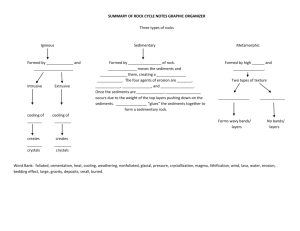
Vanadium in foraminiferal calcite as a tracer for changes
advertisement

PALEOCEANOGRAPHY, VOL. 11, 11,NO. NO.6, PAGES 665-678, 665-678, DECEMBER DECEMBER 1996 PALEOCEANOGRAPHY, VOL. 6, PAGES 1996 Vanadium in foraminiferal calcite as as aa tracer Vanadium in foraminiferal calcite tracer for for changes changes in in the the area! areal extent extent of ofreducing reducingsediments sediments 11and and Steven Steven R. David DavidW. W. Hastings R.Emerson Emerson School of of Oceanography, University Seattle School Oceanography, Universityof of Washington, Washington, Seattle Alan Alan C. C. Mix Mix College Oregon Collegeof of Oceanography, Oceanography, OregonState StateUniversity, University,Corvallis Corvallis Abstract. a tracer tracer Abstract. We Wehave haveused usedthe thevanadium vanadium concentration concentration in in cleaned cleaned foraminiferal foraminiferal calcite calcite as a of seawater V changes in the past. Since the benthic flux of vanadium is sensitive to the redox poof seawaterV changesin thepast.Sincethebenthicflux of vanadiumis sensitiveto theredoxpotential of changes concentration of of seawater seawater should should be be aa reflection reflection of of tential of sediments, sediments, changesin in the the vanadium vanadiumconcentration changes V/Ca in'G. in G. sacculifer sacculifer from from an an eastern eastern equatorial equatorial changesthat thatcontrol controlthe theredox redoxstate stateof of sediments. sediments. V/Ca Atlantic 17GGC) is Atlanticcore core(EN066(ENO66-17GGC) is 21.6 21.6 (±2.8) (+_2.8)nmol nmolV/mol V/mol Ca. Ca. This Thisvalue valuedoes doesnot notchange changeover over 35 change levels over this 35 kyr, kyr, indicating indicatingthat thatthere therewas wasno nomeasurable measurable changein in seawater seawatervanadium vanadiumlevels overthis period. Potential partial dissolution dissolution are are not not significant significant based based on on low, low, constant constant values values period. Potentialartifacts artifactsfrom from partial for foraminiferal (6-7%) minor correction for foraminiferalfragmentation fragmentation (6-7%) in in the thetop top50 50 cm cmof of the thecore. core:A A.minor correctionto toaccount account for with overgrowths, estimated for vanadium vanadiumassociated associated with Mn Mn carbonate carbonate overgrowths, estimatedfrom fromtwo two Caribbean Caribbeancores cores where this mixed phase dominates the deeper V/Ca values, has been applied. Changes in the the areal areal wherethismixedphasedominates thedeeper V/CaValues, -hasbeenapplied. Changes in extent of sediments are by value extent of anoxic anoxicand andsuboxic suboxic sediments arethus thusconstrained constrained bythis thisconstant constant valueand andthe thestandard standard deviation of deviation of the the measurement, measurement,±12%. +_12%.Based Based on on aa mass massbalance balance for for vanadium vanadium where where suboxic suboxic sedisediments are are are to ments areaa source sourceand andanoxic anoxicsediments sediments areaa sink sinkto to the theocean, ocean'suboxic suboxicsediments sediments arepredicted predicted to have changed by no more than 0.5-1.5 times the current value, assuming no change in the areal havechanged by nomorethan0.5-1.5timesthecurrent value,assuming nochangeintheareal extent of of anoxic anoxic sediments. This correspondsto corresponds to 1.3-3.5% sediments. Given a a conconextent sediments. This 1.3-3.5%of oftotal totalocean ocean Sediments. Given stant area area of of suboxic sediments, the areal areal extentof extent-of anoXic anoxic Sediments sediments didonot did notincrease increase by by more more than than stant suboxic sediments, the fivefold, or of floor over the 35 reductions in in deep water fivefold, or1.5% 1.5% ofthe theocean ocean floor over thepast past 35kyr. kyr.The Thesignificant significant reductions deep water oxygen levels levels and changes in conditions required by deoxygen andconsequent consequent changes insediment sedimen t redox redox conditions required bypolar polarnutrient nutrient depletion are data over over the the past past 35 35 kyr. kyr. This This pletionscenarios scenarios arenot notreflected reflectedin in the theforaniiniferal foraminiferalvanadium vanadiumdata suggests that that models which call in water oxygen are suggests models which callon onless lessor orno nochanges changes indeep deep Water oxygen aremore morelikely likely alternatives. alternatives. Introduction Introduction One geochemical One class classof of coupled coupledocean-atmosphere ocean-atmosphere geochemicalmodels models developed to explain the reduced glacial age pCO2 developed to explainthereducedglacialageatmospheric atmospheric pCO2 [Barnola et et al., [Barnola el., 1987; 1987; Jouzel Jouzel et et al., el., 1993] 1993] relies relies on on more more effieffi: cient cient utilization utilization of of nutrients nutrientsin inhigh-latitude high-latitudesurface surfacewaters waters [Knox and and McElroy, McElroy, 1984; Sarmiento and and Toggweiler, [Knox 1984; SarmientO Toggweiler,1984; 1984; Siegenthaler and Wenk, 1984; Martin et al., Siegenthalerand Wenk, 1984; Martin et el., 1990]. 