Lecture notes: Statistical Mechanics of Complex Systems Lecture 7-8
advertisement
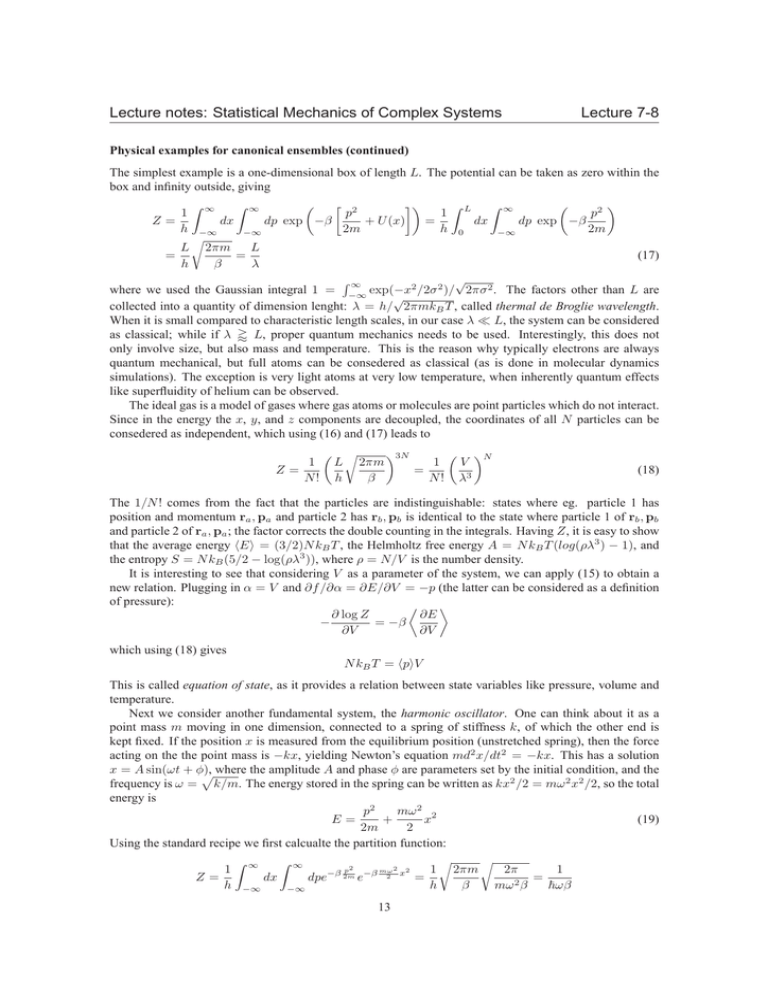
Lecture notes: Statistical Mechanics of Complex Systems Lecture 7-8 Physical examples for canonical ensembles (continued) The simplest example is a one-dimensional box of length L. The potential can be taken as zero within the box and infinity outside, giving 2 Z ∞ Z ∞ Z Z 1 ∞ p2 p 1 L Z= dp exp −β dp exp −β dx dx + U (x) = h −∞ 2m h 0 2m −∞ −∞ r L L 2πm = (17) = h β λ √ R∞ where we used the Gaussian integral 1 = −∞ exp(−x2 /2σ 2 )/ 2πσ 2 . The factors other than L are √ collected into a quantity of dimension lenght: λ = h/ 2πmkB T , called thermal de Broglie wavelength. When it is small compared to characteristic length scales, in our case λ ≪ L, the system can be considered as classical; while if λ ' L, proper quantum mechanics needs to be used. Interestingly, this does not only involve size, but also mass and temperature. This is the reason why typically electrons are always quantum mechanical, but full atoms can be consedered as classical (as is done in molecular dynamics simulations). The exception is very light atoms at very low temperature, when inherently quantum effects like superfluidity of helium can be observed. The ideal gas is a model of gases where gas atoms or molecules are point particles which do not interact. Since in the energy the x, y, and z components are decoupled, the coordinates of all N particles can be consedered as independent, which using (16) and (17) leads to 1 Z= N! r 3N N 1 L 2πm V = h β N ! λ3 (18) The 1/N ! comes from the fact that the particles are indistinguishable: states where eg. particle 1 has position and momentum ra , pa and particle 2 has rb , pb is identical to the state where particle 1 of rb , pb and particle 2 of ra , pa ; the factor corrects the double counting in the integrals. Having Z, it is easy to show that the average energy hEi = (3/2)N kB T , the Helmholtz free energy A = N kB T (log(ρλ3 ) − 1), and the entropy S = N kB (5/2 − log(ρλ3 )), where ρ = N/V is the number density. It is interesting to see that considering V as a parameter of the system, we can apply (15) to obtain a new relation. Plugging in α = V and ∂f /∂α = ∂E/∂V = −p (the latter can be considered as a definition of pressure): ∂ log Z ∂E − = −β ∂V ∂V which using (18) gives N kB T = hpiV This is called equation of state, as it provides a relation between state variables like pressure, volume and temperature. Next we consider another fundamental system, the harmonic oscillator. One can think about it as a point mass m moving in one dimension, connected to a spring of stiffness k, of which the other end is kept fixed. If the position x is measured from the equilibrium position (unstretched spring), then the force acting on the the point mass is −kx, yielding Newton’s equation md2 x/dt2 = −kx. This has a solution x = A sin(ωt + φ), p where the amplitude A and phase φ are parameters set by the initial condition, and the frequency is ω = k/m. The energy stored in the spring can be written as kx2 /2 = mω 2 x2 /2, so the total energy is mω 2 2 p2 + x (19) E= 2m 2 Using the standard recipe we first calcualte the partition function: r r Z ∞ Z 2 2 p2 1 2πm 1 1 ∞ 2π −β 2m −β mω x 2 dpe dx e = = Z= 2 h −∞ h β mω β ~ωβ −∞ 13 Lecture notes: Statistical Mechanics of Complex Systems Lecture 7-8 where we introduced ~ = h/(2π). Then hEi = − ∂ ln Z ∂ 1 =− ln = kB T ∂β ∂β β and CV = ∂hEi = kB ∂T This last result is a realisation of the principle of equipartition: each quadratic half-degree of freedom [like x and p in (19)] contributes kB T /2 to the average energy, and consequently kB /2 to the heat capacity. We will now apply these results to calculate the heat capacity of solids. Far away from the the melting temperature the many-body potential of the atoms in a crystal can be considered quadratic. Collecting all 3N coordinates of the N atoms into a vector x = (x1 , x2 , . . . , x3N ), the potential is U (x) = U0 + 3N 3N X 1 X ∂2U ∂U (xi − x0i ) + (xi − x0i )(xj − x0j ) + . . . ∂x 2 ∂x ∂x i i j i,j=1 i=1 where the series expansion