Myxobacteria motility: a novel 3D model of rippling behaviour in Myxococcus... Antony B. Holmes , Sara Kalvala and David E. Whitworth
advertisement
2nd International Conference on Bioinformatics and Systems Biology (BSB 2009)
The Second SIWN Congress (SIWN 2009), Leipzig, Germany, 23-25 March 2009
Myxobacteria motility: a novel 3D model of rippling behaviour in Myxococcus xanthus
Antony B. Holmes1 , Sara Kalvala2 and David E. Whitworth3
3
{1 MOAC; 2 Department of Computer Science}, University of Warwick, Coventry, CV4 7AL, UK
Institute of Biological, Environmental and Rural Sciences, Aberystwyth University, Ceredigion, SY23 3DD, UK
Email: {a.b.holmes; Sara.Kalvala}@warwick.ac.uk, dew@aber.ac.uk
Abstract: Bacterial populations provide interesting examples
of how relatively simple signalling mechanisms can result in
complex behaviour of the colony. A well studied example of
this phenomenon can be found in myxobacteria; cells can coordinate themselves to form intricate rippling patterns and fruiting bodies using localised signalling. Our work attempts to understand and model this emergent behaviour. We developed an
off-lattice Monte Carlo simulation of ripple formation using a
using a software framework we have created and show how
rippling is a consequence of the combined effect of cell biochemistry and cell physics.
Keywords: monte carlo, morphogenesis, myxobacteria, rippling.
1. Introduction
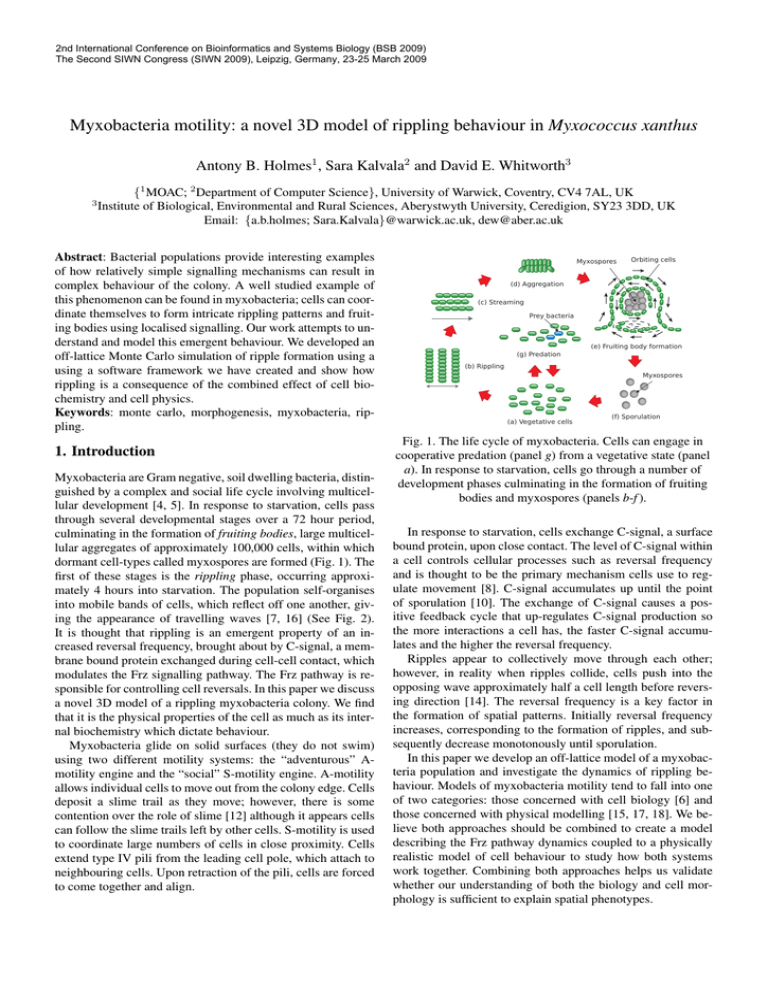
Myxobacteria are Gram negative, soil dwelling bacteria, distinguished by a complex and social life cycle involving multicellular development [4, 5]. In response to starvation, cells pass
through several developmental stages over a 72 hour period,
culminating in the formation of fruiting bodies, large multicellular aggregates of approximately 100,000 cells, within which
dormant cell-types called myxospores are formed (Fig. 1). The
first of these stages is the rippling phase, occurring approximately 4 hours into starvation. The population self-organises
into mobile bands of cells, which reflect off one another, giving the appearance of travelling waves [7, 16] (See Fig. 2).
