( ) The Curtin-Hammett Principle e
advertisement

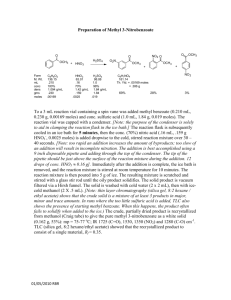
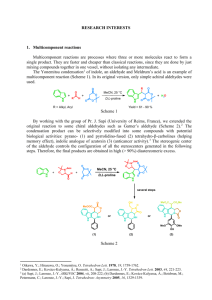
The Curtin-Hammett Principle ∂[B2] ∂t GTS2 - GTS1 ∂[B1] ( =e −(GTS2 - GTS1) RT ) ∂t ΔG1‡ ΔG2‡ ΔG21O B1 k1 A1 A2 k2 B2 So, relative reaction rates depend only on relative transition-state energies, and not on starting-material ground-state energies. The Curtin-Hammett Principle Example: Dynamic Kinetic Resolution Question: O How can enantioselective chemistry convert a racemic mixture into exclusively one diastereomer? O O OCH3 O + OCH3 (R) (S) H2 enantioselective reduction of ketone (S) to (S,S) makes sense— just incorporate new chiral center by enantioselective reduction. But how to convert (R) to (S,S)? H OH O OCH3 (S,S) Ryoji Noyori, Nagoya University (Nobel Prize, 2001) The Curtin-Hammett Principle Example: Dynamic Kinetic Resolution O O (S)-BINAP-RuBr2 OCH3 H OH O OCH3 H2 (S) (S,S) Ph (R) is converted to (R,S) much more slowly than (S) to (S,S) with this catalyst. Diastereospecific yield of reaction on racemic mixture could not exceed 50%. Ph P Ru P Ph (S)-BINAP-RuBr2 Br Br Ph (S)-BINAP BINAP ligand creates two sterically hindered quadrants. Tokunaga, M.; Kitamura, M.; Noyori, R. J. Am. Chem. Soc. 1993, 115, 144-152. Noyori, R.; et al. J. Am. Chem. Soc. 1989, 111, 9134-9135. The Curtin-Hammett Principle Example: Dynamic Kinetic Resolution O O (S)-BINAP-RuBr2 OCH3 H OH O OCH3 H2 (S) (R) is converted to (R,S) much more slowly than (S) to (S,S) with this catalyst. (S,S) ‡ lower-energy (rate-determining) transition state (S)-BINAP + ‡ steric congestion (S)-ketoester (S)-BINAP + higher-energy (rate-determining) transition state (R)-ketoester The Curtin-Hammett Principle Example: Dynamic Kinetic Resolution O O (S)-BINAP-RuBr2 OCH3 OH O OCH3 H2 (S) (S,S) + O O ? OCH3 (R) So, how to convert (R)-ketoester to (S,S)-β-hydroxyester? The Curtin-Hammett Principle Example: Dynamic Kinetic Resolution O O (S)-BINAP-RuBr2 OCH3 O OH OCH3 H2 (S) O O (S,S) + OCH3 achiral ester enolate O O (S)-BINAP-RuBr2 OCH3 (R) Equilibrate two enantiomeric starting materials through achiral enolate, by tuning Lewis acidity in solvent. H2 OH O OCH3 (R,S) Curtin-Hammett ensures that all racemate goes to (S,S), as long as equilibration is faster than catalysis. The Curtin-Hammett Principle Example: Dynamic Kinetic Resolution (S)-BINAP-Ru (S)-ketoester (S)-BINAP-Ru (R)-ketoester ‡ O ‡ O OCH3 OH O O O (S) OH O OCH3 OCH3 OCH3 (S,S) O O (R) OCH3 (R,S) (not observed) Kinetic vs. Thermodynamic Control increase frequency of going over reaction barriers (e.g., by lowering TS energy w/ catalyst, or by increasing T) E B1 A1 A2 B2 Under kinetic control, make product with lowest-energy transition state B1 A1 A2 B2 Under thermodynamic control, make product with lowest ground-state energy Kinetic vs. Thermodynamic Control Br more δ+ on 2o carbon + -80 °C δ+ HBr δ− Br δ+ δ+ δ− δ+ + 40 °C Br kinetic product δ+ Br thermodynamic product δ+ Br Br Principle of Microscopic Reversibility The lowest-energy pathway from reactant to product must also be the lowest-energy pathway from product to reactant. Pathway I Pathway II A→P and P→A choose this path. If I were hiking, I’d take Pathway I from A → P, and Pathway II from P → A. But molecules aren’t hikers. They’d always take Pathway II. Principle of Microscopic Reversibility An interesting corollary: Catalysts activate both forward and reverse reactions. O + H2O N H subtilisin, trypsin, papain subtilisin, trypsin, papain O OH Enzymes that catalyze protein cleavage… + H2N …also catalyze protein synthesis! So, how does our body selectively degrade the proteins it wants to eat, and synthesize the proteins it wants to use? Principle of Microscopic Reversibility Answer: Isolate different reactions in different places. In the gut… Inside cells… O O + H2O N H N H subtilisin, trypsin, papain O + H2O + ADP + Pi ribosome O OH + H2N …proteolysis is driven by water. OH + H2N + ATP …protein synthesis is driven by energy (ATP). Enzymes work both ways, but thermodynamics drives reactions in desired directions.