7.012 Problem Set 1 Solutions Name Section
advertisement

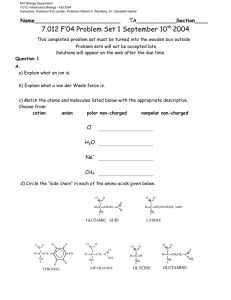
MIT Biology Department 7.012: Introductory Biology - Fall 2004 Instructors: Professor Eric Lander, Professor Robert A. Weinberg, Dr. Claudette Gardel Name__________________________ Question 1 TA____________Section____ 7.012 Problem Set 1 Solutions A. a) Explain what an ion is. An ion is an electrically charged particle that forms when an atom gains or loses one or more electrons. b) Explain what a van der Waals force is. A weak attraction between molecules resulting from a momentary shift of the electron density in a molecule thus establishing a dipole moment. The dipoles induced by the effect of one molecule on the others lead to a net electrostatic attraction. c) Match the atoms and molecules listed below with the appropriate descriptive. Choose from: cation anion polar non-charged nonpolar non-charged Cl- Anion H2O Polar non-charged Na+ Cation CH4 Nonpolar non-charged d) Circle the “side chain” in each of the amino acids given below. O O C C NH3 + O- GLUTAMIC ACID O H O H O C H C CH2 OH NH3 + H TYROSINE H C O H C CH2CH 2 O O O C H C NH3 + H C CH2 CH2 CH2CH 2 NH3 + LYSINE O O NH2 ASPARAGINE H C H NH + 3 GLYCINE O O C C O CH2 C NH3+ H C CH2 CH2 O C NH3 + GLUTAMINE NH2 e) Check the following terms to describe the side chains of the amino acids you circled above. Side Chain of… Glutamic Acid Charged Lysine Non Charged √ √ Polar Non Polar Hydrophobic Hydrophilic √ √ √ √ √ √ Tyrosine Asparagine Glycine Glutamine √-/+ √-/+ √ √ √ √ √ √ √-/+ √ √ f) Explain why ethane (C2H6) is a non-polar molecule while ethanol (C2H5 - OH) is a polar molecule. (A diagram of each molecule may help.) Ethanol is a polar molecule because there is a partial charge separation associated with the bond between the hydrogen and the oxygen. The higher electronegativity of oxygen pulls the electrons in the bond closer to the oxygen resulting in a greater electron density around the oxygen. This provides a slight negative charge for the oxygen and a slight positive charge for the carbon. The molecule is polar because these opposite charges are separated at the two ends of the bond. Ethane, on the other hand, is nonpolar because there are no such charge separations present in this molecule. The electronegativity difference between carbon and hydrogen are not significant enough to cause this type of charge separation. B. Match the letter of the organelle on the left with the function or characteristic on the right. Complex or Organelle Function or Characteristic a. Mitochondria C b. Nucleus E c. Smooth Endoplasmic Reticulum d. Golgi Apparatus F Site of lipid synthesis; where hydrophobic toxins‡ water soluable and thus excretable Digests macromolecules and degrades worn-out organelles Catalyzes protein synthesis B Contains all genomic DNA in eukaryotic cell e. Lysosome G Uses sunlight to build sugar f. Ribosome H Where ribosomal RNA is made g. Chloroplast D Prepares proteins for export from the cell h. Nucleolus A Powerhouse of the cell 2 Question 2 One day in lab while studying your favorite enzyme, Votase, you discover the following potential interactions that could occur between this amazing enzyme and its substrate of choice. Gln O HO Asp C-O- Substrate Arg H3 N + NH3+ Lys H3C CH3 CH3 Ile Votase a) At each site between the chemical group on the substrate and the closest side chain of an amino acid on Votase determine if a favorable interaction is likely to take place. If a