Advance Journal of Food Science and Technology 4(6): 352-356, 2012
advertisement

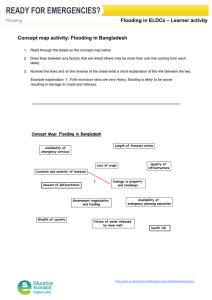
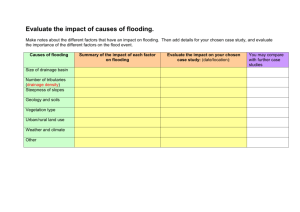
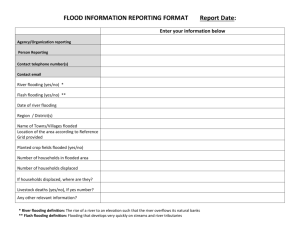
Advance Journal of Food Science and Technology 4(6): 352-356, 2012 ISSN: 2042-4868; E-ISSN: 2042-4876 © Maxwell Scientific Organization, 2012 Submitted: August 31, 2012 Accepted: October 03, 2012 Published: December 20, 2012 Physiological Responses Differences of Different Genotype Sesames to Flooding Stress 1 1 Feng-ying Xu, 1Xiao-ling Wang, 1Qi-xia Wu, 2Xiu-rong Zhang and 2Lin-hai Wang Department of Agronomy, Yangtze University, Jingzhou 434025, Hubei, P.R. China 2 Department of Oil Crops Research, Chinese Academy of Agricultural Sciences, Wuhan 430062, Hubei, P.R. China Abstract: The objective of this study was to elucidate the physiological reaction variation of different genotype sesames to flooding stress. Different sesame genotypes, including two Water logging Tolerant Genotypes (WTGs), WTG-2541 and WTG-2413 and one water logging sensitive genotype, WSG-EZhi2, were used as tested materials. Flooding was applied for 48 h when plants were at the three-leaf stage before a number of parameters were assessed. Characteristics that were detected included photosynthetic characteristics of seedlings, contents of proline and Malondialdehyde (MDA), activities of Peroxidase (POD), Catalase (CAT) and Superoxide Dismutase (SOD). Results showed the net photosynthetic rate (PN) of the three tested sesame seedlings all decreased. After a 15 days restore growth under normal field conditions, the PN of WTGs was lower than control with no significant difference. In contrast, the PN of WSG was lower than control significantly. In flooding, both the WTGs and WSG showed the higher proline contents than that of control, however, a significant lower MDA contents were observed in seedlings flooding treated than that of control. The POD activities of the WTGs increased rapidly while of the WSG decreased with flooding stress. The opposite reaction was observed in activities of CAT, of which the WTGs showed a less percentage decreasing than that of the WSG. The SOD activities of three tested materials increased in flooding, with the WTGs increasing more than of the WSG. In general, the WTGs of sesames showed a substantial tolerance to flooding stress by increasing organic osmoregulation content and activities of active oxygen scavenger enzymes. Keywords: Antioxidant enzymes, flooding stress, net photosynthetic rate, physiological parameters, sesame important aims for sesame breeding. Also, highly resistant plants mingled with desired agronomical trails will be the demand of future crops (Hohd et al., 2010). Previously, some authors observed significant genotypic differences among wheat cultivars in response to flooding stress and suggested that traits associated with flooding tolerance are simply inherited. As an important economic crop, sesame has been cultivated in the Jianghan plains of Hubei province over 2,000 years. However, studies on the inheritance of water logging tolerance in sesame are also limited. In this study, a traditional sesame cultivar (WSG-EZhi2) and two newly cultivated sesame lines (WTG-2541 and WTG-2413) were used to study the characteristics of sesame under flooding stress by measuring their photosynthetic characteristics, anti-oxidative enzymatic activities and contents of proline and malnodialdehyde. The study could provide theoretical basis for screening of water logging resistant sesame species, expansion of genetic resources and improvement of sesame yields. INTRODUCTION Poor soil aeration associated with excessive moisture usually influences plants growth in a negative way (Boru et al., 2001). In waterlogged soils carbon dioxide, ethylene, manganese and iron may accumulate in concentrations potentially toxic to plants. However, oxygen deficiency is the most