is truncated at the quadratic term. The equation of motion involves the 3N × 3N dynamical matrix ∂ 2 U/∂xi ∂xj , which separates into 3N independent one-dimensional harmonic oscillators corresponding to the normal modes and eigenfrequencies. This leads to C = 3N kB , known as Dulong-Petit law, which turns out to be correct at high temperatures. At low temperatures quantum mechanical effects have to be taken into account, which we do simply by replacing the classical harmonic oscillators with quantum harmonic oscillators. For our purposes the quantum harmonic oscillator is a system with discrete energy levels: in the ith state Ei = (i + 12 )~ω, where i = 0, 1, . . . . Being a discrete system the partition function involves just a sum, which here is a geometric sum: 1 ∞ X 1 e− 2 β~ω −β (i+ 21 )~ω = = e Z= −β~ω 1 − e 2 sinh β~ω i=0 2 The average energy and heat capacity are ~ω ~ω ∂ ln Z = coth ∂β 2 2kB T 2 ∂hEi ~ω 1 C= = kB ∂T 2kB T sinh2 2k~ωT hEi = − B At high temperature (small β) the argument of sinh is small, which expands to sinh x ∼ x. This leads to C → kB , which is the classical result. At low temperature (large β) however, the argument of sinh is large, expanding to sinh x ∼ 21 ex . 2 − ~ω e kB T , resulting in exponential suppression at low temperatures. Naively This gives C ≈ kB k~ω BT applying this result to crystals leads to the Einstein model of solids, which at low temperatures simply gives 2 C = 3N kB 2k~ω . / sinh2 2k~ω BT BT This is still incorrect, however, since all quantum harmonic oscillators are assumed to have the same frequency. In the Debye model of solids the proper spectrum of frequencies is used, which indeed reproduces experimental measurements at low temperatures as well. The Reader is referred to standard solid state physics textbooks for details. The grand canonical ensemble We now allow the exchange of two conserved quantities with the external environment: to follow the physical example of grand canonical ensembles, these are the energy and the particle number. In the maximum entropy formalism this corresponds to constraining the average energy and the average particle number. As before the units of entropy is kB , and the ith state has energy Ei and particle number Ni . The Lagrange multipliier conjugate to energy is β = 1/(kB T ) as in the canonical ensemble. The other one, however, is conventionally denoted by −µβ = − kBµT . 14 Lecture notes: Statistical Mechanics of Complex Systems Lecture 7-8 Accordingly the grand canonical partition function (denoted by Ξ) and the probabilities of the states are X Ξ(β, µ) = e−β(Ei −µNi ) i 1 − 1 (E −µNi ) pi = e k B T i , Ξ while the entropy, now function of the average energy and average particle number, using (10) becomes hEi µhN i − . SGC hEi, hN i = kB ln Ξ + T T The simple relations (8) and (11) become more complicated due to the fact that the physical variables, especially µ, are not simply the Lagrange multipliers but functions of them: ∂ ln Ξ ∂ ln Ξ ∂ ln Ξ hEi = − =− + µkB T ∂β −µ·β ∂β µ ∂µ β ∂ ln Ξ ∂ ln Ξ hN i = − = kB T ∂ − µβ β ∂µ β ∂SGC 1 = T ∂hEi hN i µ ∂SGC − = T ∂hN i hEi In the grand canonical ensemble not only the energy fluctuates, but also the particle number: ∂hN i ∂ 2 ln Ξ 2 = k T σN = Var(N ) = B ∂(−µβ)2 β ∂µ β The reciprocity relations also become more complicated, for example ∂hEi ∂hN i = ∂ − µβ β ∂β −µ·β becomes ∂hN i ∂hN i ∂hEi = − µkB T −kB T ∂µ β ∂β µ ∂µ β An important quantity is the grand free energy (we will see soon the relevance of the free energies), which is defined as Φ(T, µ) := −kB T ln Ξ = hEi − µhN i − T SGC It is interesting to note that the partition function can be written as e−βΦ = Ξ = X e−βEi eβµNi = N =0 i = X N −β A(T ;N )−µN e ∞ X eβµN X e−βEj,N j In this expression microscopic states with the same particle number N are lumped together into a macroscopic state, and the sum of their Boltzmann factors is replaced by a single Boltzmann factor where the role of the energy is played by an appropriate free energy. This manipulation is called partial trace, a terminology borrowed from the quantum formalism of statistical mechanics. 15