It is thought that rippling is an emergent property of an increased reversal frequency, brought about by C-signal, a membrane bound protein exchanged during cell-cell contact, which
modulates the Frz signalling pathway. The Frz pathway is responsible for controlling cell reversals. In this paper we discuss
a novel 3D model of a rippling myxobacteria colony. We find
that it is the physical properties of the cell as much as its internal biochemistry which dictate behaviour.
Myxobacteria glide on solid surfaces (they do not swim)
using two different motility systems: the “adventurous” Amotility engine and the “social” S-motility engine. A-motility
allows individual cells to move out from the colony edge. Cells
deposit a slime trail as they move; however, there is some
contention over the role of slime [12] although it appears cells
can follow the slime trails left by other cells. S-motility is used
to coordinate large numbers of cells in close proximity. Cells
extend type IV pili from the leading cell pole, which attach to
neighbouring cells. Upon retraction of the pili, cells are forced
to come together and align.
Myxospores
Orbiting cells
(d) Aggregation
(c) Streaming
Prey bacteria
(g) Predation
(b) Rippling
(e) Fruiting body formation
Myxospores
(a) Vegetative cells
(f) Sporulation
Fig. 1. The life cycle of myxobacteria. Cells can engage in
cooperative predation (panel g) from a vegetative state (panel
a). In response to starvation, cells go through a number of
development phases culminating in the formation of fruiting
bodies and myxospores (panels b-f ).
In response to starvation, cells exchange C-signal, a surface
bound protein, upon close contact. The level of C-signal within
a cell controls cellular processes such as reversal frequency
and is thought to be the primary mechanism cells use to regulate movement [8]. C-signal accumulates up until the point
of sporulation [10]. The exchange of C-signal causes a positive feedback cycle that up-regulates C-signal production so
the more interactions a cell has, the faster C-signal accumulates and the higher the reversal frequency.
Ripples appear to collectively move through each other;
however, in reality when ripples collide, cells push into the
opposing wave approximately half a cell length before reversing direction [14]. The reversal frequency is a key factor in
the formation of spatial patterns. Initially reversal frequency
increases, corresponding to the formation of ripples, and subsequently decrease monotonously until sporulation.
In this paper we develop an off-lattice model of a myxobacteria population and investigate the dynamics of rippling behaviour. Models of myxobacteria motility tend to fall into one
of two categories: those concerned with cell biology [6] and
those concerned with physical modelling [15, 17, 18]. We believe both approaches should be combined to create a model
describing the Frz pathway dynamics coupled to a physically
realistic model of cell behaviour to study how both systems
work together. Combining both approaches helps us validate
whether our understanding of both the biology and cell morphology is sufficient to explain spatial phenotypes.
Start
Select cell
Interaction step
Transform step
Precondition Handler
Create events
Search for precondition
handler module
Process events
No
Events have
preconditions?
Yes
Yes
No
Reject event
Fig. 2. Magnification of myxobacteria ripple formation on a
surface adapted from [13]. The dark bands are areas of
densely aligned cells travelling as waves.
2. System design
All models were created and simulated using FABCell, a multipurpose simulation environment we created, initially to focus
specifically on myxobacteria and later generalised to work with
a larger class of problems. FABCell is a high performance open
source tool developed in C++ for agent based simulations. It
uses a highly modular plug-in framework to provide functions
for particular roles. Most components can be expanded or replaced to suit the needs of the user. It can cope with both on
lattice and off-lattice models in a 3D environment. It currently
supports simulating cellular automata (CA), lattice gas cellular automata (LGCA), Cellular Potts Models (CPM), off-lattice
models and more general agent based interaction models. Support for Monte Carlo methods within simulations has also been
added. All models have a consistent design regardless of which
type of simulation is being run so that it is straightforward to
take a simple CA model and run it as a CPM for example.
