Collaboration Request Form
advertisement

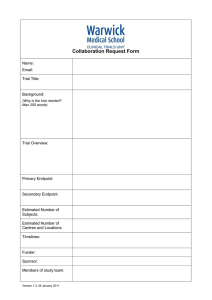
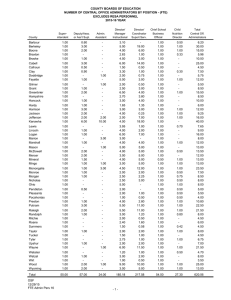
Collaboration Request Form Name: Email: Trial Title: Background: (Why is the trial needed? Max 250 words) Trial Overview: Primary Endpoint: Secondary Endpoint: Estimated Number of Subjects: Estimated Number of Centres and Locations: Timelines - Submission Date: Length of Study: Funder: Sponsor: Members of study team: Version 2.0; Date 12.10.15 Collaboration Requested: Randomisation Statistics Database/Programming Health Economics Training specify) Governance advice Senior Project Manager Support Full in-house Management (please Other (please detail including information on any preliminary conversations that have taken place with any member of CTU staff): Charges are made for all services. We will provide a cost estimate that must be included in any grant applications. Version 2.0; Date 12.10.15 Collaboration Request Form - Explanatory Notes Randomisation service: You will need to provide a clear specification of the type of randomisation required. This should always be specified in consultation with a statistician. Randomisation services covers the cost of programming associated with randomisation, supervision and quality control of randomisation procedures, administration of the randomisation service, reports of randomisation status, provision of final random allocation folder and a code security service. At the current time the service operates from 9.00am to 5.00pm, and is administered by telephone. We will provide email or faxed confirmation of random allocation. The service does not include out of hours randomisation or cover clinical queries, nor enquiries relating to emergency code breaking. If additional services are required to specify the type of randomisation that is required, please indicate these on your request. A minimum cost of £500 for set up and £10 per patient should be included in the grant application. Statistics: Subject to capacity, we can offer a complete statistical service for clinical trials. Typically a minimum cost of 0.05 FTE of a Senior Statistician and 0.33 FTE of a Study Statistician per year should be included in the grant application. Database/Programming: Programming services include a consultation process to gather and document system requirements, database and application development for data collection and trial management, randomisation software, reporting services, secure datacentre hosting, user acceptance testing, trial coordinator/system owner training (and other trial employees as agreed), continued support throughout the lifespan of the project. A typical cost of 0.33 FTE of a Programmer per year should be included in the grant application but this will depend on the needs of the trial and early discussions are advised. Health Economics: We are able to offer economic evaluation of large RCTs to assess costs of new interventions compared to existing alternatives. Typically costs of 0.05 FTE of a Senior Health Economist and 0.33 FTE of a Study Health Economist per year should be included in the grant application. Training/Governance: Warwick Clinical Trials Unit provides a high quality and up to date information for clinical trials through its web pages and Standard Operating Procedures (http://www2.warwick.ac.uk/fac/med/research/ctu/process/). Online training assessments are available for some topics. All individuals conducting trials within Warwick Medical School must comply with the SOPs. If you require additional training or advice to ensure compliance with the SOPs this can be made available to you. All staff involved in research projects will be expected to have attended a compulsory Good Clinical Practice course and refresher courses as appropriate. All Warwick based Chief Investigators will have to have completed the Clinical Trials Chief Investigators Training course in order to be given approval to run a clinical trial. The University will not sponsor or co-sponsor the trial unless this has been completed satisfactorily. A minimum cost of 10% / 0.1 FTE per year (depending on the needs of the project) of the Quality Assurance Manager’s time should be included in the grant application. Senior Project Manager Support: Subject to capacity we can provide support and advice from our SPM team. These senior staff have considerable experience in the planning and development of new trials to ensure that they are appropriately resourced and have clear operational feasibility. A cost of 15% / 0.15 FTE per year should be included. Full in-house Management: All of the above as required, plus a typical cost of 5% / 0.05 FTE of the Clinical Trials Unit Manager’s time to be included in the grant application to cover this service. All of the above should only be used as a guide. CTUcollaborations@warwick.ac.uk Version 2.0; Date 12.10.15 For further information please email: