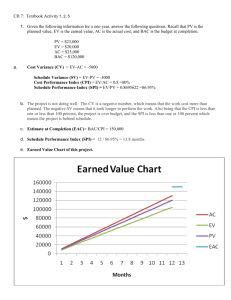
Advance Journal of Food Science and Technology 5(11): 1443-1449, 2013
advertisement

Advance Journal of Food Science and Technology 5(11): 1443-1449, 2013 ISSN: 2042-4868; e-ISSN: 2042-4876 © Maxwell Scientific Organization, 2013 Submitted: June 28, 2013 Accepted: July 08, 2013 Published: November 05, 2013 Effects of Synthetic and Natural Extraction Chemicals on Functional Properties, Polyphenol Content and Antioxidant Activity of Soy Protein Isolates Extracted from Full-Fat and Defatted Flours 1, 2 Moses Vernonxious Madalitso Chamba, 1Yufei Hua, 1Quirino Dawa, 1 Odilon Djakpo and 1Caimeng Zhang 1 School of Food Science and Technology, Jiangnan University, 1800 Lihu Avenue, Wuxi 214122, Jiangsu Province, P.R. China 2 Department of Human Ecology, Domasi College of Education, University of Malawi, P.O. Box 49, Domasi, Zomba, Malawi Abstract: The number of consumers preferring natural and organic foods to those that involve synthetic chemicals has recently shown dramatic growth. As such, a native isoelectrically precipitated soy protein isolates (SPIs) were prepared from full-fat and defatted flours using amaranth (Amaranthus tricolor L.) lye (pH>12.5) and lemon extract (pH<2.5) as natural food-plant-based chemicals, replacing the conventional synthetic ones (NaOH and HCl, respectively). Functional properties, total polyphenol content and antioxidant activity of the natural SPIs were compared to those of synthetic ones. All the SPI samples qualified to be soy protein isolates with a minimum protein content of 91.21%. The natural SPI showed significant increase in emulsion stability (p≤0.05). While higher values with narrow margins were shown by the synthetic than the natural SPIs in oil absorption (0.66±0.02, 0.50±0.01%, respectively), emulsion capacity (56.53±0.57, 55.50±0.39%), foam stability (11.33±0.61, 10.40±0.40%). No significant difference was observed in water absorption capacity. The DPPH assay showed increased antioxidant activity in the natural SPI although its total polyphenol content was lower. Thus, SPI with functional properties similar to those of conventional ones can be naturally processed using amaranth ash and lemon extracts as alternative chemicals to addressing the fear of consuming synthetic chemical by health-conscious natural and organic food consumers. Keywords: Amaranth ash extract, conventional soy protein isolate, lemon extract, natural chemicals, natural soy protein isolate, synthetic chemicals INTRODUCTION The past few years have shown dramatic growth in organic, natural and environmentally friendly or "green" food market. As many people are becoming more conscious about their health, uncertainty about safety of the highly processed foods, especially in terms of its long term effect as they interact with one another in the body, is rising. Studies on food preferences have shown fear of exposure to non-food synthetic chemicals and environmental consciousness as major factors influencing this new food consumer behavior (DicksonSpillmann et al., 2011). Recent years have witnessed an attempt by the agricultural industry to produce food naturally without using synthetic chemicals. Likewise, food manufacturers are also trying to explore new food processing techniques to address the need for safer food and compete for consumer acceptance (Zink, 1997). Various modern and partially traditional techniques are currently being employed by the food industry to process plant agricultural products, such as soybeans, into fat-reduced, protein-enriched compositions for use in food manufacturing. They include solvent extraction and a variety of press-based methods, e.g., extruder, expeller, continuous and cold presses, to separate at least a portion of the fat from the remaining plant material. Nevertheless, at some stage strong non-food synthetic chemicals are still used to finalize the process. Some of these chemicals are not natural, or plant based and cannot be used to produce certified organic food products under United States Department of Agriculture (USDA) guidelines for organic food labeling (Gold, 2012). As such, a lot of improvements are yet to be made on highly processed foods. Soy protein isolate (SPI), a soybean derivative protein powder, is one of the most important products in food processing as well as many other industrial uses. Its preference is attributed to its ability to enhance nutritional (especially protein) and functional qualities