It is REALLY IMPORTANT that to learn how to draw... other way, like the pictures here. These ChemDraw's were...
advertisement
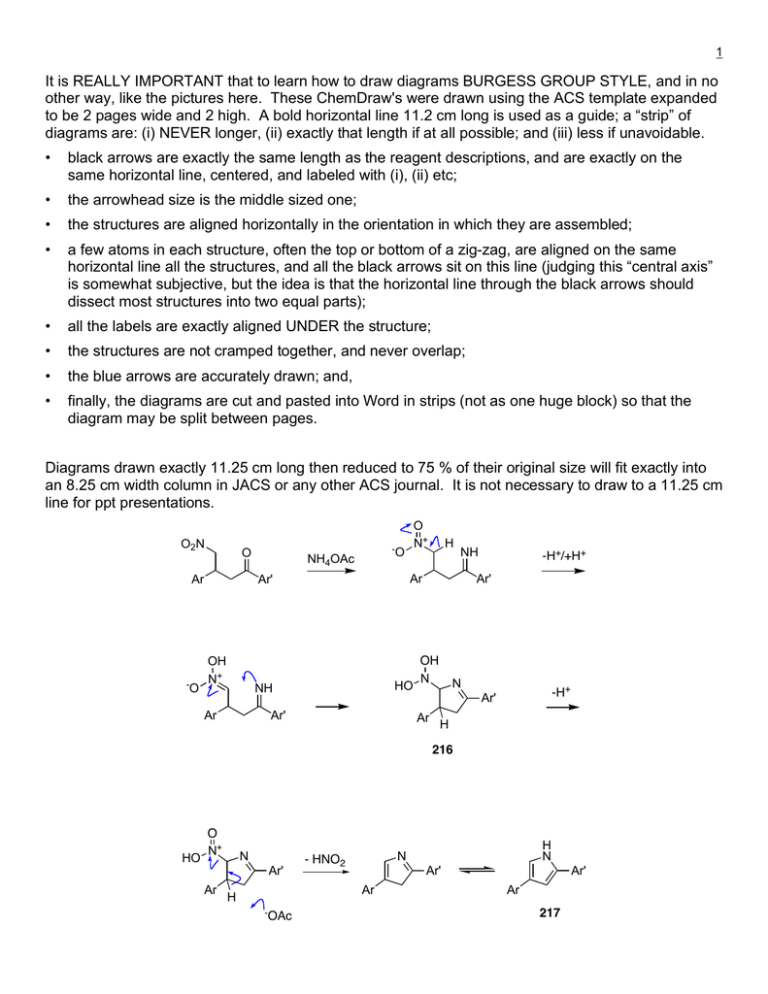
1 It is REALLY IMPORTANT that to learn how to draw diagrams BURGESS GROUP STYLE, and in no other way, like the pictures here. These ChemDraw's were drawn using the ACS template expanded to be 2 pages wide and 2 high. A bold horizontal line 11.2 cm long is used as a guide; a “strip” of diagrams are: (i) NEVER longer, (ii) exactly that length if at all possible; and (iii) less if unavoidable. • black arrows are exactly the same length as the reagent descriptions, and are exactly on the same horizontal line, centered, and labeled with (i), (ii) etc; • the arrowhead size is the middle sized one; • the structures are aligned horizontally in the orientation in which they are assembled; • a few atoms in each structure, often the top or bottom of a zig-zag, are aligned on the same horizontal line all the structures, and all the black arrows sit on this line (judging this “central axis” is somewhat subjective, but the idea is that the horizontal line through the black arrows should dissect most structures into two equal parts); • all the labels are exactly aligned UNDER the structure; • the structures are not cramped together, and never overlap; • the blue arrows are accurately drawn; and, • finally, the diagrams are cut and pasted into Word in strips (not as one huge block) so that the diagram may be split between pages. Diagrams drawn exactly 11.25 cm long then reduced to 75 % of their original size will fit exactly into an 8.25 cm width column in JACS or any other ACS journal. It is not necessary to draw to a 11.25 cm line for ppt presentations. O2N O Ar -O -O NH4OAc HO NH Ar H NH Ar Ar' OH N+ O N+ Ar' -H+/+H+ Ar' OH N N -H+ Ar' Ar H 216 HO O N+ N Ar' Ar Ar' Ar H -OAc H N N - HNO2 Ar' Ar 217 2 HO OH N N Ar' Ar H2+O + H+ -OAc O N+ H N -H+ Ar' - H2O Ar H 216 O N+ O N -H+ N H N Ar' Ar' Ar Ar 218 Ar Ar N O NH HOAc Ar + HN NH Ar' Ar' 218 -OAc OH H Ar N Ar' N Ar' 217 Ar Ar N NH Ar' N Ar' The above diagram is a mechanistic one. For reactions, draw all reagents and catalysts first, then solvent, temperature, time. Do not use abbreviations like DCM or TES or TEA. 3 OTBDPS MeO OH 6 50 bar H2 0.01 Ir* CH2Cl2 25 oC, 12 h 1.0 K2CO3 >99% conv. OTBDPS OTBDPS OH MeO + O 7 Ir* = 7:8 8 1 2 3 99:1.0 6.7:1.0 1.3:1.0