2.1 The Program Loop
All FABCell simulations execute in a similar way. An outline
of the main program loop is given in Fig. 3. During an iteration, cells are selected (either randomly or sequentially) for
updating. They go through an Interaction step to deal with cell
interactions, a Transform step to deal with physical changes
such as movement and an Update step to update the system environment with any changes. How each stage actually executes
depends on the modules that are loaded. For example CA models execute their Interaction and Update steps synchronously
whereas CPM models are often asynchronous.
State update. Each model is updated by a set of StateUpdater objects. StateUpdaters update a specific part of the
model, for example the position of a cell. A FABCell provides a library of updaters for common functions such as
cell interactions and cell movement (both synchronous and
asynchronous) or users can create their own. The two most
important StateUpdaters are MigrationStateUpdate and
InterationStateUpdate. During the migration step, cells
are moved around on the lattice. This can be done synchronously where all cells are moved based on the current
Precondition
handler found?
Run precondition
module
Update step
Terminate
simulation?
No
Simulation
finished?
No
Yes
Yes
End
Fig. 3. The FABCell program loop. An iteration consists of
selecting cells to process and generating events (and any
associated preconditions) to update the system state.
state (i.e. changes are not reflected until the next iteration), or asynchronously where changes to each cell are
immediately observable by other cells.
During the interaction step, cells interact with neighbouring cells. FABCell uses an agent paradigm, where agents
can only partially observe their environment and are limited to interactions within a local neighbourhood.
Event Model. An update is prescribed by a sequence of Event
objects which are processed and monitored using a transaction system. Events control exactly what happens to a
cell, for example a translation or reversal. Events are created using EventCreators which are passed to the migration state object. During each iteration, the MigrationStateUpdate object runs the event creators for each cell and
produces a set of Events to update the cells. There is a corresponding set of EventHandlers which process events and
update the system.
Precondition handling. A given event may have a number of
preconditions that must be met before it can be executed.
One of the functions of the event transaction system is a
precondition handling system (Fig. 3) to deal with such
situations in a controlled manner. Each event handler can
choose to either process an event or raise a precondition
event. If a precondition is raised, execution of the simulation is halted and the precondition event is routed to an oracle which attempts to match a handler to the precondition.
A precondition handler is allowed to create new events.
The new events are placed in the execution stack before
the current event and the simulation is restarted. Once the
new events have executed, the preconditions of the current
event are met and it is allowed to execute.
3. Myxobacteria rippling
We use FABCell to investigate the rippling behaviour of
myxobacteria using an off-lattice Monte Carlo simulation. At
each step, changes to the system are proposed which are accepted using the Metropolis function [11]. Each cell consists
of 8 linked segments, each with a finite volume (see Fig. 4).
Segment
centre
Ealign (a) = α · bm
(
ĉ · ê
if (ĉ · ê) ≥ 0,
bm =
− (ĉm · ê) else.
sa,1 − sa,N
ĉ =
ksa,1 − sa,N k
e
ê =
kek
X
e=
si,1 − si,N
(2)
(3)
(4)
(5)
(6)
i neighbours
Segment
volume
d
Fig. 4. Schema of a segmented model cell. Each segment
consists of a centre (black dot) and a neighbourhood of lattice
nodes that represent the segment volume (grey hexagon).
Neighbouring segment centres are a distance d from each
other.
where α is a dimensionless alignment coefficient, ĉ is the
normalised average direction of the cell, ê is the average
direction of all the cells in a local neighbourhood surrounding cell a. bm reflects that cells tend to turn through
the acute angle to align with other cells in either direction.
Bending energy. Cells have a semi flexible body with a certain stiffness which is enforced by limiting its radius of
curvature.
Ebend (a) = σ
Cell physics and interactions are described with energy terms
in the following Hamiltonian:
bm,n
dm,n
êm,n
= Estretch + Ealign + Ebend + Epropulsion
em,n
+Eclimbing + Eslime
fˆm,n
fm,n
Stretching energy. Cells have a finite volume and fixed shape
which are enforced by constraints preventing segments
getting too close or too far apart.
Estretch (a) = λ
b2a,i
(7)
i=1
3.1 The Hamiltonian
H
N
X
N
−2
X
(ksa,i+1 − sm,i k − d0 )
2
(1)
b
if n = 0.
m,n+1
= bm,n−1 if n = N .
dm,n
else.