1990]. A A conseconsequence quenceof of these thesemodels modelsis is that thatthe theincreased increasedexport exportof of organic organic matter remineralization matter to to the thedeep deepocean oceanand andsubsequent subsequent remineralizationrere' sults in in aa large sults largedepletion depletionin in deep deepwater wateroxygen oxygenduring duringglacial glacial periods. periods.A A three-dimensional three-dimensionalocean ocean circulation circulation model model of of the the Reimer, 1994] which predict predict less less or Reimer, 1994] which or no nooxygen oxygendepletion depletiondurduring glacial _ing glacialtimes. times.The Thediverse diversemodeling modelingefforts effortstherefore thereforeprepredict the last dictdifferent differentdeep deepwater wateroxygen oxygenlevels levelsduring duringthe lastglacial glacial maximum. Which approach maximum, Which approachis is valid? valid? Several proxies exist exist to Several proxies to assess assesschanges changesin in sediment sedimentredox redox conditions these conditionsin order orderto to evaluate evaluateand andconstrain constrain thesedifferent different models. The most indicator for water m0dels.' The mostunambiguous unambiguous indicator forbottom bottom water anoxia is the existence of laminated anoxia is laminated sediments sediments and the absence absence Based on on the of of benthic benthicforamimfera. foraminifera.Based theexistence existence of benthic benthic fauna in in most most sediment cores, itit is is safe fauna sediment cores, safe to to rule rule out out vast vast areas areas of of ocean anoxia anoxia for for at years. ocean at least leastthe thelast lastten tenor ortwenty twentythousand thousand years. Evidence of of decreased decreased bottom bottom water water oxygen oxygen has has been been evaluated evaluated Evidence carbon and Orr, carboncycle cycle [Sarmiento [Sarrnientoand Orr, 1991] 1991]predicts predictsaa decrease decreasein in by determination of trace metal enrichment in sediments [e.g., bydetermination oftrace metal enrichment insediments [e.g,, average deep deep water average water oxygen oxygen by by 40 40 LtM, !.tM, with with bottom bottom water water' Dean, Dean, 1989; 1989; Calvert Calvert and and Pedersen, Pealersen,1993; 1993; Sarkar Sarkar et et al., el., 1993; 1993; anoxia resulting in the southwestern Indian Ocean. Other modanoxiaresultingin the southwestern Indian Ocean,Other modColodner et er el. al.,' 1994; Piper and and Issacs, 1995; Rosenthal et et ColOdner 1994;Piper Issacs, 1995;Rosenthal eling efforts to explain reduced pCO2 during the last glacial al., 1995; Yang er al., 1995; Crusius et al., 1996] and faunal eling efforts reduced pCO2 during lastglacial el.,1995; Yang etel.,1995' Crusius etel.,1996] andfaunal age are are basedto onexplain alkalinity changes in the the oceanthe [Broecker and age based on alkalinity changes in ocean [BrOecker and . indices such as as the the number indices such numberof of fecal fecal pellets pelletsand and size sizeand andabunabunPeng, 1989; Opdyke and Walker, 1992; Archer and MaierPeng, 1989; Opdyke and Walker, 1992; Archer and Maierdance [Pedersen er dance of of benthic benthicforaminifera foraminifera [Pedersen et al., al., 1988]. 1988].These These indicators are limited indicatorsare limitedin in that thatthey theyreflect reflectlocal localdepositional depositional conditions primarily in equatorial upwelling areas. The The record record 1Now at Department of Earth and Ocean Sciences University of primarilyin equatorial upwelling areas. 1Nowat Department of EarthandOceanSciences University of conditions Biitish can British Columbia, Columbia, Vancouver, Vancouver, Canada. Canada. can easily easily be be disturbed disturbedby byphysical physicalerosion, erosion,chemical chemicalalteralteration, and bioturbation ifif overlain by oxygenated sediments ation, and bioturbation overlain by oxygenated sediments Copyright Union. Copyright1996 1996by by the theAmerican AmericanGeophysical Geophysical Union. [Thomson et al., al., 19951. Using these these approaches, it would be [Thomsonet 1995].. Using approaches, it would be necessary comprehensively in necessaryto to sample sampleocean oceansediments sediments comprehensively in order order to to evaluate evaluateglobal global changes changesin in the the extent extentof of sediments sediments Paper number Paper number96PA01985. 96PA01985. 0883-8305/96/96PA-01985512.00 0883-8305/96/96PA-01985$l2.00 665 665 666 666 HASTINGS ET F AL.: HASTINGS AL.:VANADIUM VANADI•,'I IN INFORAMINIFERAL FORAMINIFERAL CALCITE CAI.121• overlain by anoxic water. Since Since the the areal overlain by anoxic bottom bottom water. areal extent extent of of anoxic is presently very small, anoxic sediments sedimentsis presentlyvery small, about about 0.3% 0.3% of of the the derived Fe are highly of derived Fe oxyhydroxides oxyhydroxides are highlyefficient efficientscavengers scavengers of oxyanions such and for 60 oxyanions suchas asvanadium vanadium andcan canaccount account forremoving removing 60 ocean floor, increase would ± ocean floor,an anorder orderof of magnitude magnitude increase wouldbe beonly only3% 3% _+30% 30% of of the theriverine rivefineinput input[Trefry [Trefryand andMetz, Metz,1989; 1989;Kadko, Kadko, of the sediment of sediment surface. Such Such an an increase increase would would be be difficult difficult to to 1993; Rudnicki and and Elderfield, time 1993;Rudnicki Elderfield,1993]. 