t i o n s : (I)b .p . 64°C, which corresponds t o 22^ (29%); 68°C, which corresponds to 39^ (71%). and ( I I ) b .p . 97 NMR Data: 5-m ethyl - 7 - e t h y l - 6 , 8 - d i o x a b i c y c l o [ 3 . 2 . 1 ] o c t - 4 - o n e Z 5- S O6 9 10 Chemical S h i f t (6) Number o f Protons Nature o f Peaks 1 .1 -2 .0 4 6 mu I t i p l e t I 3.97 I mult i p l e t 7 3.70 I mu I t i p l e t 10 0.90 3 trip le t 11 1.35 3 s in g le t 2 ,3 ,9 I .R . Data ( t h i n f i l m ) cm"^ 2899; 1767; 1453; 1381; 1351; 1241; 1176; 1111; 1075; 1033; 970.9; 9 2 7 .6 ; 8 8 1 .1 ; 847.5 Mass Spectrum m/e ( r e l a t i v e i n t e n s i t y ) 170(6.8% ); 154(7.1% ); 142(5.3% ); 128(6.33% ); 114(37.2% ); 99(8.4% ); 98(22.3% ); 86 (2 1 .3 % ); 85(100%); 72(6 .1 % ); 71(12.3% ); 69(44.2% ); 68(39.2% ); 58(13 .1% ); 57(24%); 5 5(12 .3% ); 43(91.2% ); 42(20%); 41(6.3%) 98 MMR D a ta : 5 - m e t h y l - 7 - e t h y l - 6 , 8 - d i o x a b i c y c 1 o [ 3 . 2 . I ] o c t - 3 - e n e (22) 3 4 2 I O -------- 6 7 y I9 H MO Chemical S h i f t (6) 3,4 2,9 5.66 I .30-2.81 Number o f Protons Nature o f Peaks 2 ( v in y l) m u ltip le t 4 mu I t i p l e t I 4.08 I broad s i n g l e t 7 3.64 I mult i p l e t 10 0.90 3 trip le t 11 I .40 3 s in g le t L R . Data ( t h i n f i l m ) cm ^ 3050; 1642; 1385; 1194; 1179; 1109; 1036; 1014; 9 7 4 .4 ; 9 3 2 .6 ; 8 8 0 .8 ; 712 Mass Spectrum m/e ( r e l a t i v e i n t e n s i t y ) 154(3.2% ); 127(3.6% ); 98(18.4% ); 86(32 .4% ); 85(39 .2% ); 56(19.4% ); 114 (36.1% ); 43(100%) 99 P re p a ra tio n o f e x o / e n d o - 7 - e t h y l- 5 - m e t h y l- 6 , 8 - d io x a b i c y c lo [ 3 . 2 . 1 ] o c t a n e 111 In 200 ml o f d ry THF was d is s o lv e d 4 .0 grams (0.0 12 mole) o f 4 - p h e n y ls e l e n o -5 -m e th y l- 7 - e t h y l - 6 , 8 - d i o x a b i c y c l o [ 3 . 2 . 1 ] o c t a n e to which was added 25 grams o f a c t iv a t e d Raney n i c k e l c a t a l y s t which was s t i r r e d under n it r o g e n f o r 24 hours a t the end o f which tim e the c a t a l y s t was removed from the r e a c t io n m ix tu re by f i l t r a t i o n , and the THF was then removed under reduced p ressure v ia the r o t a t o r y e v a p o r a to r. was then chromatographed on a 46 cm x 2.5 cm s i l i c a The re s id u e gel column prepacked w it h methylene c h l o r i d e o f which s o lv e n t was used to e l u t e the column. The purpose o f the column chromatography was to remove the p h e n yls e le n o l which remains behind in the s i l i c a g e l. The methylene c h l o r i d e was then removed under reduced p re ssu re v ia the r o t a t o r y e v a p o ra to r le a v in g a l i g h t y e llo w r e s id u e which upon d i s t i l l a t i o n c o l o r l e s s l i q u i d , b .p . gave 1.72 g o f c le a r ( i g mm) 64°C, a y i e l d o f 91.8%. Glc a n a ly s is on 15% SE-10 dem onstrated the two isomers o f 7 - e t h y l - 5 - m e t h y l - 6 , 8 - d io x a b i c y c l o [ 3 . 2 . 