= cos−1 êm,n · fˆm,n
em,n
=
kem,n k
= sm,n+1 − sm,n
fm,n
=
kfm,n k
= sm,n − sm,n−1
(8)
(9)
(10)
(11)
(12)
(13)
where σ is a dimensionless bending coefficient, dm,n returns the angle between em,n and fm,n , em,n is the average direction of segment n of cell m and fm,n is the
vector between the segment ahead of n (sm,n−1 ) and the
segment behind (sm,n+1 ).
Propulsion energy. Cells move in the direction of their long
axis reflecting the propulsion effect of slime extrusion.
i=0
Epropulsion (a) = −
N
X
(ûi · ê)
(14)
i=2
where λ is a dimensionless stretching coefficient, N is the
number of segments in cell a, d0 is the optimal distance
between segments, sk,l is the vector position of segment l
in cell k and λ a dimensionless stretching coefficient. We
define stretching energy as a squared sum which compares
the distance between the centres of neighbouring segments
sm,i and sm,i+1 to d0 and penalises a cell for allowing
segments to get either too close or too far apart.
Alignment energy. Cells tend to align themselves with their
neighbours reflecting the effect of pili hooking and retraction.
sa,1 − sa,N
ksa,1 − sa,N k
sa,n−1 − sa,n
ûn =
ksa,n−1 − sa,n k
ê =
(15)
(16)
where is a dimensionless propulsion coefficient, ê is the
normalised average direction of the cell and ûn is the
update direction of segment n of cell a. Each segment
moves towards where its head segment was previously
except if this causes segments to become unaligned.
Climbing energy. In highly populated areas, we allow cells
to climb over blocking cells. The more populated an area
is, the more likely it is cells will climb to avoid obstacles.
Climbing is dependent on space above the cell being available.
ct+1 =
st =
Eclimbing (a) = −η (cells(b, c) · space(d, n))
d=b+e
(
1 if cells(f , h) = 0,
space(f , h) =
0 else.
X
cells(g, h) =
occupied(i)
(19)
(20)
i∈h
occupied(j) =
(
1
0
0
X
(27)
else.
a
(28)
i neighbours
(17)
(18)
(
ct + n + (νst ) if ct < cmax ,
where ct+1 is the new clock value, ct is the current clock
value, n is a basal increase factor, ν is the signal strength, st
the level of C-signalling a cell is experiencing at time t defined
as a measure of the collisions a cell is experiencing and a a
collision factor.
4. Results
if a cell occupies position j,
(21)
else.
where η is a dimensionless climbing coefficient, b is the
current location of cell a, c is a neighbourhood, d a location a distance e above b to check for empty space,
space(f , h) determines whether there is any space below
the cell it can move into, cells(g, h) returns the number of
cells occupying neighbourhood h centred around location
g and occupied(j) returns whether a position j is occupied
by a cell.
Slime trail energy. Cells can preferentially follow the slime
trails left by other cells. Cells typically follow the newest
and the largest slime trails they encounter.
Eslime (a) = φ b̂a · ĉ
sm,1 − sm,N
ksm,1 − sm,N k
c
ĉ =
kck
X
c=
slime(i)
b̂m =
(22)
(23)
(24)
(25)
i neighbours
slime(m) =
tm
ktm k
(26)
where φ is a dimensionless slime following coefficient, ĉ
is average direction of the slime trails in a neighbourhood
and slime(m) is the normalised direction vector of the
slime trail at location m.
3.2 Simulating C-signalling
Having established a model of myxobacteria cell physics, we
also model the effects of cell biochemistry. Reversals are
thought be controlled by C-signal stimulating the complex
Frz pathway, however the exact function of each component
has yet to be determined [19]. To overcome this problem, we
model the macroscopic behaviour of the pathway and do not
discuss its function in detail. Each cell has a model of Csignalling to control its reversal characteristics. An internal
phase clock is used as an abstract representation of C-signal.
The clock increments until it reaches cmax at which point it
resets and the cell reverses. The clock can be perturbed by signalling between neighbouring cells to make reversals happen
quicker and simulate the effect of C-signalling.
