Document 13491295
advertisement

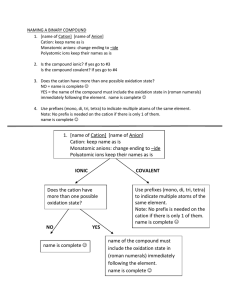
1' FPP PPO squalene synthase 2' FPP * electrophilic addition results tertiary cation allylic cation * H H * 3' * OPP loss of proton with formation of cyclopropane ring H * OPP * H presqualene PP loss of diphosphate results in primary cation * H W-M 1,3-alkyl shift H * * * H H * H 1,3-alkyl shift generates new cyclopropane ring and more favorable tertiary cation bond cleavage produces alkene and favorable allylic cation H H * Figure by MIT OCW. * squalene * H (NADPH) cation quenched by attack of anhydride squalene O2 NADPH cyclizations O squalene oxide protonation of epoxide allows ring opening to tertiary cation electrophilic addition gives tertiary cation + six-membered ring electrophilic addition gives tertiary cation + six-membered ring H H HO HO O H HO H squalene oxide electrophilic addition gives tertiary cation + five-membered ring H H H H H HO H protosteryl cation H HO electrophilic addition gives tertiary cation H H W-M rearrangement; ring expansion despite tertiary --> secondary cation HO H Figure by MIT OCW H O HO H H H chair - boat - chair - boat squalene oxide H H sequence of W-M 1,2-hydride and 1,2-methyl shifts HO HO H H H H H H protosteryl cation H H H H H HO HO H cyclopropane ring formation and loss of proton from C-10 methyl sequence of W-M 1,2-hydride and 1,2-methyl shifts H H H H H protosteryl cation H loss of H-9 creates double bond H H HO H HO H H H H H H HO H cycloartenol HO H lanosterol Figure by MIT OCW Aromatase-catalyzed conversion of androstenedione to estrone O CH3 O O2 NADPH O O HR HS HO O2 O + +H -H2O H O -H2O NADPH O + +H O HO H HO H O -HOH NADPH O2 + +H O H2O + HCOOH + HO A Three possible mechanisms for the last step in the aromatase-catalyzed oxygenation of androstenedione. O O Fe+3 +3 Fe O O O +3 Fe O OH O +3 +3 Fe O OH Fe OH H 1 A O O O O +4 Fe O H +4Fe O OH O 2 A HO O C H OH Fe O O +4Fe O O O O + HCOOH O +4Fe CH O O H H CH A 3 O 3+ HO +4Fe +4Fe +4Fe + O O HO + 3+ Fe + HCOOH +4Fe OH Figure by MIT OCW. PLANT HO HO OH O H HO HO H cycloartenol loss of C-4α methyl H C-24 alkylation opening of cyclopropane H HO H O OH HO H 24-methylenecycloartanol cycloeucalenol H obtusifoliol loss of C-14α methyl and allylic isomerization (migration of Δ8 to Δ7) 24-ethyl sterols H HO H loss of C-4 methyl HO H avenasterol H H further side chain alkylation HO H citrostadienol (24-ethylidenelophenol) H H loss of C-4 methyl H H HO episterol H gramisterol (24-methylenelophenol) H 24-methyl sterols YEAST C-24 alkylation demethylations as C-4 C-14 demethylation H HO H lanosterol HO H eburicol (24-methylenedihydrolanosterol) HO H H HO 4,4-dimethylfecosterol H fecosterol H Figure by MIT OCW. HO H ergosterol side chain and ring B modifications A cyclization is initiated by protonation of a double bond resulting in tertiary cation + H squalene B rings A and B are formed as 5 membered rings via Markovnikov addition; they then expand to 6 membered rings via W-M rearrangements H2O H2O OH H H H H H OH H H H Figure by MIT OCW H H H HO hopan-22-ol tetrahymanol H H GGPP PPO GGPP electrophilic addition resulting in tertiary cation allylic cation H H OPP loss of proton with formation of cyclopropane ring H loss of diphosphate results in primary cation OPP H H H W-M 1,3-alkyl shift generates new cyclopropane ring and more favorable tertiary cation prephytoene PP H H H H proton loss generates alkene H bond cleavage produces alkene and favorable allylic cation Z Z-phytoene sequence of desaturation reactions; in plants and fungi, the central double bond is also isomerized Z --> E E lycopene Figure by MIT OCW. protonation of double bond results in tertiary cation; then electrophilic addition H+ O2 NADPH a HO ε-ring H c H b b H a c opening of epoxide ring γ-ring O2 NADPH O2 NADPH O O HO HO β-ring pinacol-like rearrangement generates ketone H+ OH HO O2 NADPH OH H O H+ HO (a) HO OH allene generated from opening of epoxide ring and loss of proton Figure by MIT OCW. (b) β-carotene central cleavage generates two molecules of retinal excentric cleavage (b) central cleavage (a) oxidative chain shortening O retinal excentric cleavage can generate one molecule of retinal O Abietadiene Synthase bifunctional diterpene cyclase from fir cannot separate functional domains cloned (a.a. sequence is) don't know structure "homology modeling' ep.-aristolochene synthase enzyme as a model (sequiterpene synthase) Figure removed due to copyright reasons. Gibberrellin phytohormone bifunctional enzyme in fungi two enzymes in plant 2 individual enzymes Higher plants ionization protonation OPP CPS OPP FCPS/KS KS FCPS/KS Phaeosphaeria sp. L487 Figure by MIT OCW. yeast 1 bifunctional enzyme Figure by MIT OCW. Index of figures removed due to copyright reasons Jennewein, S., and R. Croteau. Figure 2 in “Taxol: biosynthesis, molecular genetics, and biotechnological applications.” Applied Microbiol Biotech 57 (2001): 13-19. Jennewein, Stefan et al. “Random sequencing of an induced Taxus cell cDNA library for identification of clones involved in Taxol biosynthesis.” PNAS 101 (2004): 9149-9154. Brown, Geoffrey D. Scheme 8 in “The biosynthesis of steroids and triterpenoids.” Natural Product Reports 15 (1998): 653-696. Abe, Ikuro, Michel Rohmer, and Glenn D. Prestwich. Scheme VI in “Enzymatic cyclization of squalene and oxidosqualene to sterols and triterpenes.” Chemical Reviews 93 (1993): 2189 – 2206. Rilling, H.C., and Chayet, L.T. “The 19-reaction conversion of lanosterol to cholesterol.” Danielsson, H., and Sjovall, J., eds. Sterols and Bile Acids. New York, NY: Elsevier, 1985. ISBN: 0444806709. Hoshino, Tsutomu, and Tsutomu Sato. Figure 3 in “Squalene–hopene cyclase: catalytic mechanism and substrate recognition.” Chemical Communications (2002): 291 – 301. Peters, Reuben J. Scheme 1, Figure 1, and Table 1 in “Bifunctional Abietadiene Synthase: Mutual Structural Dependence of the Active Sites for Protonation-Initiated and Ionization-Initiated Cyclizations.” Biochemistry 42 (2003): 2700-2707. Williams, David C. et al. Figures 1, 3 and 6 in “Intramolecular proton transfer in the cyclization of geranylgeranyl diphosphate to the taxadiene precursor of taxol catalyzed by recombinant taxadiene synthase.” Chemistry & Biology 7 (2000): 969 - 977.