1 Joctane in a 1:1 r a t i o . NMR Data 3 100 Chemical S h i f t (6) Number o f Protons Nature o f Peaks I 3.98 I m u ltip le t 7 3.79 I m u lt i pi e t 10 0.88 3 trip le t 11 I .38 3 s in g le t 8 m u ltip le t 2,3,4,9 1 .1 - 1 .9 8 . L R . Data ( t h i n f i l m ) cm™^ 2899; 1462; 1383; 1333; 1238; 1190; 1176; 1109; 1032; 1006; 9 70 .9; 9 2 7 .6 ; 9 0 0 .9 ; 880. .3; 8 5 1 .8 ; 782.5 Mass Spectrum m/e ( r e l a t i v e i n t e n s i t y ) 156(4.7% ); 127(4.3% ); 114(28%); 9 9(6.3% ); 98(14.8%); 86(10.3% ); 85 (32%); 7 2(3.4% ); 7 1(7.8% ); 68(14.1% ); 57(10%); 55(6 .3 % ); 43(100%); 41(13.4%) C a lc u la tio n s : Found: C, 6 9 .2 0 ; C, 6 9 .1 7 ; H, 10.31 H, 10.45 O x id a tio n o f 4 - p h e n y ls e le n o - 5 - m e th y l- 7 - e t h y l - 6 , 8 - d i o x a b i c y c l o [ 3 . 2 . 1 ] 0 octane w i t h m -c h lo ro p e rb e n z o ic a c id To a s o l u t i o n o f 1 .0 grams (0.0032 mole) o f 4 - p h e n y ls e le n o -5 m e th y l- 7 - e t h y l - 6 , 8 - d i o x a b i c y c l o [ 3 . 2 . 1 ]o c ta n e in 25 ml o f d ry methylene c h l o r i d e was s lo w ly added w h ile s t i r r i n g a s o l u t i o n o f 0.63 grams (0.0032 mole) o f m -c h lo ro p e rb e n z o ic a c id o ver a p e r io d o f 30 m in u te s , 101 w ith p e r io d ic c o o lin g to m a in ta in the r e a c t io n tem p era ture a t 25°C. A f t e r -one hour the r e a c t io n was stopped w i t h the a d d i t i o n o f 20 ml o f 10% aqueous sodium s u l f i t e . S e p a ra tio n o f the m ethylene c h l o r i d e la y e r f o llo w e d by washing w it h th r e e 50 ml p o r t io n s o f 5% aqueous sodium b ic a rb o n a te , d r ie d o v e r anhydrous magnesium s u l f a t e and removal o f the methylene c h l o r i d e under reduced p ressure v ia the r o t a t o r y e v a p o ra to r l e f t a re s id u e which upon d i s t i l l a t i o n c o l o r l e s s l i q u i d b .p . gave 0.42 grams o f a c l e a r , 64°C which corresponds to the p r e v io u s ly is o l a t e d 5 - m e t h y l- 7 - e t h y l- 6 , 8 - d i o x a b i c y c l o [ 3 . 2 . 1 ] o c t - 3 - e n e in 86% y i e l d by NMR, IR and mass s p e c t r a l d a ta . H yd rog enolysis o f 5 - m e t h y l - 7 - e t h y l - 6 , 8 - d i o x a b i c y c l o [ 3 . 2 . I ] o c t- 3 - e n e (22) 0 .5 grams (0.0032 mole) o f 5 - m e t h y l - 7 - e t h y l - 6 , 8 - d io x a b i c y c lo [ 3 .2 .1 ] o c t- 3 - e n e was placed i n 50 ml o f r e d i s t i l l e d e t h y l a c e ta te w i t h 0 .5 grams o f 10% Pd/C. This was then placed in a P a rr C a t a l y t i c H ydrogenation apparatus under a hydrogen atmosphere o f 45 lb s . A fte r shaking a t room tem p era ture f o r 24 h o u rs , the r e a c t io n was stopped and the c a t a l y s t f i l t e r e d from the r e a c t io n m ix tu re and the e t h y l a c e ta te removed under reduced p re s s u re . The rem a in in g re s id u e was d i s t i l l e d - under vacuum to y i e l d 0.46 grams o f a c l e a r c o l o r l e s s l i q u i d , b .p . 