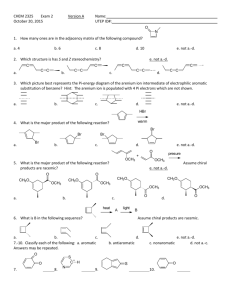
International Journal of Application or Innovation in Engineering & Management... Web Site: www.ijaiem.org Email: , Volume 3, Issue 1, January 2014
advertisement

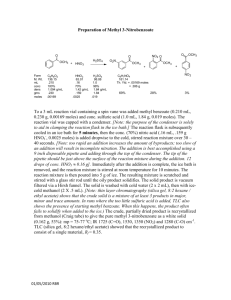
International Journal of Application or Innovation in Engineering & Management (IJAIEM) Web Site: www.ijaiem.org Email: editor@ijaiem.org, editorijaiem@gmail.com Volume 3, Issue 1, January 2014 ISSN 2319 - 4847 Synthesis, antimicrobial and antifungal study of 2,4-substituted-1,5-substitutedbenzothiazepine Swati.V. Gaikwad* ,DrA.B.Bhakea, DrS. E. Bhandarkarb *research student,Department of Applied Chemistry, DBACER ,Nagpur–11 a AssociateProfessor,Department of Applied Chemistry,YeshwantraoChavan C.O.E., Nagpur–33 b Associate Professor, Department of Chemistry, G.V.I.S.H., Amravati ABSTRACT Synthesis of 2,4-substituted-1,5-substituted- benzothiazepine was first carried out by the acetylation of substituted α – napthol in presence of glacial acetic acid and zinc chloride. The 1-(1-hydroxy-naphthalene-2-yl)ethanone obtained is then heated with aromatic aldehyde using ethanol as a solvent and 40% KOH which on condensation gave 1-(substituted-1-hydroxynaphthalen -2yl)-3-aryl-prop-2-en-1-one.This compound on microwave irradiation in presence of substituted aminobenzothiol and zinc acetate gave 2,4-substituted-1,5-substituted- benzothiazepine .The synthesized compounds are characterized by elemental analysis, 1H NMR, IR Spectroscopy. Newly synthesized compound shows an excellent antimicrobial and antifungal activities. Keywords: Synthesis, benzothiazepine,elementalanalysis,antimicrobial INTRODUCTION Benzothiazepines are seven membered heterocyclic compounds.Benzothiazepines have received a considerable attention nowadays because of its versatile biological activities like anticonvulsant [1],CNS depressant [2,3,4] ,anticancer[5],antifungal[6] and antimicrobial activities[7,8].As disease poses a major threat to human beings and scientists are fighting to find solutions in the form of various medications, benzothiazepine finds its importance in bioinorganic and medicinal chemistry in designing new therapeutic agents with improved pharmacological properties.[9,10].Present work deals with the synthesis of 2,4-substituted-1,5-substituted- benzothiazepine and their characterization by spectral analysis ( IR, 1H NMR ). EXPERIMENTAL All the melting points were taken in silicon oil bath with open capillary tubes and are uncorrected. IR spectra were recorded on a Nicolet-Impact 400 FT-IR spectrometer 1 H NMR spectra were recorded on a Brucker AC300 FNMR spectrometer(300 MHz), using TMS as an internal standard. Microanalysis of nitrogen was obtained by Kjeldahal’s Method. Thin Layer Chromatography on silica gel-G, was used to check the purity of the compounds. Procedure for the synthesis of 2-acetyl-substituted-1-naphthol: In hot glacial acetic acid, fused ZnCl2 was added and refluxed till dissolved, then powdered substituted 1-naphthol was added and the mixture was refluxed for about 8 hours then cooled & poured in acidulated water. The solid obtained was filtered, washed, dried and recrystallized from rectified spirit to obtain compound (2a). Synthesis of 1-(substituted-1-hydroxynaphthalen -2-yl)-3-aryl-prop-2-en-1-one (3a): 2-acetyl-substituted-1-naphthol and aromatic aldehydes were added in ethanol solvent. To this mixture KOH (10%) solution was added dropwise with constant stirring. The reaction mixture was kept overnight. Then the mixture was poured over crushed ice & little HCl. The product was filtered and recrystallized from ethanol to obtain the compounds (3a). Synthesis of 2,4-substituted-1,5-substituted- benzothiazepine (1-25): 1-(substituted-1-hydroxynaphthalen -2-yl)-3-aryl-prop-2-en-1-one and substituted 2-aminobenzenethiol was heated in presence of catalyst zinc acetate to obtain a compound 2,4-substituted-1,5-substituted- benzothiazepine.The physical data of newly synthesized compounds is given in Table.I Table I - Physical and analytical characterization data of newly synthesized compounds Volume 3, Issue 1, January 2014 Page 478 International Journal of Application or Innovation in Engineering & Management (IJAIEM) Web Site: www.ijaiem.org Email: editor@ijaiem.org, editorijaiem@gmail.com Volume 3, Issue 1, January 