1993].The Theresidence residence timeof of V V detect. Another with detect. Another approach approachwhich which reflects reflectsglobal global changes changesis is with respect respectto to riverine rivefineinput inputis is 100 100kyr kyr[Shiller [Shillerand andBoyle, Boyle, based on between 31 8 of oxygen 1987]. based onthe thedifference difference between/5180 of atmospheric atmospheric oxygen 1987]. and seawater (the Dole Especially relevant to to this this work and seawater (the Dole effect) effect) as as recorded recorded in in the the Vostok Vostok ice ice Especially relevant work is is that that several several data data sets sets core over over the the past past 130 from sediments off of Africa core 130 kyr kyr [Bender, [Bender, 1994]. 1994]. Since Since the the Dole Dole fromreducing reducing sediments off the thecoast coast ofnorthwest northwest Africasugsugeffect on timescale, out of of effectis isnearly nearlyunchanged unchanged onaaglacial-interglacial glacial-interglacial timescale, gest gestthat that there thereis is aa significant significantbenthic benthicflux flux of of vanadium vanadiumout the authors conclude that there cannot have been major suboxic sediments, where Mn is also diffusing into overlying the authors concludethat there cannot have been major suboxicsediments, whereMn is alsodiffusinginto overlying changes bottom waters changesin in global globalmarine marineproductivity productivityand andhence henceminimal minimal bottom waters[Seralarhan [Seralathanand andHartmann, Hartmann,1986; 1986;Legeleux Legeleuxet et excursions excursions in in oxic oxic versus versus suboxic suboxic or or anoxic anoxic sedimentation. sedimentation. al., al., 1994; 1994; Hastings Hastingsand andEmerson, Emerson,1995]. 1995]. This Thisisisconsistent consistent In tracer with the V is In this thiswork workwe wedevelop developaanew newpaleoceanographic paleoceanographic tracerfor for with the observation observationthat that in in hemipelagic hemipelagicsediments, sediments,V is changes in redox on using coupled with the redox cycle of Mn. In suboxic sedichanges in sediment sediment redoxconditions conditions onaaglobal globalscale scale using tightly tightly coupledwith the redox cycle of Mn. In suboxicsedivanadium in foraminiferal ments, defined in in this vanadiumincorporation incorporationin foraminiferalcalcite calciteas asan anindicator indicator ments,operationally operationally defined thiswork workas asthose thosewhere wherepore pore of in The of of past pastV V concentrations concentrations in the theocean. ocean. Thepotential potential ofusing using water wateroxygen oxygenis is depleted depletedwithin withinthe theupper upper1-3 1-3cm, cm,and andwhere where forarniniferal vanadium as proxy foraminiferalvanadium asaapaleoceanographic paleoceanographic proxyfor forseasea- no no surficial surficial enrichment enrichmentof of Mn Mn oxides oxidesisisobserved, observed,lower lowerbulk bulk water has concentrations imply a diffusive flux of vanadium watervanadium vanadiumconcentrations concentrations hasrecently recentlybeen beendemonstrated demonstrated V V concentrationsimply a diffusive flux of vanadiuminto into the the [Hastings culture show yr1. The [Hastingset et al., al., 1996a]. 1996a].Laboratory Laboratory cultureexperiments experiments show overlying overlyingbottom bottomwaters watersof of about about66 nmol nmolcm-2 cm-2yr-1. Theareal areal that extent that living living planktonic planktonicand andshallow shallowwater waterbenthic benthicforaminifera foraminifera extent of these these suboxic suboxic sediments sediments is estimated estimated to be 2.5% 2.5% of incorporate vanadium into their tests in direct to ocean based that incorporatevanadiuminto their testsin directproportion proportion to oceansediments sediments basedon onsediments sediments thathave havean anoverlying overlying seawater concentrations. V/Ca V/Ca values values for for the the same of productivity greater than than 200 g C not seawaterconcentrations. samespecies speciesof productivity greater C rn-2 m-2yr1 yr-1atatdepths depths notexexforaminifera in different for foraminifera in differentocean oceanbasins basinsare areconstant constant forcore-top core-top ceeding ceeding1500 1500rn. m. The The depth depthand andproductivity productivitycriteria criteriaare arebased based samples on those there is is a diffusive samplescollected collectedabove abovethe theforaminiferal foraminiferallysocline lysoclinein in differdifferthose sediments sediments where there diffusive flux flux of V out out of of ent ent ocean oceanbasins. basins.The The mean meancore-top core-topvalues valuesare are 21.7 21.7 (± (+ 3.6; 3.6; n=15) and n=15) and112 112 (±9; (+9; n=3) n=3) nmol nmolV/mol V/mol Ca Ca for for Globigerinoides Globigerinoides sacculifer and wuellerstorfi, Coresacculifer andCibicidoides Cibicidoides wuellerstorfi,respectively. respectively. Core- the the sediments. sediments. Other Other estimates estimates of of suboxic suboxic sediments sediments are are higher, higher, ranging ranging from