4.1 Ripple formation
Fig. 5 shows the output from a Monte Carlo simulation of
5500 cells using the Hamiltonian described in Section 3. Csignalling was modelled using a variable phase clock function
[2]. Cells were initially randomly distributed and randomly
aligned. The emergent behaviour of the system is the formation of 3 ripple bands. In the pre-rippling phase the majority
of cells reverse approximately every 10 minutes with a small
percentage of cells reversing very quickly (every 4-6 minutes).
This corresponds to increased signalling due to the proximity
of a large number of randomly oriented cells which collide. A
proportion of cells experience many collisions and reverse in
6 minutes or less. A much greater proportion of cells reverse
in either 7 or 8 minutes. This can be down to a number of factors. Cells which have climbed over others and are nearer the
top level will naturally experience fewer C-signalling events
as there will be fewer cells to interact with. Cells nearer the
bottom will be more crowded together and are therefore likely
to experience more signalling. It might be expected that signalling would be a lot higher initially but two factors limit
this: polar sensitivity and traffic jams. C-signalling is thought
to only occur at the cell poles, implying signalling does not
take place over a large proportion of the cell so even cells
in close proximity, actually experience few signalling events.
Cells tend to stall in a closely packed environment since there
is limited space for cells to move. Once a traffic jam forms,
cells can get stuck temporarily until they either naturally reverse or the jam disperses. During this waiting time, there is
again little opportunity for signalling because the position of
cell heads relative to each other changes very little. These two
factors combined ensure that at a given time, only a small percentage of cells will actually be close enough that their heads
interact. There will be always be some cells which receive minimal signalling either from being in a jam or else isolated from
other cells. The majority of cells reverse every 7 to 8 minute reflecting that most cells will experience some signalling which
will speed them up slightly.
4.2 Slime trails
In our models, cells are biased towards following the newest
and thickest slime trails to match observed behaviour. The
slime trail machinery was found to be important in maintaining
spatial cohesion between cells. Ripples do not form when the
slime machinery is switched off. Ripple formation appears to
2h
4h
8h
a)
250
250
225
225
225
200
200
200
175
175
175
150
150
150
125
x (µm)
250
x (µm)
x (µm)
b)
125
125
100
100
100
75
75
75
50
50
50
25
25
3
6
9
12 15 18 21
Time (minutes)
24
27
30
25
3
6
9
12 15 18 21
Time (minutes)
24
27
30
3
6
9
12 15 18 21
Time (minutes)
24
27
30
Fig. 5. Time evolution of ripple formation in a Monte Carlo off-lattice simulation of 5500 cells in a simulation volume
measuring 250 × 40 × 10 µm. Starting from an initial random distribution, cells organise into 3 distinct ripple bands after
approximately 4h. (a) Top down view of simulation. (b) Corresponding space-time plot of cell movement in the x dimension
during a 30 minute interval measured around the given time point.
be strongly dependent on cell alignment, both within ripples
and in troughs when cells are acting under A-motility. The
slime serves to direct cells, so in regions of low cell density
they follow straight paths between ripple fronts. Without slime,
cells move more randomly unless they are close enough to
other cells for S-motility to cause alignment. Leading cells
within a ripple front or densely populated area break away
and expand from the pack in “Frizzy” like patterns as cell
orientations become more diverse. Ripples break down as other
cells follow the leading cells with S-motility. A-motility and
S-motility are not sufficient by themselves to maintain cell
order over long distances which seems to be a requirement of
rippling.
4.3 Signalling and reversing
In the segmented cell model, only the head and tail region
are sensitive to signalling, as C-signalling is thought to occur
only at the poles [9] and only when cells are colliding. This
means the majority of the cell body is insensitive to signalling.
It became apparent that the duration of a signalling pulse is
important. Evenly in a highly dense population, a cell will not
actually receive that many signalling events. Therefore cells
must be very sensitive to signalling if only a few events are
required to trigger reversal and the effect of signalling must
last several seconds if it is to sufficiently perturb the reversal
cycle and cause a premature reversal.
5. Conclusion
We were able to show that even in a dense region, cells do not
necessarily experience increasing C-signalling due to the Csignalling mechanism being only at the head and tail of the cell.
The small surface area of the C-signalling region implies that
either cells are very sensitive to C-signalling if reversals can
be triggered in after 4-5 collisions or else C-signalling occurs
along more of the body.