of food products to which it is used as an ingredient (Kinsella, 1979; Mariotti et al., 1999; L’hocine et al., 2006). SPI has been added to baked foods, breakfast cereals and meat products among others. It can also be made into a nutritious drink once Corresponding Author: Yufei Hua, School of Food Science and Technology, Jiangnan University, 1800 Lihu Avenue, Wuxi 214122, Jiangsu Province, P.R. China 1443 Adv. J. Food Sci. Technol., 5(11): 1443-1449, 2013 added to water. A soybean product qualifies to be called a protein isolate only if it contains at least 90% crude protein (N×6.25) on dry basis (Codex Alimentarius Commission, 1996). To achieve this purity, conventional technique of processing SPI involves use of synthetic chemicals such as n-hexane, ethanol, sodium hydroxide (NaOH) and hydrochloric acid (HCl) (Kinsella, 1979). These chemicals have well known risks if handled without taking adequate precautions and their health hazard cases, especially with direct contact in adequate doses have been widely reported. Although some of these chemicals are removed during the process, having some small residues in the final product cannot be exclusively denied. Furthermore, a health conscious natural food consumer cannot be convinced that foods processed using these chemicals are safe to eat. Dickson-Spillmann et al. (2011) reported that laypeople perceive chemicals as either safe or hazardous and think that even minor doses of chemicals are likely to cause harm. To such people, ‘synthetic equals dangerous’. The challenge lies therefore on identifying natural and food-based reagents and chemicals that would produce similar or improved results as the synthetic ones. At this time, when consumers are increasingly becoming skeptical about consuming food processed using synthetic chemicals, more exploration on the traditional chemistry may be considered a favorable option just as it is in traditional medicine. Amaranth ash solution has been used traditionally for food preparation as an alternative to bicarbonate of soda (alkaline). In countries like Malawi, amaranth (Amaranthus hybridus) plant is the most preferred source of ash for food alkali due to its pH strength and safety for consumption. However, information on the same can scarcely be found. Lemon extract as citric acid is a well known food acid, but rarely mentioned in soybean products studies. In our previous study it was observed that soy protein isolate with acceptable yield, composition and nutritive quality can be produced using purely natural, food-plant-based chemicals such as amaranth ash and lemon extracts as alternative to NaOH and HCl, respectively (Chamba et al., 2013). The aim of this study was therefore; to examine the functional properties (water and oil absorption capacity, emulsifying capacity and stability, foam formation and stability, gelation), total polyphenol content (TPC) and antioxidant activity of the SPIs prepared using these natural chemicals with comparison to those in which the conventional synthetic chemicals were involved. purchased from a local market. Soybean grains were supplied by Suzhou Golden Village Food Co., Ltd. Jiangsu province, China. The soybeans were sorted, cleaned (to remove unrelated materials), dehulled then ground into native full fat flour, fine enough to pass through an 80 mesh sieve. On dry basis, the flour had 35% protein (N×6.25), 24% lipid, 3% ash, 7% moisture and Protein dispersibility index (PDI) of 72%. One part of the flour was treated with n-hexane and 95% alcohol to remove fat and soluble sugars. Amaranth ash extract (pH≥12.50) was prepared by filtering distilled water through burnt amaranth plant ash at the ash to water ratio of 1:5(w/v) after optimization. Lemon extract (pH<2.20) was prepared by squeezing the lemons then vacuum filtering the extract to remove the pulp. DPPH (2, 2-diphenyl-1-picryl hydrazyl) was purchased from Sigma, Shanghai. All other reagents and chemicals were of analytical grade. Deionized or distilled water was used in all laboratory procedures and sample preparations. Preparation of soy protein isolate: Four SPI samples namely; Synthetic Chemical Full Fat flour (SCFF), Synthetic Chemical Defatted Flour (SCDF) Natural Chemical Full Fat flour (NCFF) and Natural Chemical Defatted Flour (NCDF) were prepared as described by Chamba et al. (2013). Appropriate flour (full fat or defatted) was suspended in distilled water at the flour to water ratio of 1:10 (w/v). The pH