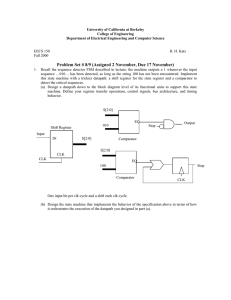
N CO CN
advertisement
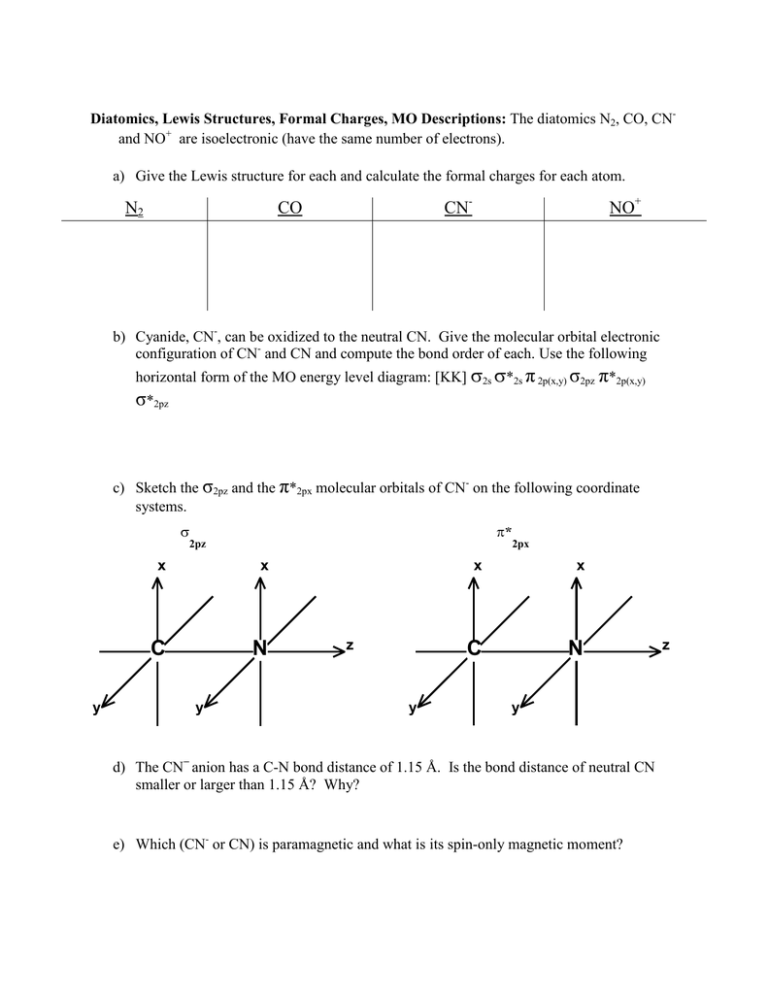
Diatomics, Lewis Structures, Formal Charges, MO Descriptions: The diatomics N2, CO, CNand NO+ are isoelectronic (have the same number of electrons). a) Give the Lewis structure for each and calculate the formal charges for each atom. N2 CN- CO NO+ b) Cyanide, CN-, can be oxidized to the neutral CN. Give the molecular orbital electronic configuration of CN- and CN and compute the bond order of each. Use the following horizontal form of the MO energy level diagram: [KK] σ2s σ*2s π 2p(x,y) σ2pz π*2p(x,y) σ*2pz c) Sketch the σ2pz and the π*2px molecular orbitals of CN- on the following coordinate systems. σ y π* 2pz x x C N y z y 2πx x x C N y d) The CN− anion has a C-N bond distance of 1.15 Å. Is the bond distance of neutral CN smaller or larger than 1.15 Å? Why? e) Which (CN- or CN) is paramagnetic and what is its spin-only magnetic moment? z CHEMISTRY 362-Worksheet 1. Give the energy level diagrams and the electronic configurations (KK σ2s2 …) of C2 and B2. What is the bond order and what is the ground state term symbol for each? 2. Give the electronic configuration (KK σ2s2 …) of O2, O2+, O2-, and O2=. What is the bond order and what is the ground state term symbol for each?