important cause of flooding injury (Kozlowski, 1984). In rainy and humid regions, sesame yield is limited by waterlogged soils resulting from excessive rainfall. In the Jianghan plains of Hubei province in China, water logging commonly damages sesame during seedling establishment. Water logging occurs periodically in irrigated regions due to poor drainage. Inadequate soil aeration affects photosynthesis and activities of metabolizing enzymes. The degree of stress on sesame in water logging soils depends on the crop stage, duration of flooding, soil type, growth conditions and genotype. Genotypic differences in response to flooding have been reported in wheat and pepper and so on. However, studies on the resistance ability of different genotype sesames against water logging are limited. Therefore, selecting water logging resistant germplasms along with expanding genetic resources and increasing genetic diversities is one of the most MATERIALS AND METHODS Materials: Two sesame lines tolerant to water logging (WTG-2541 and WTG-2413) and one sensitive cultivar (WSG-EZhi2) were included in the study. All of the Corresponding Author: Xiao-ling Wang, Department of Agronomy, Yangtze University, Jingzhou 434025, Hubei, P.R. China, Tel.: +86-716-8066899 352 Adv. J. Food Sci. Technol., 4(6): 352-356, 2012 three materials were provided by Oil Crops Research Institute, Chinese Academy of Agricultural Sciences, Wuhan, China. Methods: Experimental design: Three sesame genotypes, including WTG-2541, WTG-2413 and WSG-EZhi2, were used as tested materials. One field experiment was conducted in practice base of Agricultural College, Yangtze University and Jingzhou, China. The soil type is classified as fine clay, medium fertility (8 mg/g organic matter) with a pH of 8.7 in the plow-layer. Each material was planted in a single basin of 3×5 m size in a completely randomized design with three replications. Seeds were planted with 40 cm row spacing and 20 cm plant spacing on 1 June 2011. The basin’s outer ridges were subsequently constructed with mound and waterproof plastic to avoid leakage. Initial irrigation was applied immediately to commence germination and the excess water drained after 45 min. For flooding treatment, one more flooding-irrigation was given at the three-leaf stage when all basins were filled with water to 0.5-1 cm above the soil surface. This level was maintained for 48 h by replenishing the basins frequently. Then the water was drained from the basins. Subsequently, a 15 days restore growth under normal field conditions was conducted. Physiological parameters of seedling samples both before and after the restore growth were assessed. Indicators determination: Seedling samplings were collected after drained 3 h to determine related indicators. Net photosynthetic rate (PN) was determined between 09:00-11:00 h (Beijing time) at the three-leaf stage using a portable photosynthesis system (LI-6400, Li-COR, Inc., Lincoln, NE, USA) with a 3×2 cm (length × width) leaf chamber with air humidity of 49.5±1.8%. The PN was measured at the irradiance of 1,000 μmol m2/s, temperature of 30.5±0.7°C and the natural CO2 concentration. Proline content and enzyme activity of Superoxide Dismutase (SOD) were determined according to Ou et al. (2011). Enzyme activity of Catalase (CAT) was determined according to Hwang et al. (1999) by measuring the rate of decomposition of H2O2 at 240 nm. Peroxidase (POD) activity was measured according to literature (Zhang et al., 2007) by recording the increase in absorbance due to oxidation of guaiacol. Content of Malondiadehyde (MDA) was determined according also to Zhang et al. (2007) to estimate the lipid peroxidation. Statistical analysis: Means and variance were computed in Excell 2003 to compare parameters above mentioned using the ANOVA of SAS Procedure. P values less than 0.05 was considered as statistically significant difference. RESULTS Effect of flooding