of the suspension was adjusted to and maintained at 7.0 with either amaranth extract or NaOH, while stirred for 1 h at ambient temperature 22±2°C. After centrifugation, at 10000×g and 4°C for 30 min (for SCFF, SCDF and NCDF SPIs), the supernatant was recovered. Soy protein was precipitated by adjusting pH to 4.5 with lemon extract or HCl then centrifuged at the above conditions. The precipitate was washed and centrifuged twice before suspended again in distilled water and neutralized to pH 7.0 with either amaranth extract or NaOH. All resolubilized SPI samples were freeze-dried, sealed in polythene bags and stored at 4°C until further analysis. METHODOLOGY Proximate analyses: Proximate analyses of the four samples were conducted as follows: Crude protein was determined by micro-Kjeldahl method with the common conversion factor of N×6.25 (AOAC, 2000). Crude oil was extracted the traditional Soxhlet extraction apparatus and determined according to AOAC official method (AOAC, 2000). Total ash was analyzed using the conventional method by dry-ashing in muffle furnace at 550°C. Moisture content was calculated by drying weighed samples for 3 h in an oven at 120°C (Smith et al., 1966). Materials: Edible green and purple colored amaranth (Amaranthus tricolor L.) plants were obtained from local farmers in the outskirt of Wuxi city, Jiangsu province, China. Ripe lemon (Citrus limon) fruits were Functional properties: Oil and water absorption capacities: Oil absorption capacity (OAC) and Water Absorption Capacity (WAC) were assessed in triplicate using the procedure 1444 Adv. J. Food Sci. Technol., 5(11): 1443-1449, 2013 described by Lin et al. (1974). A protein sample (0.3 g) was mixed with soy oil or distilled water (1.5 mL) in a pre-weighed 15 mL graduated centrifuge tubes and centrifuged for 30 min at 4000 rpm. The supernatant was discarded and the tubes were reweighed. The OAC and WAC (%) were expressed using the following equation: FAC/WAC (%) = (W 0 + W 1 ) /W 0 ×100 where, W 0 = The initial weight of the sample W 1 = The weight of sample plus the absorbed liquid Foaming capacity and stability: Foams were prepared according to Sathe and Salunkhe (1981) with minor modifications. Briefly, 2 g of each SPI sample was solubilised in 200 mL of distilled water to make a 1% solution. Aliquots of 50 mL were poured into graduated 250 mL, 3 cm diameter cylinder and whipped by high shear stepless speed homogenizer (model: FA 25, Fluco Equipment Co. Ltd., Shanghai, China) at 10000 rpm, ambient temperature (20±2°C) for 3 min. A smooth round end glass rod was used to level the foam for easy reading. Foam capacity was calculated as percentage rise in total volume at 0 times. Stability was determined as a factor of foam drainage recorded after 30 min: Foaming capacity (%) = (V 1 - V 0 ) /V 0 ×100 Foam stability (%) = V 2 /V 0 ×100 where, V 0 = Initial sample volume before whipping V 1 = Total sample volume after whipping at time 0 V 2 = The water volume still trapped in foam after 30 min Emulsifying capacity and stability: For emulsion properties, the method described by Yasumatsu et al. (1972) was used with minor modification. Protein isolate solution (1%, w/v) was prepared in 30 mL of distilled water in a beaker, magnetically stirred for 1 h to solubilize the protein. Then, 30 mL of soy oil was added. The mixture was homogenized by a rod homogeniser (model: FA 25, Fluco equipment co. Ltd., Shanghai, China) for 0.5 min at 10000 rpm. Triplicate aliquots (12 mL each) of the freshly prepared emulsion was poured into calibrated centrifuge tubes and centrifuged at 2500 rpm for 15 min. The ratio of the height of water layer to the total liquid layer was used to calculate emulsifying activity (EA). The emulsion was then heated at 70°C for 30 min in a water bath, followed by 15 min of cooling under running tap water and centrifugation at the same conditions. The stability was worked out using the height of the two layers. Results were calculated as follows: EA/ES (%) = (V 0 - V 1 ) /V 0 ×100 where, V 0 = The initial volume of the total liquid in the centrifuge tube V 1 = The volume of water layer Least gelation concentration: The least gelation concentration was determined by the method of Coffman and Garcia (1977). Test tubes containing 5 mL protein suspensions of 5, 6, 7, 8 up to 15%, respectively (w/v) in distilled water were heated for 1 h in boiling water, followed by cooling in ice bath and further cooling for 2 h at 4°C. The