Collaboration Request Form
advertisement
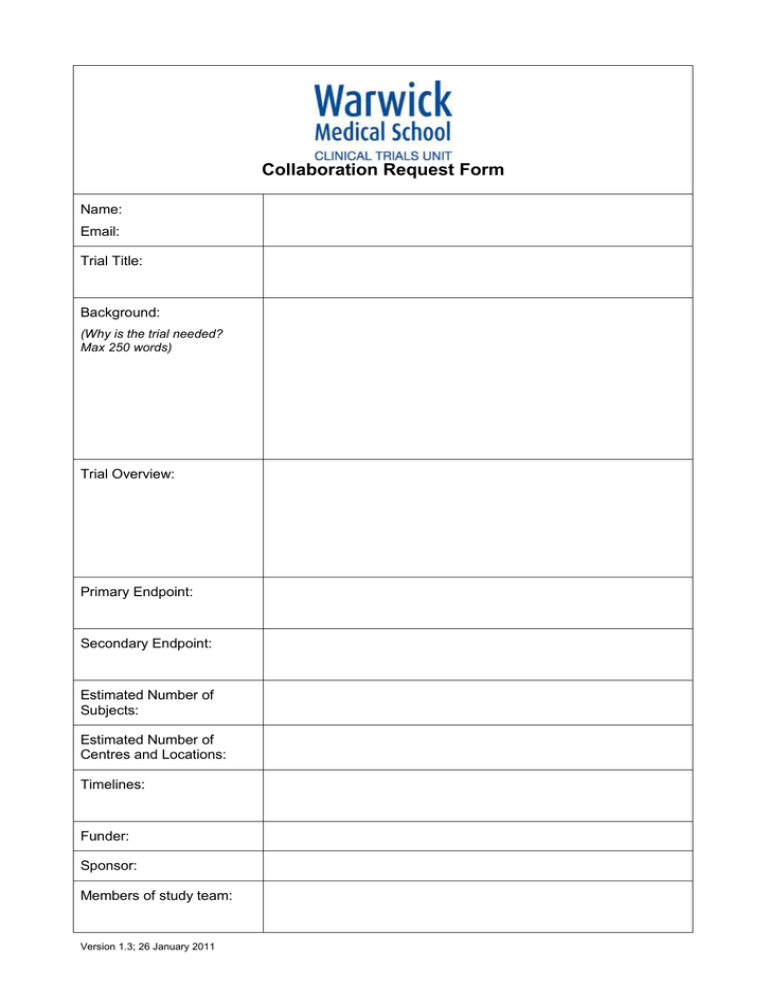
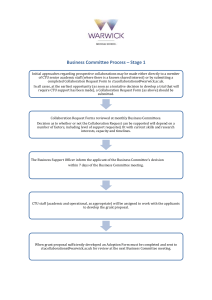
Collaboration Request Form Name: Email: Trial Title: Background: (Why is the trial needed? Max 250 words) Trial Overview: Primary Endpoint: Secondary Endpoint: Estimated Number of Subjects: Estimated Number of Centres and Locations: Timelines: Funder: Sponsor: Members of study team: Version 1.3; 26 January 2011 Collaboration requested: Randomisation Training (please specify) Statistics Governance advice Programming Senior Project Manager Support Full in-house management Other (please detail including information on any preliminary conversations that have taken place with any member of CTU staff): Charges are made for all services. We will provide a cost estimate that must be included in any grant applications. Randomisation service: You will need to provide a clear specification of the type of randomisation required. This should always be specified in consultation with a statistician. Randomisation services covers the cost of programming associated with randomisation, supervision and quality control of randomisation procedures, administration of the randomisation service, reports of randomisation status, provision of final random allocation folder and a code security service. At the current time the service operates from 9.00am to 5.00pm, and is administered by telephone. We will provide faxed confirmation of random allocation. The service does not include out of hours randomisation or cover clinical queries, nor enquiries relating to emergency code breaking. If additional services are required to specify the type of randomisation that is required, please indicate these on your request. A cost of £500 for set up and £10 per patient should be included in the grant application. Programming service: Programming includes the costs of initial design of the study databases, construction of the databases, training in the use of the database to trial co-ordinator (and other trial employees as agreed), maintenance and electronic data storage and trouble shooting. A cost of 0.33 FTE per year should be included in the grant application. Statistics: Subject to capacity, we can offer a complete statistical service for clinical trials. A cost of 0.33 FTE per year should be included in the grant application. Access to Services: Warwick Clinical Trials Unit provides a high quality and up to date information for clinical trials through its web pages and Standard Operating Procedures (http://www2.warwick.ac.uk/fac/med/research/ctu/process/). Online training assessments are available for some topics. All individuals conducting trials within Warwick Medical School must comply with the SOPs. If you require additional training or advice to ensure compliance with the SOPs this can be made available to you. All Chief Investigators, Trial Co-ordinators and Data Entry Clerks will be expected to have attended a compulsory Good Clinical Practice course once every 3 years or if major changes occur. All Warwick based Chief Investigators will have to have completed the Clinical Trials Chief Investigators Training course in order to be given approval to Version 1.3; 26 January 2011 run a clinical trial. The University will not sponsor or co-sponsor the trial unless this has been completed satisfactorily. A cost for 5% per year of the Quality Assurance Manager’s time should be included in the grant application to cover this service. Version 1.3; 26 January 2011