Resonant vibrations in HEAT repeats: the tune for the mitotic dance? UK
advertisement

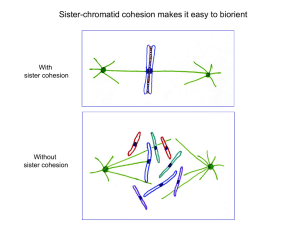
Resonant vibrations in HEAT repeats: the tune for the mitotic dance? Nigel Dyer UK Professor Herbert Frohlich • My normal starting point is Professor Herbert Frohlich, who proposed in the early seventies that there may be a way in which cellular components (proteins/lipid chains?) may interact with each other through a resonant vibrational mechanism. • He suggested that could provide a way in which large numbers of such components may oscillate in a coherent way, and this could provide a basis for an attractive force between such components. • He also suggested that Quantum Mechanics may also provide a basis for such assemblies to act in some respects as a single entity. This proposal echos some of Frohlichs early work on superconductivity, • Evidence for this has been elusive, but neither has it been possible to prove that this could not occur Consider a single polarised macromolecule, e.g. a protein • Frohlich showed that in the right conditions there would be a preferential excitement of large amplitude low frequency modes. • Later papers by others come to the same conclusion, but the question has always been whether this is biologically significant? Consider a set of such polarised proteins • Frohlich argued that a coherent vibrational mode can be set up that involves all these proteins, as a result of dipole-dipole interaction HEAT repeat structure – e.g. Importin beta • Some four years ago I started to look at whether the recently discovered ‘HEAT’ repeat motif found in some proteins might be the substrate for Frohlich style vibrational modes • ‘HEAT’ are the initial letters of the first 4 proteins where this was found. • HEAT repeats consist of a ladder of helix-turn-helix motifs • The picture below shows the outer ‘A’ helixes and inner ‘B’ helixes of the HEAT repeat in Importin beta, which surrounds a central importin beta alpha helix (blue) Examples of proteins containing HEAT repeats Examples I will consider Condensin: These are involveded in the compacting of Chromosomes Importin: Transports cargo into the cell nucleus TOGp (XMAP215 etc): Associated with the ends of microtubules as they grow towards the chromosomes during mitosis. Others… Delangins: Locates the cohesin complex within chromosomes Huntington: ATM/ATR: Repair of breaks in DNA The Condensin Family of proteins • This is the first, and probably most important of the proteins containing HEAT repeats I will look at. • There are condensins in prokaryotes and eukaryotes. They are quite different, and only the eukaryotic version contains proteins with HEAT repeats. Prokaryotic Condensin Structured Maintainance of Chromosomes (SMC) proteins with long coiled coil legs Eukaryotic Condensins Proteins containing HEAT repeats Effect on mitotic chromosomes of disabling Condensin I and II (Ono, Losada et al. 2003) •e: Control •f: Condensin I disabled: The chromosomes are puffy and bent •g: Condensin II disabled: The chromosomes are straighter but curly/twisted Can Frohlich’s ideas provide a model for how condensin functions? • I think they can, but the original ideas may need to be modified/extended • Consider a group of proteins, vibrating coherently, as per Frohlich’s original model, but where one of them is not quite aligned with the rest Interaction brings proteins into alignment • I believe that a slight extension of Frohlich’s original model/physics can provide a mechanism that will bring the proteins into alignment. • Frohlich’s attractive force draws them into a closer ‘state’, not location. • More on this later! Mitotic chromosome: Condensins disabled • Consider a mitotic chromosome where the condensins are disabled, so the arms are bent and twisted. • Condensin I is represented by green rods/cylinders, that are assumed to vibrate along the axis of the rods, and that these rods are aligned along chromosome axis Action of Condensin I • If Condensin I is now ‘enabled’ then the alignment mechanism I proposed would draw them into alignment with each other • Consequently this straightens the chromosome arms, although they are still twisted around their axis Condensin II • Now represent condensin II by blue rods that are orthogonal to the main axis • They are not aligned with each other because main axis is twisted Action of Condensin II • If Condensin II is now ‘enabled’ the alignment force will try and move the rods such that they are aligned with each other • This process of alignment has the effect of removing the twist from the chromosome arms • Both Condensin I and II are now aligned, but orthogonal to each other, within each arm. Action of Condensin I and II • If we assume that the alignment effect extends between the chromosome arms, then the action of both condensins will help draw the chromosome arms so that they lie parallel with each other The role of Condensin I and II in defining chromsome axes • We are now in the position where condensin I and II are defining two key axes on the chromosome • Condensin I defines long axis of Chromosome • Condensin II defines a perpendicular axis/plane that passes through both Chromosome arms • But is there more? Lets return to some recent experimental data… Investigation of the effect of depleted Condensin I on the metaphase plate • Control, with well defined metaphase plate. All the chromosomes are aligned. • Two examples with depleted Condensin 1 There are poorly defined metaphase plates. The chromosomes are no longer aligned well with each other (From Ono, Fang et al. 2004) Condensin 1 and the definition of the metaphase plate • Perhaps these results tell us that the condensin I alignment not only works within the chromosomes, but also between them, and helps define the plane of the metaphase plate, shown by the green ‘condensin 1’ arrow. The diagram shows a rather linear plane to simplify the diagram. • The diagram below is by necessity somewhat simplistic, in that metaphase plates are complex, variable, and still not fully understood, often with only parts of the chromosomes aligned along the plate Investigation of the effect of depleting Condensin II on metaphase plate • Control, with centrosomes (arrowed) symmetrically placed on either side of the metaphase plate (dotted line) • Two examples with depleted condensin II • The centrosomes are now poorly positioned in relation to metaphase plate, indeed in these examples they are both on the same side of the metaphase plate. (From Ono, Fang et al. 2004) Condensin II and the definition of the mitotic spindle • Perhaps these results tell us the not only does condensin II define an axes within the chromosome, but that the effect of this axis extends beyond the metaphase plate and helps define the location of the mitotic spindle and the location of the centrosomes • This is shown as the extending blue plane, and associated blue arrow centrosomes HEAT repeat structure – Importin beta • This is fairly accurate representation of the structure that has been determined experimentally. • There is however, evidence that the in vivo structure is more compact, so I tried some modelling with corks and beads… A more compact HEAT repeat structure? • The final more compact ‘cork and bead’ model. Corks for alpha helixes, beads for individual amino acids • After many unsuccessful attempts, I found it came together if I assumed that the importin alpha at the centre was no longer an alpha helix, but an extended linear structure. HEAT repeat structure – A more compact form? • In this more compact model there are then four rings of 5 inner (yellow) helixes around the elongated core • One of the helixes (green) in importin beta is longer than the others. This fits well if it is assumed to extend across the central two rings, thus explaining why there are 19 HEAT repeats. Computer model of the HEAT repeat structure • I then modelled the structure using the Swiss pdb Viewer program. Which appeared to validate the original cork and bead model View along the length of Importin alpha/beta complex End on view of Importin alpha/beta complex, with no side chains on the IBB domain of importin alpha How does the HEAT repeat vibrate? • It then struck me that the way this might vibrate could well be completely different from the mode that Frohlich envisaged, and this new mode may overcome some of the problems that had been raised about the original model • I wonder whether the structure can be seen as am inner core (yellow) which vibrates backwards and forwards inside an outer sheath. • The two would oscillate 180° out of phase, such that there is no movement of centre of mass • Vibrations would then spread as evanescent wave into the surrounding substrate HEAT repeat resonant energy transfer • I think that this model can then show how energy can be coupled from excited structure (on right) to adjacent structure (on left). • This is effectively a resonant energy transfer, and would be very efficient providing certain conditions are met. • This model results in both structures being in a coherent synchronous oscillation. This overcomes one of the weakness in Frohlichs original proposal involving dipole dipole interaction, where the coupling results in adjacent dipoles oscillating out of phase. How can HEAT repeat oscillations create an alignment force • Consider two HEAT repeats oscillating synchronously, but slightly misaligned • Non-linear interaction of evanescent waves generates forces that bring HEAT repeats into alignment, in that the aligned vibrational mode is a lower energy state than when they were misaligned. But where does the energy come from • Perhaps the answer comes from looking at the simpler, more ancient prokaryotic condensin. • Unlike eukaryotic condensin, this is able to condense prokaryotic DNA without an external energy source. Once compacted, the DNA, can be pulled apart and it will recompact, over and over again. Structured Maintainance of Chromosomes (SMC) proteins Prokaryotic Condensin Eukaryotic Condensins Proteins containing HEAT repeats How does SMC proteins/Prokaryotic Condensin compact DNA? • It is known that the condensin (brown) ‘grabs’ the DNA (red) at widely separated points. • There will be thermally induced breathing modes in long coiled coil legs of the prokaryotic • I believe that this creates a ‘Brownian ratchet’ that pulls DNA together during contraction phase of oscillation. There are other known examples of this effect, but this has not previously been considered for prokaryotic condensin SMC head binding with ATP • When the DNA is condensed, it is known that ATP binds the heads of the Prokaryotic condensin at Walker A/B domains • It is also known that Walker A/B domains in ABC transporter proteins are associated with mysoin like power stroke, where the ATP is not just used to bind the Walker A/B domains together, but also to generate movement. • Perhaps in Eukaryotic condensin, ATP binding in the SMC proteins, is used to pump vibrational modes in the coiled coil SMC proteins, and which is then coupled into the HEAT repeats Walker A/B domain and binding ATP Other proteins containing HEAT repeats: TOGp/XMAP 215 • TOGp is associated with the growing ends of the microtubules as they extend towards the chromosomes in the metaphase plate • Perhaps these resonate with the vibrational mode of condensin II and so is able to make use of the axis defined by condensin II to draw the microtubules towards the chromosomes. • Condensin II is particularly concentrated at the centromeres, the ultimate objective for the microtubules, which makes sense. TOGp (red) Other proteins containing HEAT repeats: Importin beta • Importin beta (shown as a green cylinder) attaches to cargos (the grey blob) and transfers them into the nucleus • During interphase, when this happens, condensin I is still active in the nucleus, so perhaps Importin beta resonates with the coherent vibrations in the nucleus, and a Frohlich like attractive force then draws the importin, with its cargo, into the nucleus. Cell nucleus Importin beta (green) But what happens when it meets the Nuclear pore complex • The nuclear pore complex is the ‘gate’ into the cell, letting some things through but keeping other (often smaller) things out. • The core of the pore complex is filled with nucleoporin proteins, a mix of multiple FG repeats and hydrophilic linkers • So perhaps the pore is blocked by water gell created by nucleoporin proteins? Unless I have missed something, this does not appear to have been considered. Passage of Importin through the Nuclear pore complex • This raises the interesting possibility that the vibrations from the importin are coupled into the nucleoporin proteins, and so into the attached water. Triggering a Gel/water transition and so allowing the importin through the gate. Central formers for HEAT repeats • I now think that all HEAT repeats, when active, take the more compact form around a suitable former. This often only happens when the former is suitably phosphorylated, which is how the operation of the HEAT repeat is controlled. • I think there are prime candidates for all the HEAT repeats considered so far: HEAT repeat Former • Importin beta: Importin alpha • Condensin: Histone H3 tails (Jager, Rauch et al. 2005) • TOGp/XMAP215 Tubulin tails And finally: Orthogonal condensin axes and orthogonal centrioles • There is a long standing enigma as to why the centriole consists of two cylinders, at right angles to each other. • The model that has emerged is of condensin defining two axes, at right angles to each other through coherent orthogonal vibrational modes. The centrosome, with its centriole has to coordinate its location/movement with these planes/axes, but this would need two highly sensitive, orthogonal detectors. • Perhaps these are the centrioles? There are a number of HEAT repeat proteins that are associated with the centrioles during mitosis, perhaps they align themselves on the surface of the centriole in order to perform this function. centrosomes Centriole pair Thanks to: Vermont Photonics Warwick University Lila Gierasch, Gerry Pollack, Mae-wan Ho and countless others who I have visited and emailed The creaters of Blender