results were reported as liquefied (-), gluey (±) and gel (+). The least gelation concentration was the minimum concentration at which the sample did not fall down or slip when the test tube was inverted. Determination of total polyphenol content and antioxidant activities: Total polyphenol contents (TPC): To extract polyphenols, a sample (1 g) was suspended in 25 mL methanol-water 80:20 v/v acidified with 0.1% HCl and magnetically stirred for 1 h at room temperature. Later, the mixture was centrifuged at 1800×g for 15 min, the methanol was decanted and the residue extracted again with 25 mL of fresh aqueous methanol. After centrifugation at same conditions, the two extracts were combined (Akowuah et al., 2005; Mujica et al., 2009). Total phenols content (TPC) of the SPIs was determined by Folin-Ciocalteau phenol reagent as described by Škerget et al. (2005) with some modification, using garlic acid monohydrate (0-60 µg/L, N = 7) as a standard. To 0.5 mL of appropriately diluted sample, 2.5 mL of Folin-Ciocalteu reagent was added. After that (within time interval from 0.5 to 8 min), 2 mL of Na 2 CO 3 (7.5%) was added. The mixture was incubated for 5 min at 50°C and then cooled. For a control sample, 0.5 mL of distilled water instead of sample was used. The absorbance was measured at 760 nm by UV/Vis spectrophotometer (UV-2450, Shimadzu Corporation, Japan). The content of total phenols was expressed as garlic acid equivalents (GAE) in µg/g SPI. All analyses were run in triplicate and mean values were calculated. The calibration equation of garlic acid was y = 0.05997x - 0.03535, R2 = 0.9997. DPPH free radical-scavenging assay: DPPH free radical-scavenging assay was determined according to the method of Brand-Williams et al. (1995) with modification. A 2 g of each SPI sample was extracted twice (2 h each time) with 20 mL aqueous methanol (80% containing 0.1% HCl) centrifuged at 2500 rpm for 15 min and supernatant combined. Then, a series of working solutions of different concentrations (0.5, 1, 2, 3, 4 and 5 mg/mL, respectively) were prepared. A 1445 Adv. J. Food Sci. Technol., 5(11): 1443-1449, 2013 Table 1: Proximate analyses of the SPI samples Content NCFF NCDF Protein (%) 91.21 93.64 Lipid 0.71 <0.05 Ash 5.42 5.91 Moisture 3.38 3.24 SCFF 92.12 0.65 3.83 2.49 SCDF 98.45 <0.05 2.08 2.84 3.5 mL of methanolic DPPH (2, 2-diphenyl-1-picryl hydrazyl, 0.06 mM) solution was added to 1.5 mL diluted sample or methanol (for control) and vortexed. The mixture was incubated for 30 min at room temperature in the dark. The absorbance was read at 517 nm by UV-Vis spectrophotometer (UV-2450, Shimadzu Corporation, Japan) against the DPPH solvent as blank. The inhibition percentage of free radical by sample was calculated using the following formula and the results presented as a comparative graph: Inhibition % = (A 0 - A 1 ) /A 0 ×100 where, A 0 = The absorbance of the control (blank, without sample) A 1 = The absorbance in the presence of the sample Statistical analysis: One way analysis of variance (ANOVA), with Duncan's multiple range test, was conducted using a SAS program (version 8.1, SAS Institute Inc., Cary, NC, USA) to asses significance of differences (p<0.05) among means of triplicate sample runs. RESULTS AND DISCUSSION Proximate analysis: Values for proximate analysis are presented in Table 1. The SPIs prepared from either full-fat soybean flour or natural chemicals (ash and lemon extracts) showed slightly lower protein compositions as compared to the one from both defatted flour and conventional synthetic chemicals (NaOH and HCl), respectively. A soy product is recognized as an isolate only if it contains not less than 90% protein (Preeti et al., 2008). Thus all samples, including the NCFF, qualified to be SPIs. The rest of the compositions were within the recommended ranges of a good quality SPI according CODEX STAN 175-1989 general standards (Codex Alimentarius Commission, 1996). The lower protein composition observed in NCFF may be attributed to high fat in the starting material (full-fat flour), ash composition in the solvent (ash extract) and remaining pulp in the acid (lemon extract). Functional properties: Oil and water absorption capacities: Results of the oil and water absorption capacities are presented in Table 2. The WAC was significantly higher in SCFF SPI (0.62±0.05 mL/g) followed by SCDF and NCFF (0.55±0.04, 0.55±0.03 mL/g, respectively). NCDF SPI showed the lowest value. In their study, Fleming