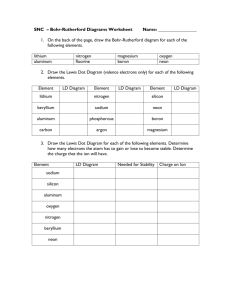
Name: Section (E or G)

Properties of p-Block Elements
Are p-block elements more diverse or less diverse than s-block elements?
Group 3A: The Boron Group
What two elements make laboratory glassware able to withstand extreme differences in temperature?
What is the main source of boron?
Why is it cost-effective to recycle aluminum?
Crystals of aluminum oxide form what kinds of gems?
Group 4A: The Carbon Group
What is the branch of chemistry that studies carbon compounds?
What is a mineral?
What is an ore?
What is an allotrope? Name two allotropes of pure carbon.
What are three uses of lead that have resulted in toxic exposure in humans?
What is the primary use of lead today?
Group 5A: The Nitrogen Group
Name three nitrogen compounds that have important industrial uses, and list those uses.
Nitrogen is the most abundant element in the atmosphere, but animals cannot use nitrogen in that form. How do animals obtain the nitrogen they need to build compounds such as proteins?
How are red, white, and black phosphorus related to each other? What are the properties of each?
Why are phosphate-containing fertilizers harmful to the environment?
Group 6A: The Oxygen Group
What are the two allotropes of oxygen?
How is pure oxygen gas obtained?
Name two oxides formed from oxygen and carbon.
What compound causes acid rain?
What compound makes rotten eggs smell so bad?
Group 7A: The Halogens
What does “halogen” mean?
What is the most reactive element in the periodic table?
What element is used in bleach?
Group 8A: Noble Gasses
Why were noble gasses among the last elements to be discovered?
Noble gasses do not usually form compounds. However, in 1962, a chemist named Neil
Bartlett succeeded in making a compound of fluorine and xenon. Why do you think he chose fluorine for his experiments?
Helium is the second most common element in the universe, yet there is very little of it on the Earth. Why?
Excited atoms of neon always glow bright orange, yet neon lights come in other colors.
How is this possible?
Thinking Critically
Although carbon and lead are in the same group, one is a nonmetal and the other is a metal. Explain how two elements with the same number of valence electrons can have such different properties.
A place has been left for element 113 on the periodic table. To what group does it belong? How many valence electrons will it have? What element will it most closely resemble?