stress on PN of sesame seedlings: Under flooding stress, the Net Photosynthetic rates (PN) of leaves for all tested sesame seedlings were decreased significantly: WSG-EZhi2 had the biggest decrease (decreased by 98.32%); WTG-2541 had the second smallest decrease and WTG-2413 had the least decrease (decreased by 59.23 and 55.15%, respectively), indicating the two newly cultivated sesame lines are less susceptible to flooding injury than the former. The PN increased gradually three days after restore growth (under normal field conditions). At 15 days after restore growth, PN of the WTG-2541 and WTG-2413 increased greatly to 90.72 and 96.42% compare to controls, respectively. In contrast, PN of WSG-EZhi2 increased only to 71.71% (Table 1). Effect of flooding stress on proline content: There were no significant differences among proline contents of all the tested materials before flooded with the values of 15.10, 17.13 and 16.53 μg·g-1FW (FW: fresh weight) for WTG-2541, WTG-2413 and WSG-EZhi2, respectively. Under flooding stress, proline contents of leaves increased significantly by 72.19, 80.96 and 22.37% for WTG-2541, WTG-2413 and WSG-EZhi2, respectively (Fig. 1). Effect of flooding stress on malondialdehyde content: There were no significant differences among MDA contents of the three tested materials before flooding treatment with the values of 17.21, 18.06 and 16.06 µmol·g-1FW for WTG-2541, WTG-2413 and WSG-EZhi2, respectively. Under flooding stress MDA contents of leaves in three tested materials increased (WTG-2541, WTG 2413 and WSG-EZhi2 increased by 7.74, 2.10 and 28.73%, respectively) (Fig. 2). Effect of flooding stress on antioxidant enzyme activity: SOD activities of WTG-2541 and WTG-2413 were slightly lower than that of WSG-EZhi2 before Table 1: Effect of flooding stress on PN of three sesame seedlings’ leaves (μmol CO2/m2/s) Cultivar (lines) Treatment 48 h after flooding stress 3 days after restore growth 15 days after restore growth WTG-2541 FS 6.330* (59.23%) 12.634* (39.70%) 21.733* (9.28%) Control 15.528 20.953 23.794 WTG-2413 FS 7.260* (55.15%) 12.515* (35.87%) 24.620* (3.58%) Control 16.186 19.515 25.535 WSG-EZhi2 FS 0.260* (98.32%) 10.405* (50.36%) 16.887* (28.29%) Control 15.447 20.961 23.550 WTG: Water logging tolerant genotypes; WSG: Water logging sensitive genotype; FS: Flooding stress; *: Mean significance at 0.05 level compared to control; Values in parentheses are relative percentage of flooding injury 353 Adv. J. Food Sci. Technol., 4(6): 352-356, 2012 Control FS 35 70 Peroxidase activity (U • g-1FW) Proline content (μg•g-1FW) 30 25 20 15 10 5 50 40 30 20 10 0 0 WTG-2541 WTG-2413 WTG-2541 WSG-EZhi2 Fig. 1: The effect of flooding stress on Proline (Pro) contents in leaves of three sesame genotypes The grey columns represent Pro contents of controls and the white columns represent Pro contents of sesames treated by flooding stress, respectively 7 6 Catalase activity (U • g-1FW) Malonialdehyde content (μmol•g-1FW) 20 WSG-EZhi2 Fig. 4: The effect of flooding stress on Peroxidase (POD) activities in leaves of three sesame genotypes The grey columns represent POD activities of controls and the white columns represent POD activities of sesames treated by flooding stress, respectively Control FS 25 WTG-2413 Different genotype sesames Different genotype sesames 15 10 5 0 5 Control FS 4 3 2 1 0 WTG-2541 WTG-2413 WSG-EZhi2 WTG-2541 Different genotype sesames Fig. 2: The effect of flooding stress on Malondialdehyde (MDA) contents in leaves of three sesame genotypes The grey columns represent MDA contents of controls and the white columns represent MDA contents of sesames treated by flooding stress, respectively 100 Superoxidase activity (U • g-1FW) Control FS 60 80 Control FS 60 40 20 0 WTG-2541 WTG-2413 WSG-EZhi2 Different genotype sesames Fig. 3: The effect of flooding stress on Superoxide Dismutase (SOD) activities in leaves of three sesame genotypes