from 8 8 to to 13% 13% [Klinkhammer [Klinkhammer and and Palmer, Palmer, 1991; Reimers et et al., al., 1992], using 1991;Reimers 1992],but butare areevaluated evaluated usingdifferent different criteria. Based Based on on the theestimate estimatethat thatsuch suchsuboxic suboxicsediments sedimentsacaccount count for for 2.5% 2.5% of of ocean oceansediments, sediments,the the V V flux flux is isestimated estimatedto to partial dissolution of the depth to examipartial dissolution of thetests. tests.With Withincreasing increasing depthof ofdepodepo- be beequivalent equivalent to45% 45%of ofthe theriverine rivefineinput. input.An Anin-depth in-depth examisition, more corrosive nation corrosive bottom waters, waters, and and thus thus more more extensive extensive nation of of the the marine marine mass massbalance balance for for vanadium vanadium and andassociated associated partial dissolution of the test, V/Ca increases by [D. et in partialdissolution of the test,V/Ca increases by up upto to aafactor factor data datasets setsis isgiven givenelsewhere elsewhere [D. Hastings Hastings etal., al.,manuscript manuscript in of 4 in G. sacculifer and by a factor of 2 in C. wuellerstorfi. preparation, 1996]. Assuming that other terms in the oceanic of 4 in G. sacculiferandby a factorof 2 in C. wuellerstorfi. preparation,1996]. Assumingthat other termsin the oceanic This effect V over in This dissolution dissolution effectcan canbe beminimized minimizedby bycareful carefulchoice choiceof of V cycle cycleare areconstant constant overthe thepast pastglacial glacialperiod, period,changes changes in study to the vanadium concentration in should reflect studymaterial materialfrom fromdepths depthsshallow shallowenough enough toavoid avoidsignifisignifi- the vanadium concentration in seawater seawater should reflectchanges changes cant By that in the redox state state of of the the surface surface sediments. sediments. cantcalcite calcitedissolution. dissolution. Bydemonstrating demonstrating thatincorporation incorporation in of vanadium is to of vanadiumin in cultured culturedforaminifera foraminifera isdirectly directlyproportional proportional to seawater concentrations and and that Methods seawaterconcentrations that global globalcore-top core-topV/Ca V/Ca values values Methods above the foraminiferal lysocline are constant, we abovethe foraminiferallysoclineare constant,we have haveshown shown Foraminifera were were obtained by washing that vanadium is is a a valuable Foraminifera obtainedby washingand andsieving sieving(>63 (>63 thatforaminiferal foraminiferalvanadium valuabletool tool to to reveal revealpast past J.tm) bulk ocean sediment several times in 5% changes in the vanadium levels in seawater. In this paper we gm) bulk oceansediment severaltimesin 5%(NaPO3)6 (NaPO3) 6 changesin the vanadiumlevelsin seawater. In thispaperwe buffered pH 88 with bufferedto to pH withNaOH NaOHto toremove removeclays claysand andfines. fines.After After examine the downcore V/Ca record changes examinethe downcoreV/Ca recordto to constrain constrain changesin in was (<250, drying,the thesediment sediment wassieved sievedinto intofive fivesize sizefractions fractions (<250, seawater V over seawaterV over the the past past35 35 kyr, kyr,which whichin inturn turnprovides provides drying, 250-355, information regarding changes changes in in the 250-355, 355-425, 355-425,425-495, 425-495,and and>495 >495J.Lm), gm), and andsingle single informationregarding the redox redoxstate stateof of marine marine species of were under sediments. sediments. species of foraminifera foraminifera werehand-picked hand-picked underaadissecting dissecting microscope from the the 355 tm fraction. microscopefrom 355 to to 425 425 gm fraction.Those Those individuals visibly contaminated by black specks, assumed to individuals visibly contaminated by black specks, assumed to The Marine Vanadium Cycle The Marine Vanadium Cycle top analyses analyses along along two rises across the top two submarine submarine rises across the foraminiferal lysocline lysocline indicate indicate aa significant foraminiferal significanteffect effectrelated relatedto to Vanadium is aa useful the flux Vanadium is useful tracer tracer because because the flux to to and and from from ocean sediments is sensitive to the ocean sediments is sensitive to the redox redox state state of of bottom bottom water. Since water. Sincethe the vanadium vanadiumconcentration concentrationis is nearly nearlyconservaconservative throughout the world tive throughoutthe world ocean ocean[Collier, [Collier, 1984], 1984], aa single singlepapaleoceanographic record record is is sufficient sufficient to to provide leoceanographic provideaa global globalestiestimate of of changes The mate changesin in the theareal arealextent extentof ofreducing reducingsediments. sediments. The major source source of of dissolved to the major dissolved vanadium vanadium to the ocean ocean is is riverine rivefine input, which which accounts accounts for for aa global global flux flux of of 55 xx 108 mol V V yr-1 yr1 input, 108mol [Shiller and and Boyle, It is [Shiller Boyle, 1987]. 1987]. It is well well established establishedthat thatanoxic anoxic sediments, defined defined here here as sediments, as those thoseoverlain overlainby by anoxic anoxicbottom bottom water, are a known sink for vanadium [Holland, water, are a known sink for vanadium [Holland, 1984; 1984; Breit Breit and responsible for for removing removing88 +± 5% 5% of of the and Wanty, Wanty,1991] 1991] responsible the riverine riverineinput input[Emerson [Emersonand andHuesred, Huested,1991]. 1991].Hydrothermally Hydrothermally be ferromanganese ferromanganeseoxide, were not not used. used.Foraminifera Foraminiferawere were gently crushed to chambers and gently crushed toopen openthe theindividual individual chambers andrigorously rigorously cleaned modified from cleanedusing usingaamethod method modified fromBoyle Boyleand andKeigwin Keigwin [1985/86] to to eliminate these phases. Two cleaning [1985/86] eliminate these phases. Tworeductive reductive cleaning steps before step Following steps beforeand andafter afterthe theoxidative oxidative stepwere wereused. used. Following the procedure, all was in a a thecleaning cleaning procedure, all sample samplehandling handling wasperformed performed in HEPA laminar flow flow hood hood in in aa class HEPA laminar class100 100 clean cleanroom. room.A A comcom- plete description of the is plete description of thecleaning cleaningprocedure procedure is given givenby by Hastings et Hastings et al. al. [1996a]. [1996a]. Cleaned samples were gravimetrically spiked Cleaned samples weredissolved, dissolved, gravimetrically spiked with an an 50V isotope spike spike enriched enriched to to 44 with 50Visotope 44 atom atom%, %,and andtaken takento to dryness. Dried were eluted through cation dryness. Driedsamples samples wereredissolved, redissolved, eluted through cation exchange interferences, and exchangeresin resinto to remove removeCa Caand andisobaric isobaric interferences, and analyzed analyzedfor for V V by byisotope isotopedilution dilutionthermal thermalionization ionizationmass mass 667 667 HASTINGS Fr AL.: CALCITE HASTINGSET AL.:VANADIUM VANADIUMIN INFORAMINTFERAL FORAMINIFERALCALCITE spectrometry (TIMS) or spectrometry(TIMS) or by by isotope isotopedilution dilutionelectrothermal electrothermal vaporization inductively coupled plasma vaporizationinductively coupled plasmamass massspectrometry spectrometry (ETV-ICP-MS) [Hastings et et al., al., l996b]. (ETV-ICP-MS) [Hastings 1996b]. Drying Drying the the sample samplein in the ETV the ETV unit unit before beforeanalysis analysisquantitatively quantitativelyeliminates eliminates the the ClO+isobaric C10 isobaricinterference interferencewith with V V at atmass mass51, 51, which whichis is aasigsignificant nificant problem problemfor for V V determination determinationby byICP-MS. ICP-MS. Analyses Analyses samples sample samplesof of the thedissolved dissolved samplewere werediluted dilutedinto into25 25mL mLof ofLa La solution (400 ppm La in 0.05 N HC1 and 0.002 N HNO3). solution(400 ppm La in 0.05 N HC1and0.002 N HNO3).The The bulk metal bulk metal content content of of marine marinesediments sedimentswas was determined determinedby by digesting dried sediments in strong digestingdried sedimentsin strongacids acids(2 (2 mL mL concentrated concentrated HNO3, HF, HC1O4) HNO3, 33 mL mL concentrated concentrated HF,and and11mL mLconcentrated concentrated HC104) and analysis et al., and subsequent subsequent analysisby by ICP-MS ICP-MS [McLaren [McLarenet al., 1987; 1987; Longerich et et al., al., 1990]. with anomalously high at with anomalously highisobaric isobaricinterferences interferences at mass mass50 50(50Cr (50Cr Longerich 1990]. Details Detailson on the theprocedure procedureare are given givenby by and 5OTi) that interfere interfere with with the the isotope Hastings [1994]. and 50Ti) that isotopedilution dilutionmeasurement measurement Hastings [1994]. are not not included mean values values or or shown shown in in the the are included in in the the calculated calculated mean figures. This This problem occurred in in 5% figures. problemoccurred 5% of of the the samples. samples. Measurement precision on on the the same Measurementprecision samesample sampleof of aadissolved dissolved CaCO3 CaCO3 standard, standard,equivalent equivalentto to 2.5 2.5 mg mgcalcite calciteafter aftercleaning, cleaning, was was ±3% +3% (la; (lo; nn=10) =10) over overthe thecourse courseof ofone oneICP-MS ICP-MS run runand and ±7% foramimferal sam+7% (la; (lo; n=9) n=9)by byTIMS. TIMS.Using Using55toto15-mg 15-mg foraminiferal samples ples that that have havebeen beencleaned cleanedindividually individually reduces reducesthe the average average precision on duplicate samplesto to +8% ±8% (lo) (la) by precisionon duplicate samples by ICP-MS ICP-MS and and ±9% +9% (la) (lo) by byTIMS. TIMS. This Thisreflects reflectssome someof ofthe thenatural naturalvarivariability ability in in actual actual samples samplesand and is is also alsoan anindication indicationof of how how effective effective the the cleaning cleaningprocedure procedureis is in inremoving removingcontaminant contaminant phases. phases.The The procedural proceduralblank blankvalue, value,determined determinedby by nonisotope nonisotope dilution dilution method, method,was was 0.27 0.27 pg pg V. V. The The detection detectionlimit limit for forETVETVICP-MS, ICP-MS, defined defined as as 33 times times the the standard standarddeviation deviation of of the theblank blank at at mass mass51, 51, was was0.3 0.3 pg pgV V without withoutthe theisotope isotopespike spikeand and66 pg pgV V using isotope dilution. The isobaric interferences at mass using isotope dilution. The isobaric interferencesat mass50 50 from from Cr Cr and andTi Ti significantly significantlyaffect affect the the value valuefor for the thedetection detection limit. limit. A A detailed detailedexplanation explanationof of the the analytical analyticalmethod methodis is given given elsewhere elsewhere[Hastings [Hastingset et al., al., 1996b]. 1996b]. Magnesium, calcium, Magnesium, calcium, and and manganese manganesewere were measured measuredsimulsimultaneously taneouslyby by inductively inductivelycoupled coupledplasma plasmamass massspectrometry spectrometry (ICP-MS) subsamplesof of the the dissolved dissolved sample (ICP-MS) on on25-I.LL 25-gL subsamples samplediluted diluted into et al., al., 1996a]. into 2 2 mL mL of of 0.1 0.1 N N HNO3 HNO 3 [Hastings [Hastings et 1996a]. These These values values were were used usedfor forMg/Ca Mg/Ca and andMn/Ca Mn/Cadeterminations. determinations. Calcium Calcium analyses analysesused usedto to calculate calculateV/Ca V/Ca ratios ratioswere wereperformed performed by spectrophotometry. Fifty-.LL by flame flameatomic atomicabsorption absorption spectrophotometry. Fifty-gL subsub- Site Description and Site Description and Chronology Chronology Caribbean Sea Caribbean Sea Two shallow cores cores were were selected selectedin inthe theCaribb6an Caribban Tworelatively relatively shallow Sea. (1l°39.83'N, 79°35.52'W; Sea. TT9108-1GC TT9108-1GC (11ø39.83'N, 79ø35.52'W; 2540 2540 m) m) is is located located in in the the western westernregion regionof of the theColumbia ColumbiaBasin Basin(Figure (Figure 1). Measurements of õ180 18O on on C. C. wuellerstorfi Measurements wuellerstorfiand andthe thevisual visual description of the the core that the the top top 50 descriptionof core indicate indicatethat 50 cm cm of of the the sedisediment ment was wassampled sampledtwice. twice. The The gravity gravitycore coreprobably probablypenetrated penetrated the the sediment, sediment,withdrew withdrew briefly, briefly, and andthen thenrepenetrated. repenetrated.These These additional 50 cm additional 50 cm are are not not included included in in data data shown shown for for this this core. core. Core m) is Core CP CP 6001-4 6001-4 (14°55'N, (14ø55'N, 71° 71 ø 50'W; 50'W; 3645 3645 m) is from from the the Beata Beata Ridge Ridge that that separates separatesthe theColumbia Columbiaand andVenezuela Venezuela Basins. Basins.The The average averagesedimentation sedimentationrate rate was was 4.2 4.2 cm/kyr. cm/kyr. Since Since the the top top 25 25 cm cm of of the thepiston pistoncore coreare aremissing, missing,the thedata datashown shown are are aa composite compositeof of the thetrigger triggerweight weightcore coreand andthe thepiston pistoncore. core. The changes in l8O, V/Ca, The changes in õ180, V/Ca,and andMg/Ca Mg/Cain in the thetop top50 50cm cmwere were used to used to estimate estimatethe the extent extentof of the thecore coretop topmissing missingfrom fromthe the piston piston core. core. The 1800-m The 1800-m sill sill separating separatingthe the Caribbean Caribbean Sea Sea from from the the Atlantic Ocean blocks the inflow of North Atlantic Atlantic Ocean blocks the inflow of North Atlantic Deep Deep Water. Water. Deep Deep Caribbean Caribbeanwaters waterstherefore thereforereflect reflect the the chemistry chemistry of of Atlantic Atlantic intermediate intermediate waters, waters, and and sediments sediments from from the the Caribbean reflect dissolution cycles that that are Caribbeanreflect dissolutioncycles are opposite oppositein in sense sense 600 60 ø ß o 30°ø 30 cP6001 -4 ß EN066 - 17GGC 0°o o• ß ß ß 30° 30 ø 1200 120ø 600 60ø 00 0o Figure Location of of cores cores used used in and water water depths depths for for these these cores cores are are Figure 1. ]. Location in this this study. study.Specific Specific locations locationsand EN066-17GGC, 5°22'N, 21ø5'W, 2l°5'W, 3050 EN066-17GGC, 5ø22'N, 3050 m; m; CP6001-4PC, CP6001-4PC, 14°55'N, 14ø55'N, 71°50'W, 71ø50'W, 3645 3645 m; m; and and Tr9108-1GC, TT9108-1GC, ll°39.83'N, 11ø39.83'N, 79°35.52'W, 79ø35.52'W, 2540 2540 m. m. 668 668 HASTINGS E AL.: VANADIUM IN CALCITE HASTINGS ET AL.: VANADIUM INFORAMINIFERAL FORAMINIFERAL CALC1TE to to the the dissolution dissolutioncycles cyclesof of the thedeep deepAtlantic Atlantic[Peterson, [Peterson,1990; 1990; see et al., see also also Imbrie Irnbrie et al., 1992]. 1992]. In In contrast contrast to to Atlantic Atlantic sedisedi• ments, ments,glacial glacialCaribbean Caribbeansediments sedimentsreflect reflectenhanced enhancedcarbonate carbonate preservation. preservation. Eastern Eastern Equatorial Equatorial Atlantic Atlantic Core (5°22'N, 21°5'W) Core EN066-17GGC EN066-17GGC (5ø22'N, 21ø5'W) is is aa278-cm 278-cmgravity gravity core raised from 3050 m water depth on the Sierra core raisedfrom 3050 m water depth on the SierraLeone LeoneRise. Rise. The is based The chronology chronologyis basedon onisotope isotopestage stageboundaries boundariesestiestimated from from oxygen isotope changes measured on on C. mated oxygenisotope changesmeasured C. wuellerwueller- storfi [Curry and storfi and andon onchanges changesin in percent percentcarbonate carbonate [Curry and Lohmann, Lohrnann,1986]. 