Although C-signal accumulates within a cell up until the
point of sporulation, the current understanding of the Frz pathway would suggest that it is not the amount of C-signal within
the cell but rather the change in C-signal that causes reversal. If
cells are continuously stimulated by constantly increasing levels of C-signal, they will keep reversing more quickly leading
to hyper-reversals. This does not happen as reversal frequencies return to their pre-rippling values as cells become synchronised within a ripple. This suggests cells only signal when they
are approximately head on as otherwise rippling cells would
signal continuously and the ripple wavelength would be very
short. We therefore suggest C-signal interaction with the Frz
pathway is not so straightforward. If the C-signalling effect
is finite, then how long a pulse lasts becomes important. In
our off-lattice model, time is resolved in the order of seconds;
however, a signal lasting one second has minimal impact on
the system. Combined with the effect of the small signalling
region only at cell poles, we found that a C-signal pulse has
to be effective for several seconds if it is to adjust the reversal
characteristics enough to cause spatial pattern formation.
Mesh based models [1, 2, 3] typically restrict the degrees
of freedom of cells. Cell flexibility and shape are not really
considered; however, we find that these are important physical properties determining cellular behaviour. As ripples form,
areas of low cell density develop. Due to the stochastic motion of cells, small perturbations in the travelling direction often lead cells to gradually but significantly alter their course
in a low density region with few other cells to interact with.
We found that slime serves an essential purpose of maintaining long range cohesion between cells. S-motility is too short
range; cells will remain aligned within small clusters but the
macroscopic behaviour allows cells to change course leading
to the same dispersal problem as with single cells in a low density environment. Ripple formation is dependent on cells remaining aligned to maximise collisions in counter-propagating
waves and troughs; if cells drift off course the ripple fronts
break down.
Rippling can occur in a monolayer population [14]. Our
experiments suggest that in a tightly packed population, ripples
do not form if a strict monolayer of cells is maintained. When
tightly packed, cells have little room to manoeuvre and are
frequently obstructed by other cells. Some cells are able to
move around obstacles but this is dependent on there being
enough space to allow them to change course; however, most
cells are very close to each other and cannot avoid stalling. This
issue is not addressed in lattice models where cells are typically
restricted to moving in fixed planes which reduces the overall
number of cells that can actually cause obstruction. Ripples
are not a consequence of cells colliding, blocking each other
and then reversing as data tracking individual cells showed
they typically do not pause for long periods [14]. In highly
populated environments aggregation centres are much likely
to form but these are not a common artefact during rippling.
S-motility and A-motility are not sufficient by themselves to
maintain rippling. If cells alter course to avoid objects, their
alignment rapidly breaks down into small clusters and the
macroscopic alignment is lost. We therefore conclude that cells
will ripple in a monolayer provided some cells can climb over
others to avoid collision.
Alignment energy models the effects of S-motility and is
dependent on the general direction of movement of a cell’s
neighbours. Cells tend to align along the average direction of
neighbouring cells. If the angle between a the average direction
of a cell and its neighbours is obtuse, a cell will align in the opposite direction so that the alignment is direction independent.
We found that if cells only align in the direction of their neighbours, it prevents them from intermixing during collisions and
when they are tightly packed. Cells stall and attempt to fold
back in on themselves to align with the oncoming cells rather
than attempting to push past each other.
Collision resolution is important for determining how cells
deal with problems. Wu et al. [18] use prescriptive algorithms
for dealing with collisions, so cells always behave in the same
manner (with a small amount of variability). It seems unlikely
that cells have a specific mechanism for collision resolution;
their movement is a directed random process and if they do
collide, they appear to continue with normal behaviour patterns
until free rather than using a collision resolution strategy. We
did not implement a collision algorithm and instead added a
term to the Hamiltonian to make it energetically unfavourable
for a cell to occupy the space of another. The natural tendency
of cells is therefore to try and avoid collisions but take no
affirmative action if they do collide which we think reflects
the biology of the system better.
As an important secondary result, we have developed FABCell a high-performance agent based framework for studying
the population dynamics of biological cells in a 3D environment. Our general purpose tool provides a highly flexible and
modular design for scientists to create models. Future versions
of the software will extend the functionality even further.