et al. (1974) and Deshpande et al. (1982) attributed improved water absorption capacity to the increase in protein content of the sample. Similarly, higher WAC has been observed in protein isolates in comparison with protein concentrates (Fleming et al., 1974; Lin et al., 1974; Wang and Kinsella, 1976; Hutton and Campbell, 1977) probably indicating the relationship between water absorption and protein content. However, the findings of this study did not fully agree with the protein content of the samples (Table 1). Inasmuch as protein content between the two main samples (SCDF and NCFF) was substantially different, there was no difference in water absorption, indicating that other factors might had played a role. Lin et al. (1974) observed that sunflower products had lower water absorptions than did soy products, although their protein contents were similar. These finding indicate that water absorption of a particular sample need not always be parallel to the protein content; other food components may have an influence. The oil absorption capacity (OAC) differed significantly in all samples (SCDF> SCFF>NCFF>NCDF) as shown in Table 2. The differences were within a range between 0.37±0.01 mL/g (NCDF) and 0.66±0.02 mL/g (SCDF) for all samples and from 0.50±0.01 mL/g (NCFF) to 0.66±0.02 mL/g (SCDF) between the two main samples (traditional and conventional SPIs, NCFF and SCDF, respectively). Oil absorption values seemed to fall within the same ranges with those of water absorption. Oil absorption is mainly attributed to the physical entrapment of oil and is related to the number of nonpolar side chains on proteins that bind hydrocarbon chains of fat (Lin et al., 1974; Kinsella, 1979). The increased absorption in SCDF is then associated with increased nonpolar side chains in the sample due to its higher protein content. Foaming capacity and stability: Volume increase of the 1% aqueous dispersion of all the SPI samples ranged from 110.00±5.29% (NCFF) to 231.33±4.16% (SCDF) after whipping as shown in Table 2. The specific volumes of the foams were determines as indicators of air uptake during whipping. The lower air Table 2: Water and oil absorption, emulsion and foaming properties of the traditional and conventional soy protein isolates Sample Water absorption (mL/g) Oil absorption (mL/g) Emulsion capacity (%) Emulsion stability (%) Forming capacity (%) SCFF 0.62±0.05a 0.59±0.01b 55.83±0.66b 52.30±1.64b 127.33±3.06c a a b b SCDF 0.55±0.04 0.66±0.02 56.53±0.57 54.51±0.92 231.33±4.16a NCFF 0.55±0.03a 0.50±0.01c 55.50±0.39c 54.71±1.00a 110.00±5.29d NCDF 0.32±0.05b 0.37±0.01d 57.47±0.33a 53.43±0.89b 217.33±7.02b a-d : Values with different superscript letters along the same column indicate significant difference (p≤0.05) among means of triplicates tests 1446 Form stability (%) 9.80±0.70b 11.33±0.61a 10.40±0.40b 9.60±0.40b Adv. J. Food Sci. Technol., 5(11): 1443-1449, 2013 uptake of the SPI prepared with the natural chemicals and full-fat flour was associated with decrease in its foam volume. Specific volume of foam (volume per unit mass) for a given set of conditions is an indication of air uptake only and is not necessarily a measure of foam quality and stability (Deshpande et al., 1982). The foam stability was determined as a factor of foam drainage after 30 min, other than the volume maintained by of the foam. Observation has shown that foam has a capability of maintaining their volume by sticking to the side of the container even if it is weak. Usually, the amount of liquid retained in the foam is a better indication of foam strength or quality. Although the total volumes just after whipping at ambient temperature (16±1°C) differed significantly (p<0.05) in all samples (Table 2), their stabilities did not differ except in SCDF, where it was higher. Thus the stability of the foam may not only be attributed to protein concentration alone as indicated by Deshpande et al. (1982), but also to other components to which the liquid gets entrapped. The effects of pH on both foam capacity and stability have been reported (Lin et al., 1974; Sekul et al., 1978; Canella et al., 1979; Cherry and McWatters, 1981). However, the fact that all samples were treated under the same pH condition, it may not be associated with the differences. Emulsifying capacity and stability: The emulsion capacity (EC) and emulsion stability (ES) of all the SPI samples are given in Table 2. Comparison of the main natural and conventional samples (NCFF and SCDF) showed a significant decrease (p<0.05) of EC with a very narrow margin (1.86%) in NCFF than SCDF SPIs. The highest EC was observed in one of the intermediate samples (NCDF). However, the natural SPI demonstrated the most significantly (p<0.05) stable emulsion than the rest of the samples, whose ES did not differ. At the same concentration, the ES of all the samples seemed lower that those reported by others. This may be attributed to the method used to prepare the emulsions. It has been reported that in emulsions prepared using the shearing force method, the stabilization property of heated soy protein isolate decreased to a lower level when it was compared to those prepared by other methods (Zayas, 1997). Stabilization of the emulsions by protein polymers is relevant in almost all food applications. Protein polymers have two effects: They can gel in the continuous phase at high protein concentrations through protein cross-linkings and they provide strong repulsive forces when absorbed at the oil/water interface (Ma and Barbosa-Cánovas, 1995). Least gelation concentration: Gelation may be defined as a protein aggregation phenomenon in which polymers-polymer and polymer-solvent interactions and attractive and repulsion forces are so balanced that a tertiary network or matrix is formed (Schmidt, 1981). Such matrix has a capability of trapping and immobilising large amount of water. Protein Table 3: Least gelation concentration of SPI samplesa Concentration (%) SCFF SCDF NCFF NCDF 5 (-) (-) (-) (-) 6 (-) (-) (-) (-) 7 (-) (-) (-) (-) 8 (±) (-) (-) (-) 9 (±) (±) (-) (±) 10 (±) (+) (±) (+) 11 (+) (+) (±) (+) 12 (+) (+) (±) (+) 13 (+) (+) (±) (+) 14 (+) (+) (+) (+) 15 (+) (+) (+) (+) LGC 11 10 14 10 a : Gelation levels: (-) liquefied, (±) gluey and (+) gel; The values are the mean of three replicates concentration, other protein components, in a complex food system, non protein components, heat treatment conditions, pH and ionic and reducing agents are some of the factors that affect gelation (Schmidt, 1981). Comparison of the main natural and conventional SPIs indicated similarity with the findings observed in the foaming property, where higher values were observed in the SPIs prepared from defatted flour than full-fat flour. The greatest value was recorded in NCFF (14%) and the lowest in SCDF and NCDF (both 10%) as presented in Table 3. This was not surprising as it related to the protein content of the samples, which was higher in those prepared from defatted soy flour and was least in NCFF SPI. Depending on food product to which a protein isolate is used as an ingredient, both high and low gelation properties are desirable. These results showed that the natural chemical prepared SPI had lower gelation property compared to the conventional one. By using non-defatted flour and lemon and ash extracts, which were not thoroughly purified, NCFF contained a complex of particles and compound such as carbohydrates and metal elements that might interfere with formation of continuous network of the protein molecules to form a gel. A higher protein concentration thus may be required to overcome their interference with gel formation. Schmidt et al. (1978) reported that dialysis of whey protein concentrate system improved its gelation property. Nevertheless, more information is required on the effects of components other than the SPI itself on protein gelation properties before predictions regarding the gelation behavior of this natural SPI in a complex food system can be made. Determination of total polyphenol content and antioxidant activities: Total polyphenol contents (TPC): The results of the Total polyphenol content (TPC) showed a wide difference between samples prepared using different chemicals, but no significant differences were observed in SPIs prepared from the same chemicals. SCFF and SCDF contained higher TPC (23.80±1.93 and 22.69±2.67 µg GAE/g, respectively) than both NCFF (11.02±1.27 µg GAE/g) and NCDF (9.40±1.33 µg GAE/g). Consequently, it was expected that the conventional chemically prepared samples would 1447 Adv. J. Food Sci. Technol., 5(11): 1443-1449, 2013 CONCLUSION 25 NCFF SPI SCFF SPI NCDF SPI SCDF SPI Percentage inhibition 20 15 10 5 0 0 2 4 6 8 10 12 14 16 Sample concentration (mg/ml) Fig. 1: Antioxidant activity by 2, 2-diphenyl-1-picryl hydrazyl (DPPH, 0.06 mM) assay demonstrate increased antioxidant activity than their natural counterparts. Nevertheless, many compounds