1986]. Ages Agesassociated associatedwith with these thesestage stageboundaries boundaries are from Imbrie et et al. the effects effects of of the are from Irnbrie al. [19841 [ 1984] and and incorporate incorporatethe the now [Bard 14C-Uyear yeardifferences differences nowobserved observed [Bardet et al., al., 1990]. 1990].The The multilinear algorithm algorithm [Keigwin and Jones, Jones, 1994] based on on the multilinear [Keigwin and 1994] based the original linear linear equation original equation[Bard [Bard et et al., al., 1993] 1993] was wasused usedto to make make the corrections the correctionsto to calendar calendaryears. years.Carbonate Carbonatecontent contentdecreases decreases downcore from from Holocene Holocene values values of of 75% 75% to to glacial downcore glacial values valuesof of Table for G. Table 1. 1.V/Ca, V/Ca, Mn/Ca, Mn/Ca, and andMg/Ca Mg/Ca Values Values for G. sacculifer sacculifer From 'Core Core TT9108-1GC From TF9108-1GC 1 Corrected Depth, Corrected Depth, 1 cm cm V/Ca, V/Ca, nmol/mol nmol/mol Mn/Ca, Mn/Ca, ILmol/mol gmol/mol Mg/Ca, Mg/Ca, mmol/mol mmol/mol 11 20.5 20.5 4.6 4.6 3.87 3.87 1 31.0 31.0 26.6 26.6 6.8 3.98 3.98 7.4 3.75 27.2 27.2 62.4 62.4 36.9 96.2 96.2 89.0 3.8 3.56 3.56 21.5 21.5 3.66 3.66 23.8 23.8 3.58 10 10 20 20 20 :30 30 30 • 49.4 49.4 51.7 51.7 58.4 58.4 59.4 59.4 3.41 3.47 3.47 40 40 146 40 40 60 60 137 261 116 3.07 60 60 261 132 3.26 3.26 80 80 814 152 3.10 3.10 80 80 307 166 3.07 3.07 basin are are less than those basin less corrosive corrosive than those in in the the western western basin basin due due to to 100 100 229 3.11 the relatively low amount Bottom Water Water the relatively low amountof of corrosive corrosiveAntarctic Antarctic Bottom (AABW) that enters enters the the basin basin [Warren, (AABW) that [Warren, 1981]. 1981]. Thunell Thunell [1982] [1982] 100 400 400 398 487 469 552 552 216 3.07 3.07 220 220 3.37 3.37 approximately 50% approximately 50% CaCO3 CaCO3 [Curry [Curry and and Lohmann, Lohrnann,1986, 1986, 1990]. This This relatively relatively shallow core in 1990]. shallow core in the theeastern easternAtlantic Atlantic was was chosen to to avoid of calcite. chosen avoid dissolution dissolutionof calcite. Deep Deep waters waters in in this this 120 3.27 3.27 3.25 estimated the the lysocline lysocline to to be be 4800 120 231 3.32 120 3.32 estimated 4800 m m in in the the eastern easternbasin basin compared with with 4000-4300 4000-4300 mm in compared in the the western western Atlantic. Atlantic. 359 3.50 140 3.50 Foraminiferal fragmentation 140 Foraminiferal fragmentationand andfraction fractionofofdissolution-resisdissolution-resis272 3.34 tant species tant species increase increase sharply sharply at at 4600 4600 m, m,suggesting suggesting increasing levels levels of of dissolution below that increasing dissolutionbelow that depth depth[Dubois [Dubois and and 1 Depths at and below 10 cm have been corrected for the apparent 1 DePths atand below 10cmhave been corrected fortheapparent Prell, 1988]. accumulation data Prell, 1988]. Fragmentation Fragmentationand andcarbonate carbonate accumulation data double Penetration penetration of of the the gravity 50 double gravitycore coreby by subtracting subtracting 50 cm cmfrom fromthe the in the eastern basin indicate a shoaling of the lysocline by in the easternbasin indicate a shoalingof the lysocline by original depths. original depths. 1000 m m during 1000 during glacial glacial stages stages[Curry [Curry and andLohmann, Lohrnann,19861, 1986], which implies implies that which that the the foraminiferal foraminiferal lysocline lysocline was was at at about about 3700 between V/Ca V/Ca and 3700 m m during duringthe the last lastglacial glacialperiod. period. between and Mn/Ca Mn/Ca values valuesis is significant, significant,with with aa regresregres- Results Results Caribbean Sea Sea Caribbean Relatively Relatively shallow shallow cores corescollected collectedabove abovethe theforaminiferal foraminiferal lysocline were sampled in order to avoid the lysoclinewere sampledin order to avoidthe effect effectrelated relatedto to partial for core partial dissolution. dissolution.Foraminiferal Foraminiferal fragmentation fragmentationfor core CP CP 6001-4 6001-4 (3645 (3645 m) m) is is less lessthan than10% 10% throughout throughoutthe the core coreto to stage stage 5, 5, reflecting reflecting good goodpreservation preservation(L. (L. Peterson, Peterson,personal personalcomcommunication, munication, 1993). 1993). Sediments Sedimentsfrom from core core TI' T1r 9108-1, 9108-1, which which is is 1100 m shallower 1-4, should should also also be be well 1100 m shallower than than CP CP 600 6001-4, well prepreserved. served.Thus Thus partial partial dissolution dissolutionof of tests testsshould shouldnot not be be aa signifsignificant V/Ca ratios ratios in in G. G. sacculifer icant effect. effect. In In core core 'FT TT 9108-1, 9108-1, V/Ca sacculifer (355-425 increase linearly linearly with with depth (355-425 j.tm) gm) increase depth from from aa core-top core-top value value of of 20 20 nmol nmol V/mol V/mol Ca Ca to tovalues valuesgreater greaterthan than500 500 nmol/mol at 140 nmol/mol at 140 cm cm (Table (Table 1). 