The grey columns represent SOD activities of controls and the white columns represent SOD activities of sesames treated by flooding stress, respectively flooding treatment. However, under flooding stress, the SOD activities of WTG-2541 and WTG-2413 increased by 17.04 and 19.99%, respectively. By contrast, the WTG-2413 WSG-EZhi2 Different genotype sesames Fig. 5: The effect of flooding stress on Catalase (CAT) activities in leaves of three sesame genotypes The grey columns represent CAT activities of controls and the white columns represent CAT activities of sesames treated by flooding stress, respectively SOD activity of WSG-EZhi2 only increased by 6.30%, which was smaller significantly than that of the two WTGs (Fig. 3). Effects of flooding stress on different antioxidant enzyme activities were inconsistent. Peroxidase (POD) activity of WTG-2541 was higher significantly (with the value of 55.63 U·g-1FW) than WTG-2413 and WSG-EZhi2 before flooding treatment. Under flooding stress, the POD activities of WTG-2541 and WTG2413 increased respectively by 2.93 and 14.59%, while it decreased by 24.90% for WSG-EZhi2 (Fig. 4). CAT activities of WTG-2541 and WTG-2413 were lower significantly than that of WSG-EZhi2 before flooding treatment. However, under flooding stress, the CAT activities of WTG-2541 and WTG-2413 decreased by 60.90 and 35.28%, respectively. By contrast, the CAT activity of WSG-EZhi2 decreased by 78.78%, which was lower significantly than that of the two WTGs (Fig. 5). This indicated the less effect of flooding stress on WTG-2541 and WTG-2413 than on WSG-EZhi2. 354 Adv. J. Food Sci. Technol., 4(6): 352-356, 2012 DISCUSSION Hypoxia caused by flooding can induce a series of responses in plants (Ou et al., 2011). One of the earliest responses is stomata closure. Flooding can lead to stomata closure in a short period and increase leaf resistance. Stomata closure hinders CO2 absorption and inhibits synthesis of 8-amino-propyl acetate (5-ALA). Reduced CO2-fixation ability of chloroplast and chemical changes of PSП system will lead to significant decrease in leaf net photosynthetic rate (PN) (Beckman et al., 1992). Our results showed that PN of the three sesame cultivar (lines) dramatically decreased under flooding stress. In addition, PN of WTG-2541 and WTG-2413 declined at relatively lower degree compared with that of WSG-EZhi2, indicating that the former have relative stronger resistance to flooding injury. Moreover, the less effect (flooding injury) on PN in WTG-2541 and WTG-2413 than that in WSG-EZhi2 were also been observed when plants grew for 3 days and/or 15 days at normal field conditions. Therefore, the two newly cultivated sesame lines have the better ability to cover from flooding injury than WSG-EZhi2. The adaptation process of crops to adverse environment is often accompanied by changes of some osmolytes (Ou et al., 2011). Studies have shown that under stress, plants are subjected to accumulation of proline in cell (Xing and Cai, 1998). Proline accumulation can enhance resistance of plant to osmotic stress. Therefore, as an important cytoplasmic penetrate and ant dehydrating agent, proline can reduce cell osmotic potential, improve the water-holding capacity of plant tissue and protect enzymes and membrane in vivo (Ma, 1994). This study found that all of the three tested sesames have increased proline content under flooding stress, which proved the improvement of proline under stress condition is one of the self-defense reactions of plants (Wu et al., 1997). Moreover, a greater increase of proline content in WTG-2541 and WTG-2413 was observed than that in WSG-EZhi2. The result showed that the two WTGs had the higher reaction to flooding stress than WSG-EZhi2 cultivar. As mentioned above, some authors thought flooding stress can cause stomata closure, which will reduce CO2 availability in the leaves and inhibit carbon fixation (Crawford, 1978). Thus excessive excitation energy in chloroplasts could increase the generation of Reactive Oxygen Species (ROS) and induce oxidative stress (Gossett et al., 1999). Hence the ROS production in plants will increase under flooding stress (Ahmed et al., 2002). In the current research, the injury of biological lipid by ROS, as indicated by Malondialdehyde (MDA) content was clearly detected in the flooded plants, but with distinct difference between the two genotypes, implying the possible difference in their capacity of defense system against ROS. The more increase of MDA in WSG-EZhi2 than in WTG-2541 and WTG-2413 in our study indicated that the latter lines own stronger resistant to oxidative stress than WSG-EZhi2 cultivar. Since Superoxide Dismutase (SOD) is present in all aerobic organisms and sub cellular compartments that generate activated oxygen, it has been assumed that SOD has a central role in the defense against oxidative stress (Scandalias, 1993; Zhang et al., 2007). It has been generally showed that SOD activity is increased in cells in response to diverse a biotic stresses including salinity, drought, heavy metals and water logging. Meloni et al. (2003) reported that salt-tolerant cotton cultivar exhibited remarkable increase in SOD activity under NaCl stress, whereas sail treatment had no significant impact on SOD activity of salt-sensitive cultivar. By contrast, Hwang et al. (1999) found that water logging led to more increase in SOD activity for water logging-sensitive cultivar of sweet potato than for tolerant one. The results of the present study showed that SOD activity of both the water logging tolerant genotype (WTG-2541 and WTG-2413) and water logging sensitive genotype (WSG-EZhi2) were increased. Similarly, SOD activity of the two WTGs had greatly increase compared with that of WSGEZhi2. Therefore, it may be assumed that an increase in SOD activity under flooding stress could be indicative of an increased production of ROS. However, a dramatic decrease in SOD activity was also found in the plants exposed to successively longer stresses (Ahmed et al., 2002; Wu et al., 2003). Simultaneous increase in activity of H2O2scanvenging enzyme, with accompanying increase in SOD activity, is crucial for effective defense against oxidative stress, or excessive accumulation of H2O2 caused by elevated SOD activity could result in enhanced cyto-toxicity by the even more destructive hydroxyl radical generated from H2O2 in a metalcatalyzed Haber-Weiss reaction (Gossett et al., 1999). Peters et al. (1989) reported that POD activity was higher in stress-tolerant bean than in sensitive one. Meloni et al. (2003) found significant increase in POD activity in salt-tolerant cotton cultivar when the plants were exposed to salinity stress, whereas sensitive cultivar remained unchanged. In the present study, under flooding stress, POD activity increased in WTGs while decreased in WSG. It can be concluded that rapid development of higher POD activity under stress should be a trail of tolerant plant species or genotypes, enabling plants to protect themselves against the oxidative stress (Zhang et al., 2007). However, in our study, decrease dramatically of CAT activity was observed in all of the three tested materials with flooding treatment. The feasible interpretation had been given by some authors (Mittler et al., 2004; Fu et al., 2009): stress-induced decrease of photo-synthesis can lead to reduced demand for NADPH in Calvin cycle 355 Adv. J. Food Sci. Technol., 4(6): 352-356, 2012 and consequently result in over-reduction of photosynthetic electron transport, which eventually leads to the formation of reactive oxygen. When plants accumulate ROS, the contents of their endogenous ROS-scavenging enzymes also decrease. Especially, a more decrease of CAT activity in WSG-EZhi2 than in WTG-2541 and WTG-2413 was observed. It also means that more severe lipid per oxidation in WSGEZhi2, as indicated