such as vitamin C, which is contained in lemons among other sources, are also responsible for the antioxidant activity. This had to be verified by conducting an antioxidant activity determination. DPPH free radical-scavenging assay: DPPH free radical-scavenging assay, using either 2, 2-diphenyl-1picryl hydrazyl or 1, 1-diphenyl-2-picryl hydrazyl radical is one of the most common methods for evaluating antioxidant activity of food samples. It is simple, rapid, generally accurate and inexpensive (Prakash et al., 2001). Additionally, different authors have used different ways of reporting DPPH antioxidant assay results either graphically or by calculating IC 50, a value at which 50% of the used DPPH has reacted with the sample (Marinova and Batchvarov, 2011). In this study, the graphical presentation was preferred to show the exact absorption behavior of the samples. As shown in Fig. 1, the DPPH assay results of this study showed that the SPI prepared using the traditional chemical demonstrated increased antioxidant activity (with NCFF having the highest) than those using conventional chemicals. This increase may be attributed to the presence of lemon extract, which is one of the well known sources of vitamin C, a natural antioxidant. If this is really the case, then the activity may vary depending on the vitamin C composition of lemons used. It was also observed that at a certain concentration, the natural SPI (NCFF) showed serious reduction in absorbance regardless of repetition of the test for a number of times. There is possibility that, due to the impurity of the natural chemicals, the sample contained certain components such as carotenoids, which are normally present in the lemons that at increased concentration substantially interfered with coloration of the assay (Noruma et al., 1997), but not necessarily the antioxidant activity of the sample. The findings of this study suggest that instead of the conventional synthetic NaOH and HCl, traditional chemicals such as amaranth plant ash and lemon extracts can be used to prepare SPI, not only with desirable functional properties similar to those of the conventional ones, but also with increased antioxidant activity. Basic as it may seem, this method of SPI processing may have effective outcomes of improving livelihood of indigenous people in terms of food supplementation, in addition to providing a possible alternative to the synthetic chemicals, which are increasingly feared by the health-conscious food consumers. Much as the functional properties of soy protein isolates have been associated with the protein content of the product, the findings of this study also suggest that functional properties of SPI cannot apparently be attributes to the protein level, but also to its interaction with other components such as ash particles, carbohydrates and lipids. ACKNOWLEDGMENT The authors gratefully acknowledge the assistance of some students and staff of Vegetable Protein Laboratory of the School of Food Science and Technology, Jiangnan University, China. REFERENCES Akowuah, G.A., Z. Ismail, I. Norhayati and A. Sadikun, 2005. The effects of different extraction solvents of varying polarities on polyphenols of orthosiphon stamineus and evaluation of the free radical scavenging activity. Food Chem., 93: 311-317. AOAC, 2000. Official Methods of Analysis. 17th Edn., Association of Official Analytical Chemists, Gait Hersburg, Maryland, USA. Brand-Williams, W., M.E. Cuvelier and C. Berset, 1995. Use of a free radical method to evaluate antioxi dant activity. LWT-Food Sci. Technol., 28: 25-30. Canella, M., G. Castriotta and A. Bernadi, 1979. Functional and physical chemical properties of succinylated and acetylated sunflower protein. Lebensm. Wiss. Technol., 12: 95-101. Chamba, M.V.M., Y. Hua, N. Murekatete and Y. Chen, 2013. Effects of synthetic and natural extraction chemicals on yield, composition and protein quality of soy protein isolates extracted from fullfat and defatted flours. J. Food Sci. Technol, DOI 10.1007/s13197-013-1084-x. Cherry, J.P. and K.H. McWatters, 1981. Whippability and Aeration. In: Cherry, J.P. (Ed.), Protein Functionality in Foods. ACS Symposium Series 147, American Chemical Society, Washington DC, pp: 149. 