favorable interaction is likely to take place, give the name for the strongest direct intermolecular interaction. Choose from ionic interaction, covalent bond, hydrogen bond, and van der Waals force. Amino Acid Will a favorable interaction take place? Circle one. Strongest Interaction ionic interaction, covalent bond, hydrogen bond, or van der Waals force. Gln Yes/No hydrogen bond Asp Yes/No ionic interaction Ile Yes/No van der Waals Lys Yes/No -- Arg Yes/No ionic interaction b) For all cases where a potential interaction seemed unfavorable, explain why that interaction may be unfavorable. The interaction between the side chain of lysine and the NH3+ group is unfavorable because both are positively charged. The positive charge of the NH3+ group will repel and not attract the positive charge of the lysine side chain. 3 Question 3 a) In your continuing work with the enzyme votase you discover that the enzyme is also a transmembrane protein (part of the protein crosses the lipid bilayer of the cell). Circle the portion of the sequence below that you would expect to be the transmembrane region of the protein. +NH3 - glu - trp - asp - arg - his - asp - phe - glu - ser - gly – pro - thr - phe - ile - trp - leu - ile - trp - leu - val - ile - ala – val - leu - phe - leu - leu - ile - trp – ala - val - leu – arg - pro - gly - cys - ser - lys - ala - tyr - ala - lys -- val - cys - ala - gly - cys - ser - asp - lys - gly - glu - COOH b) Why will the section you circled be embedded in the membrane? The lipid bilayer of the cell is largely nonpolar and thus it is expected that the region of the protein that passes through this nonpolar layer will also be nonpolar due to the hydrophobic effect. The section underlined above represents the largest stretch of nonpolar amino acids of the residues given and would most likely be the transmembrane section. c) Draw a schematic of the phospholipid bilayer. Label the lipid hydrocarbon chains, phosphate hydrophilic heads, and water. Cell Exterior H2O H2O Hydrophilic Heads H2O Hydrocarbon Chains H2O H2O H2O Cell Interior 4 Question 4 a) What is the chemical difference between the pentose sugar in DNA and the pentose sugar in RNA? (See page 48 in text book.) The sugar in RNA has a hydroxyl (-OH) group in the 2' position while the sugar in DNA has a hydrogen in the 2' position. b) In the peptide below, circle the peptide bonds. What are the amino acids in this peptide? cysteine-alanine-tyrosine-phenylalanine c) What is the name of the macromolecule below? ____Ribonucleic Acid (RNA)_______ 5 STRUCTURES OF AMINO ACIDS at pH 7.0 O O H C CH3 ALANINE (ala) H O O H N C H + C C CH2 NH3 + O N C H H H ISOLEUCINE (ile) HISTIDINE (his) O- O C CH2CH2 CH3 H NH3 + O H OC H C C CH3 NH OH + 3 THREONINE (thr) H C CH2 C CH3 NH3 + CH3 H C CH2 O- H H C CH2 NH3+ O- O- O C H C CH2 OH NH3 + SERINE (ser) PROLINE (pro) H C H H H H O O NH3 + TRYPTOPHAN (trp) C CH2CH2CH2CH2 NH3 + H C CH2 CH2 H N CH2 H + H N C H H LYSINE (lys) O H O C C PHENYLALANINE (phe) O GLYCINE (gly) C NH3 + METHIONINE (met) NH3 + O C S C H NH2 LEUCINE (leu) H O- O C H H C C CH2CH3 NH3 CH3 + H H C O O O C O GLUTAMINE (gln) O C H O- ASPARTIC ACID (asp) O NH3 + GLUTAMIC ACID (glu) CYSTEINE (cys) NH3 + OC CH2CH2 O- O C CH2 C NH2 C H C H ASPARAGINE (asn) O NH3 + NH3 + NH3 + O C CH2CH2 C O C CH2 C NH2 + C C CH2 SH H NH2 O- O C H C ARGININE (arg) O O C CH2CH2CH2 N O- O C H NH3 + NH3 + O O C C H O O H H C C H O O C CH2 OH NH3 + H TYROSINE (tyr) H H C C NH3 H + CH3 CH3 VALINE (val) 6