by higher MDA content may be attributed to its inability in rapid development of higher CAT activity when exposed to flooding stress. CONCLUSION Taking together, our study found that the two WTGs had not only the better ability to recover from flooding injury with the higher net photosynthetic rate and proline contents, but also had the stronger activities of active oxygen scavenger enzymes than WSG-EZhi2. Therefore the new cultivated sesame lines (WTG-2541 and WTG-2413) had stronger resistant ability to flooding stress than the WSG-EZhi2. Hence, introducing and popularizing the two WTGs in the Jianghan plains of Hubei province may improve sesames’ resistant ability to adverse environments and increase crop yields. REFERENCES Ahmed, S., E. Nawata, M. Hosokawa and T. Sakuratain, 2002. Alterations of photosynthesis and some antioxidant enzymatic activities of mungbean subjected to waterlogging. Plant Sci., 163: 117-123. Beckman, T.G., R.L Perry and J.A. Flore, 1992. Shortterm flooding affects gas exchange characteristics of containerized sour cherry trees. Hort. Sci., 27: 1297-1301. Boru, G., M.G. Van, W.E. Kronstad and L. Boersma, 2001. Expression and inheritance of tolerance to water logging stress in wheat. Euphytica, 117: 91-98. Crawford, R.M.M., 1978. Metabolic Adaptation to Anoxia. In: Hook, D.D. and R.M.M. Crawford (Eds.), Plant Life in Anaerobic Environment. Ann Arbor Science Publishers Inc., Michigan, pp: 119-136. Fu, Q.S., H.L. Li, J. Cui, B. Zhao and Y.D. Guo, 2009. Effects of water stress on photosynthesis and associated physiological characters of Capsicum annuum L. Sci. Agric. Sin., 42: 1859-1866. Gossett, D.R., E.P. Millhollon and M.C. Lucas, 1999. Antioxidant response to NaCl stress in salt-tolerant and salt-sensitive cultivars of cotton. Crop Sci., 34: 706-714. Hohd, M.L., I. Shamsu, H. Qaiser, A. Shaheena and A. Aqil, 2010. Physiological and biochemical changes in plants under water logging. Protoplasma, 241(1-4): 3-17. Hwang, S.Y., H.W. Lin, R.H. Chen, H.F. Lo and F. Li, 1999. Reduction susceptibility to water logging together with highlight stress is related to increases in superoxide dismutase and catalase activities in sweet potato. Plant Growth Regul., 27: 167-172. Kozlowski, T.T., 1984. Extent, Causes and Impact of Flooding. In: Kozlowski, T.T. (Ed.), Flooding and Plant Growth. Academic Press, London, pp: 1-5. Ma, Z.R., 1994. Study on free proline accumulation of seedling in many species of plant under water stress. Prat. Sci., 11: 15-18. Meloni, D.A., M.O. Oliva, C.A. Martinez and J. Cambraia, 2003. Photosynthesis and activity of superoxide dismutase, peroxidase and glutathione reductase in cotton under salt stress. Environ. Exp. Bot., 49: 69-76. Mittler, R., S. Vanderauwera, M. Gollery and F. VanBreusegem, 2004. Reactive oxygen gene network of plants. Trends Plant Sci., 9: 490-498. Ou, L.J., X.Z. Dai, Z.Q. Zhang and X.X. Zou, 2011. Responses of pepper to waterlogging stress. Photosynthetica, 49: 339-345. Peters, J.L., F.J. Castillo and R.H. Heath, 1989. Alteration of extracellular enzymes in pinto bean leaves upon exposure to air pollution, ozone and sulfur dioxide. Plant Physiol., 89: 159-164. Scandalias, J.G., 1993. Oxygen stress and superoxide dismutase. Plant Physiol., 101(1): 7-12. Wu, L., Y.D. Li, Z.D. Zhang and R. Hao, 1997. A comparison of physiological and morphological reactions of three types of blueberries to flooding stresses. Acta Hort. Sin., 24: 287-288. Wu, F.B., G.P. Zhang and P. Dominy, 2003. Four barley genotypes respond differently to cadmium: Lipid peroxidation scavenging system. J. Exp. Bot., 9: 1097-1109. Xing, S.C. and Y.H. Cai, 1998. The relationship between stresses and proline. J. Ecol. Agric., 2: 30-33. Zhang, G.P., K. Tanakamaru, J. Abe and S. Morita, 2007. Influence of water logging on some antioxidative enzymatic activities of two barley genotypes differing in anoxia tolerance. Acta Physiol. Plant, 29: 171-176. 356