1448 Adv. J. Food Sci. Technol., 5(11): 1443-1449, 2013 Codex Alimentarius Commission, 1996. Codex Alimentarius-Cereal, Purses, Legumes and Protein Derived Products and Vegetable Proteins. Codex Alimentarius Commission, Rome, Italy. Coffman, C.W. and V.V. Garcia, 1977. Functional proper ties and amino acid contents of protein isolate from mung bean flour. J. Food Technol., 12: 473-484. Deshpande, S.S., S.K. Sathe, D. Cornforth and D.K. Salunkhe, 1982. Effect of dehulling of functional properties of dry bean (Phaseolus vulgaris L.) Flours. Cereal Chem., 59: 39-401. Dickson-Spillmann, M., M. Siegrist and C. Keller, 2011. Attitudes toward chemicals are associated with preference for natural food. Food Qual. Prefer., 22: 149-156. Fleming, S.E., F.W. Sosulski, A. Kilara and E.S. Humbert, 1974. Viscosity and water absorption characteristics of slurries of sunflower and soybean flours, concentrates and isolates. J. Food Sci., 39: 188-193. Gold, M.V., 2012. Organic Production/Organic Food: Information Access Tool. IOP USDA Web. Retrieved from: http://www.nal.usda.gov/afsic/ pubs/ofp/ofp.shtml, (Accessed on: August 10, 2012). Hutton, W.C. and A.M. Campbell, 1977. Functional properties of a soy isolate and a soy concentrate in simple system as related to performance in a food system. J. Food Sci., 42: 457-460. Kinsella, J.E., 1979. Functional properties of soy protein isolate. J. Am. Oil. Chem. Soc., 56: 242-258. L’hocine, L., J.I. Boye and Y. Arcand, 2006. Composition and functional properties of soy protein isolate prepared using alternative defatting and extraction procedures. J. Food Sci., 71: C137-C145. Lin, M.J.Y., E.S. Humbert and F.W. Sosulski, 1974. Certain functional properties of sunflower meal products. J. Food Sci., 39: 368-370. Ma, L. and V. Barbosa-Cánovas, 1995. Rheological characterization of mayonnaise. Part II: Flow and viscoelastic properties at different oil and xanthan gum concentrations. J. Food Eng., 25: 409-425. Marinova, G. and V. Batchvarov, 2011. Evaluation of the methods for determination of the free radical scavenging activity by DPPH. Bulg. J. Agric. Sci., 17: 11-24. Mariotti, F., S. Mahé, R. Benamouzig, C. Luengo, S. Daré, C. Gaudichon and D. Tomé, 1999. Nutritional value of [15N]-soy protein isolate assessed from ileal digestibility and postprandial protein utilization in humans. J. Nutr., 129: 1992-1997. Mujica, M.V., M. Granito and N. Soto, 2009. Importance of the extraction method in the quantification of total phenolic compounds in Phaseolus vulgaris. L. Interciencia, 34: 650-654. Noruma, T., M. Kikuchi and Y. Kawakami, 1997. Proton-donative antioxidant activity of fucoxanthin with 1, 1-Diphenyl-2-Picrylhydrazyl (DPPH). Biochem. Mol. Biol. Int., 42: 361-370. Prakash, A., F. Rigelhof and E. Miller, 2001. Antioxidant Activity. In: DeVries, J. (Ed.), Medallion Laboratories Analytical Progress. Medallion Laboratories, Minnesota, Minneapolis, 19(2): 1-6. Preeti, S., R. Kumar, S.N. Sabapathy and A.S. Bawa, 2008. Functional and edible uses of soy protein products. Compr. Rev. Food Sci. Food Safety, 7: 14-28. Sathe, S.K. and D.K Salunkhe, 1981. Functional properties of great northern bean (Phaseolus vulgaris) proteins: Emulsion, foaming, viscosity and gelation properties. J. Food Sci., 46: 71-75. Schmidt, R.H., 1981. Gelation and Coagulation. In: Cherry, J.P. (Ed.), Protein Functionality in Foods. ACS Symposium Series 147, American Chemical Society, Washington DC, pp: 131. Schmidt, R.H., B.L. Illingworth and E.M. Ahmed, 1978. The effect of dialysis on heat-induced gelation of whey protein concentrate. J. Food Process. Pres., 2: 111-221. Sekul, A.A., C.H. Vinnett and R.L. Ory, 1978. Some functional properties of peanut proteins partially hydrolysed with papain. J. Agric. Food Chem., 26: 855-858. Škerget, M., P. Kotnik, M. Hadolin, A.R. Hraš, M. Simonič and Z. Knez, 2005. Phenols, proanthocyanidins, flavones and flavonols in some plant materials and their antioxidant activities. Food Chem., 89: 191-198. Smith, A.K., J.J. Rackis, P. Isnardi, J.L. Cartter and O.A. Krober, 1966. Nitrogen solubility index, isolated protein yield and whey nitrogen content of several soybean strains. Cereal Chem., 43: 261-270. Wang, J.C. and J.E. Kinsella, 1976. Functional properties of novel proteins: Alfafa leaf protein. J. Food Sci., 41: 286-292. Yasumatsu, K., K. Sawada, S. Moritaka, M. Mikasi, J. Toda, T. Wada and K. Ishii, 1972. Whipping and emulsifying properties of soybean products. Agric Biochem., 36: 719-727. Zayas, J.F., 1997. Functional Property of Protein in Food. Springer-Verlag, Berlin, Heidelberg, Germany. Zink, D.L., 1997. The impact of consumer demands and trends on food processing. Emerg. Infect. Dis., 3: 467-469. 1449