64°C which was i d e n t i c a l in NMR, IR , g lc and mass s p e c t r a l data ( I ) . ^ REFERENCES REFERENCES I. W. D. Redard and D. I . Wood, M. C. B irc h Ed. "Pheromones, " American E l s e v i e r , New Y o rk, N. Y . , 1974, pp. 441-449. a. R.. M. S i ! v e r s t e i n , R. G. Brownlee, I . E. B e l l a s , D. I . I . E. Browne, S c ie n c e , 159, 889 (1968 ). b. R. M. Si I v e r s t e i n , J. Chem. E d . , 45, ?94 (1968 ). Wood and 3. B. P. Mundy, K. B. L ip k o w itz and G. W. D ir k s , H e te r o c y c le s , 6, 51 (1977 ). 4. T. E. B e l l a s , R. G. Brownlee and R. M. S i ! v e r s t e i n , T e t r . 5149 (1 9 6 9 ). ™ 5. H. H. Wasserman and E. H. B a rb e r, J. Am.. Chem. S o c ., 91 , 3674 (1969 ). 6. J. 0. Rodin, C. A. Reece, R. M. S i l v e r s t e i n , V. H. Brown and J. Degraw, J. Chem. Eng, and D a ta , 1 6 , 380 (197 ) . 7. P. J. K o cienski and R. W. Ost r o w , J . O rg. Chem. , 41 , 398 (1976). 8. J. F. Coke, H. J. W illia m s and S. N a ta r a ja n , J. O rg. Chem., 42, 2380 (1977). 9. H. C. Brown, P. J. Geoghegan, J. T. Kurek and G. J. Lynch, Q rg. M e ta l. Chem. S y n . , I , 7 (1970 ). 10 . 25, I. a. B. P. Mundy, R. D. O tzenberger and A. R. D e be rn a rd is , J. O rg. Chem., 36, 2390 (1971). b. K. B. L i p k o w it z , B. P. Mundy and D. Geeseman, Syn. Commun. , 3, 453 (1973 ). c. B. P. Mundy, K. B. L ip k o w it z and G. W. D i r k s , Syn. Commun. , 5, 7 (1975 ). d. K. P. L i p k o w it z , B. P. Mundy and I . 41 , 371 (1976 ). H. M atsko, J. O rg. Chem., 11. P. Chaguin,. J. P. M o riz u r and J . K o ssa n yi, J.. Am. Chem. S o c., 99 903 (1977 ). 12 . a. K. B. S harpless and R. I . L a u e r, J. O rg. Chern. , 3 9 , 429 (19740. 104 12. b. H. J. Reich, J. Org. Chem. , 39, 428 (1974) 13. A. T o s h im it s u , S. Vermura and M. Okano, J. C. S. Chem. Comm., 166 (1 9 7 7 ). 14. K. C. N icolaou and Z. Lysenko, T e t r . 15. K. C. M icolaou and W. E. B a r n e tt e , J .C .S . Chem. Comm. , 331 (1977). 16. K. A l d e r , H. Offermanns and F. Ruden, Chem. R er. , 74, 905 (1941). 17. a. G. S to rk and S. R. Dowd, J. Am. Chem. S o c ., 3 5 , 2178 (1953). b. G. Buchi and J. E. P o w e ll, J r . , J . Am. Chem. S o c ., 92, 3126 (1970 ). L e t t . , 1977; 1257. 18. Ring opening may proceed in the f o l l o w i n g manner 19. R. L. A u g u s tin e , E d . , M. N. R e r ic k , R e duction : Technique and A p p li c a t io n s in O rganic S y n th e s is . New Y ork: Marcel Dekker, I n c . , 1968, p. I . 20. a. H. J. R e ich, J. M. Renga and I . L. Reich, J. Am. Chem. S o c . , 97, 5434 (1975 ). b. K. B. S h a rp le s s , K. M. Gordon, R. F. Lauer, P. W. P a t r i c k , S. P. S in g e r and M. W. Young, Chemica S c r i p t a , 1975, 8A, 9. c. B. S. T h y a g a ra ja n , Mechanism o f M o le c u la r M i g r a t i o n s , p. 156, New Y ork: W iley I n t e r s c i e n c e , 1968. 21. H. D. Becker, J . Am. Chem. S o c ., 8 5 , 3410 (1963). V o l. I , MONTANA STATE UNIVERSITY LIBRARIES 3 1 762 1001 2 8 8 9 9 N378 Bornmann, William G New synthetic m e t h o d o l o g i e s for B616 C O T ). 2 natural products IS S U E D TO DATE n J t f H a i t .ife» 4 / / = P / <4 /2 / z z/ /o & y &