Rasilez
advertisement

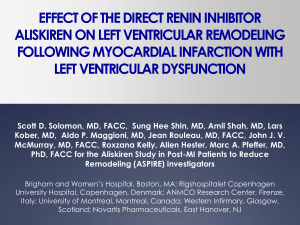
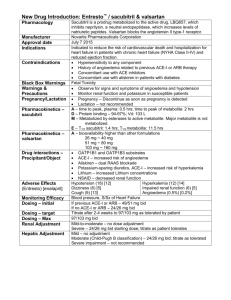
Rasilez® Active ingredient: Aliskiren Marketing authorisation holder: Novartis Europharm Ltd Availability: National Health Service and Private Sector Variations: Aliskiren is contraindicated in hereditary or idiopathic angioedema and in patients with a history of angioedema with aliskiren. Treatment with aliskiren should be used with caution in these patients and they should be monitored particularly upon initiation of treatment. Treatment should be discontinued in cases of angioedema, whilst providing adequate treatment together and monitoring till the situation is resolved. When the tongue, glottis or larynx are affected, adrenaline should be delivered in combination with other steps to maintain an open airway. Angioedema is listed as a rare adverse effect during treatment of aliskiren. This frequency is comparable to the treatment with placebo or hydrochlorothiazide in controlled clinical trials. In post-marketing surveillance, such cases were reported with most of them having a history of angioedema or symptoms suggestive of angioedema which in certain cases was related to administration of other medicines likely to cause angioedema such as Renin-Angiotensin System blockers. The SmPC and package leaflet were updated to include a blood creatinine increase as a rare adverse effect. These cases were reported in post-marketing surveillance. A study was carried out to evaluate the potential interaction between the active ingredient, aliskiren and grapefruit juice in healthy persons. Data showed a decrease in Area Under Curve (AUC) (61% in case of 150mg aliskiren, 38% in case of 300mg aliskiren) and Cmax of aliskiren. This may be due to a suppression of organic anion transporting polypeptide-mediated uptake of aliskiren by grapefruit juice in the digestive tract. Patients shall be advised not to consume grapefruit juice concomitantly with aliskiren due to a risk of therapeutic failure. From a drug-drug interaction study, it was decided to contraindicate the use of aliskiren and the highly-potent P-gp inhibitor ciclosporin and even moderate P-gp inhibitors, verapamil and quinidine. Consequently further pre-clinical data had to be submitted by MAH to evaluate the potential mechanism of interaction between P-gp inhibitors and aliskiren. The MAH applied to update contraindications section by eliminating verapamil from contraindications. Further updates were carried out in sections 4.4 and 4.5 of SmPC. The bioavailability of verapamil may be minimally decreased by aliskiren. When aliskiren 300mg is coadministered with moderate P-gp inhibitor, verapamil 240mg resulted in 97% increase in aliskiren AUC. Hence it should be used with caution. In their presence, the plasma levels of aliskiren achieved will be in the range as if its dose was doubled. Doses up to 600mg or double the highest recommended therapeutic dose of aliskiren were well tolerated in controlled clinical trials. Reference: European Medicines Agency. Rasilez. [online]. 2011 June 1 [cited 2011 July 30]; Available from: URL: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/000780/human_med_001013.jsp&murl=menus/medicine s/medicines.jsp&mid=WC0b01ac058001d124