2014 ISSN 2319 - 4847 R R2 Melt ing Poin 0 t C Compound R1 % Nitrogen % Yield Found Calculated R.F. Value 2a -- -- -- 75 85 - - 3a -- C6H5 H 85 75 - - 1 H C6H5 H 76 68 4.66 4.78 0.65 2 H OCH3 H 73 64 5.45 5.67 0.64 71 69 4.52 4.74 0.56 80 50 5.38 5.57 0.64 91 72 4.25 4.52 0.59 88 55 5.25 5.30 0.56 65 56 4.35 4.49 0.57 72 61 5.89 6.01 0.69 90 80 4.26 4.32 0.58 88 65 4.99 5.04 0.56 92 67 4.19 4.29 0.54 70 85 4.88 4.96 0.53 69 75 4.26 4.32 0.58 OCH3 88 80 4.85 5.04 0.62 R2 Melt ing Poin 0 t C % Yield 3 4 5 6 7 8 9 10 11 12 13 14 H H Br Cl H H OH C6H5 H OH OCH3 H OH OH Br Cl H H OCH3 C6H5 H OCH3 OCH3 H OCH3 Br H OCH3 Cl H H C6H5 OCH3 H OCH3 Compound R1 R % Nitrogen Found Calculated R.F. Value 15 H Br OCH3 95 55 4.20 4.29 0.63 16 H Cl OCH3 71 69 4.72 4.96 0.52 18 OH C6H5 OCH3 80 50 4.08 4.12 0.65 19 OH OCH3 OCH3 91 72 5.00 5.04 0.56 20 OH Br OCH3 88 55 4.02 4.09 0.54 21 OH Cl OCH3 65 56 4.65 4.69 0.53 22 OCH3 C6H5 OCH3 72 61 3.86 3.95 0.64 Volume 3, Issue 1, January 2014 Page 479 International Journal of Application or Innovation in Engineering & Management (IJAIEM) Web Site: www.ijaiem.org Email: editor@ijaiem.org, editorijaiem@gmail.com Volume 3, Issue 1, January 2014 ISSN 2319 - 4847 23 OCH3 24 OCH3 25 OCH3 OCH3 Br OCH3 OCH3 OCH3 Cl 90 80 70 85 69 75 4.75 4.79 0.56 3.90 3.93 0.58 4.45 4.48 0.55 SPECTRAL INTERPRETATION IR (KBr) : 1650 (C=O), 3350 (-OH) , 3131(-NH) NMR (CDCl3 + DMSO-d6) : δ 2.35 (s,3H,CH3), δ 6.88- 7.11 (m, 11H, Ar-H), δ 9.83 (s,1H,-OH) , δ 3.86(s,3H, OCH3) SCHEME OH OH O + HO O ZnCl2 CH3 CH3 (2a) R1 R1 naphthalen-1-ol acetic acid O 10%KOH R2 R1 R R SH OH S HO NH2 H O R N H R2 R1 (3a) (1-25) R1 = H, OH, OCH3; R = aryl, CH3, Br,Cl; R2 = H, OCH3 ANTIMICROBIAL STUDIES All abovebenzothiazepine derivatives have been studied for their antimicrobial activity against Escherichia coli, Proteus mirabilis, Staphylococcus aureas, Pseudomonas aeruginosa,. The culture of each species was incubated at 370C and the zone of inhibition was measured after 24 hr. Most of these compounds were found active. Volume 3, Issue 1, January 2014 Page 480 International Journal of Application or Innovation in Engineering & Management (IJAIEM) Web Site: www.ijaiem.org Email: editor@ijaiem.org, editorijaiem@gmail.com Volume 3, Issue 1, January 2014 ISSN 2319 - 4847 ACKNOWLEDGEMENT The authors are thankful to Principal, YeshwantraoChavan C.O.E., Nagpur–33 for providing necessary laboratory facilities. REFERENCES 1. Sarro J G De, Chimirri A, Sarro A De ,5H‐[1,2,4]Oxadiazolo[5,4‐d][1,5]benzothiazepins as anticonvulsant agents in DBA/2 A. R. Trivedi, D. K. Dodiya, N. R. Ravat, V. H. Shah, Arkivoc XI (2008) 131 2. Basawaraj R., Naubade K., Sangapure S.S, Synthesis of some Benzodiazepins and benzothiazepins bearing benzofuran moiety as a possible CNS depressants. Ind.J.Hetr.Chem,17,2008;217‐220 3. Nikalje AG, Pattan SR , Synthesis of 1,5‐benzothiazepins as a potential CNS and CVS agents.Int.J.Chem.Sci,2007;5(1),290‐296 4. MasafumiHagiwara,Satomi Adachi‐Akahane and TakuNagao,High‐Affinity Binding of DTZ323, a novel derivative of Diltiazem. Ind. J.Pharmacology, 1997; 281( 1) :173‐179 5. Sharma KA., Singh G., Yadav AK. , Prakash L. Improved method for synthesis of new 1,5‐benzothiazepins derivative as analogue of anticancer drugs. Molecules, 1997; 2(2):130‐135 6. DandiaAnshu, Singha R. ,Khaturia S. Efficient microwave enhanced solvent free synthesis of potent antifungal agents:Fluorinatedbenzothiazepin fused β‐lactom derivative. Cheminform,2007;38(36):236‐239 7. Giuliano G., Luana P., Valeria A. Synthesis of new 1,4‐benzothiazepins tricyclic derivative with structural analogue with TIBO and their screening for anti‐HIV activity. Eur. Journal of Medicinal Chemistry,1999; 34, (9) , 701‐709 8. Nikalje AG, Mane RA, Ingle DB , Synthesis of new 1,5‐benzothiazepins as potent antimicrobial agents,Ind. J.Het.Chem,2006;15(3),309‐310 9. K. L. Ameta, Nitu S. Rathore and Bireshkumar , Synthesis and in vitro anti-breast cancer activity of some novel 1,5-benzothiazepine derivatives, J. Serb. Chem. Soc. 77 (6) 725–731 (2012) 10. DeepikaVyawahare, MangeshGhodke and Anna PratimaNikalje, green synthesis and pharmacological screening of novel 1,5benzothiazepines as CNS agents. International Journal of Pharmacy and Pharmaceutical Sciences Vol 2, Issue 2, 2010 Volume 3, Issue 1, January 2014 Page 481