As this paper demonstrates, rippling cannot be explained
purely by the function of signalling pathways alone; it is also a
direct result of the physical characteristics of the cell. Our work
serves as a basis for future models looking at other aspects of
the myxobacteria life cycle.
References
[1] M.S. Alber, M.A. Kiskowski, and Y. Jiang. Two-stage aggregate
formation via streams in myxobacteria. Phys.Rev.Lett., 93:1–4,
2004.
[2] U. Börner, A. Deutsch, and M. Bär. A generalised discrete model
linking rippling pattern formation and individual cell reversal
statistics in colonies of myxobacteria. Phys. Biol., 3:138–146,
2006.
[3] U. Börner, A. Deutsch, H. Reichenbach, and M. Bär. Rippling
patterns in aggregates of myxobacteria arise from cell-cell
collisions. Physical Review, 89:78100–78097, 2002.
[4] M.E. Diodati, R.E. Gill, L. Plamann, and M. Singer. Initiation
and early developmental events. In D.E. Whitworth, editor,
Myxobacteria: Multicellularity and Differentiation, pages 43–
76. American Society for Microbiology, 0.
[5] M. Dworkin. Recent advances in the social and developmental
biology of the myxobacteria. Microbiological Reviews, 60:70–
102, 1996.
[6] O.A. Igoshin, A. Goldbeter, D. Kaiser, and G. Oster. A
biochemical oscillator explains several aspects of Myxococcus
xanthus behavior during development. Proc. Natl. Acad. Sci.
USA, 101:15760–15765, 2004.
[7] O.A. Igoshin, R.D. Welch, D. Kaiser, and G. Oster. Waves and
aggregation patterns in myxobacteria. Proc. Natl. Acad. Sci.
USA, 101:4256–4261, 2004.
[8] L. Jelsbak and L. Søgaard-Anderson. The cell surface-associated
intercellular c-signal induces behavioral changes in individual
Myxococcus xanthus cells during fruiting body morphogenesis.
Proc. Natl. Acad. Sci. USA, 96:5031–5036, 1999.
[9] L. Jelsbak and L. Søgaard-Anderson. Cell behavior and cellcell communication during fruiting body morphogenesis in
Myxococcus xanthus. Journal of Microbiological Methods,
55:829–839, 2003.
[10] S.K. Kim and D. Kaiser. C-factor has distinct aggregation and
sporulation thresholds during Myxococcus development. Journal
of Bacteriology, 173:1722–1728, 1991.
[11] N. Metropolis. The beginning of the monte carlo method. Los
Alamos Science, 15:125–130, 1987.
[12] T. Mignot. The elusive engine in Myxococcus xanthus gliding
motility. Cell. Mol. Life Sci., pages 1–13, 2007.
[13] L.J. Shimkets and D. Kaiser. Induction of coordinated movement
of Myxococcus xanthus cells. Journal of Bacteriology, 152:451–
461, 1982.
[14] O. Sliusarenko, J. Neu, D.R. Zusman, and G. Oster. Accordion
waves in Myxococcus xanthus. Proc. Natl. Acad. Sci. USA,
103:1534–1539, 2006.
[15] J. Starruß, T. Bley, L. Søgaard-Anderson, and A. Deutsch. A
new mechanism for collective migration in Myxococcus xanthus.
Journal of Statistical Physics, 128:269–286, 2007.
[16] R.D. Welch and D. Kaiser. Cell behavior in traveling wave
patterns of myxobacteria. Proc. Natl. Acad. Sci. USA, 98:14907–
14912, 2001.
[17] Y. Wu, N. Chen, M. Rissler, Y. Jiang, D. Kaiser, and M.S. Alber.
Ca models of myxobacterial swarming. In Lecture Notes in
Computer Science, pages 192–203. Springer-Verlag, 2006.
[18] Y. Wu, Y. Jiang, D. Kaiser, and M.S. Alber. Social interactions in
myxobacterial swarming. PLoS Computational Biology, 3:2546–
2558, 2007.
[19] D.R. Zusman, A.E. Scott, Z. Yang, and J.R. Kirby. Chemosensory pathways, motility and development in Myxococcus xanthus.
Nature Reviews Microbiology, 5:862–872, 2007.