1). Mn/Ca Mn/Ca values valuesalso alsoincrease increase from Ca •at at the from aa value valueof of less lessthan than88j.tmol gmol Mn/mol Mn/mol Ca the top top of of the the core to 270 tmol/mol at 140 cm. In core CP6001-4, V/Ca core to 270 gmol/mol at 140 cm. In core CP6001-4, V/Ca ratios ratiosin in G. G. sacculifer sacculifer(355-425 (355-4251.tm) gm) increase increasemonotonically monotonically with with depth depthfrom from aa mean meancore-top core-topvalue valueof of 20.5 20.5nmol nmolV/mol V/mol Ca Ca to to aa value valueof of 312 312nmollmol nmol/molat atstage stageSe 5e(Table (Table2). 2). Mn/Ca Mn/Ca ratios ratios also 1010J.tmol alsoincrease increasefrom fromaacore-top core-topvalue valueofofabout about •mol Mn/mol Mn/mol Ca Ca to to500 500l.tmol/mol •mol/mol at at 482 482 cm, cm,stage stageSe. 5e. In these V values values at at In these two two Caribbean Caribbean cores, cores, the the foraminiferal foraminiferal V depth depthare are 15-25 15-25 times timesgreater greaterthan thanthe thecore-top core-topvalue, value,while while the Mn/Ca values are 35-50 times higher. The the Mn/Ca values are 35-50 times higher. The correlation correlation sion coefficient coefficient of r2 r 2 = 0.98 for TT9108-1GC TT9108-1GC and and r2 r2 = = 0.80 0.80 for for CP 6001-4 (Figures 2a and 2b). Living foraminifera incorpoCP 6001-4 (Figures2a and 2b). Living foraminiferaincorporate very (<15 rate very little little Mn Mn into intotheir theirtests tests (<15j.Lmol gmol Mn/mol Mn/mol Ca), Ca), as as shown shownby by core-top core-topvalues valuesin in this thisstudy studyand andprevious previousmeasuremeasure- ments of These data ments of sediment sedimenttrap trap samples samples[Boyle, [Boyle, 1983]. 1983]. These data suggest that a Mn-rich phase, accreted after deposition, suggestthat•a•Mn-rich phase, accretedafter deposition,concontrols both in trols both the the Mn Mn and and V V contents contents of of foraminiferal foraminiferal calcite calcite in these sediments. It is oxyhydroxide these sediments. It isnot notlikely likelyto tobe beaaFeMn FeMn oxyhydroxide coating since cleaning step step used used to coating since the the reductive reductive cleaning to clean clean the the foraminiferal tests effectively removes these oxyhydroxides foraminiferal tests effectively removesthese oxyhydroxides [Boyle and Keigwin, Hastings et et al., [Boyle and Keigwin, 1985/86; 1985/86; Hastings al., 1996a]. 1996a]. Recrystallization incorporation Recrystallizationand andsubsequent subsequent incorporationof of authigenic authigenic Mn Mn in in the theforaminiferal foraminiferaltest testis isnot notplausible plausiblesince sincethe thestable stable isotopic composition of foraminifera accurately records isotopic compositionof foraminiferaaccuratelyrecordsthe the chemistry chemistryof of the thewater waterin inwhich whichthey theygrew. grew.The Themost mostlikely likely explanation is aa mixed overgrowth explanationis mixedMn, Mn, Ca Cacarbonate carbonate overgrowthon onthe the tests in sediments during teststhat thatis isdeposited deposited inthe thereducing reducing sediments duringdiagendiagenesis esisfBoy!e, [Boyle, 1983]. 1983.].A A mixed mixedMn-Ca-Mg Mn-Ca-Mg carbonate carbonatehas hasbeen been identified in many reducing environments [Lynn and identifiedin many reducingenvironments[Lynn andBonatti, Bonatti, 1965; Pedersen and and Price, and Price, 1965; Pedersen Price,1982; 1982;Shimmield $hirnrnieldand Price, 1986] 1986] and and has has been beensuggested suggestedto to control controlpore porewater waterMn Mn concenconcentrations et al., al., 1987]. carbonate trations[Middelburg [Middelburget 1987]. Such Suchmanganoan manganoan carbonate coatings are highly enriched in trace elements, including coatingsare highly enrichedin trace elements,includingBa, Ba, Cd, Cd, and and U U [Boyle, [Boyle, 1983; 1983; Russell Russell et et al., al., 1994, 1994, 1996]. Vanadium is enriched Vanadium is enrichedin in other otherMn Mn phases, phases,including includingmanmanganese ganesenodules nodules[Cronan, [Cronan,1976] 1976]and andMn-rich Mn-richmetalliferous metalliferoussedsed- 669 669 HASTINGS ET Er AL.: CALCiTE AL.:VANADIUM VANADIUM IN INFORAMINTFERAL FORAMINIF'-F_RAL CALCITE Table Table 2. 2. V/Ca, V/Ca,Mn/Ca, Mn/Ca,and andMg/Ca Mg/CaValues Valuesfor for G. G. sacculifer sacculifer Samples From Caribbean Core CP6001-4 (3654 SamplesFrom CaribbeanCore CP6001-4(3654m) m) V/Ca, V/Ca, Corrected Depth,1 Corrected Depth, 1 cm cm Mn/Ca, Mn/Ca, nniol/mol nmol/mol Mg/Ca, Mg/Ca, mmol/mol mmol/mol Itmol/mol gmol/mol Trigger TriggerWeight WeightCore Core 1 1 25.0 25.0 11 16.4 16.4 7 15.6 15.6 7 28.8 28.8 60.2 60.2 52.6 52.6 97.0 97.0 22 22 22 41 41 41 (311)) (311 3.71 3.84 8.7 8.7 47.9 47.9 47.8 47.8 3.87 3.87 3.63 3.63 3.66 3.66 3.12 3.12 3.09 3.09 125 Piston Piston Core Core 39.7 39.7 76.0 76.0 67.4 67.4 63.7 63.7 62.9 62.9 51.7 51.7 3.67 3.67 2.96 2.96 3.17 3.17 3.10 3.10 3.24 3.24 3.24 3.24 105 102 79.1 79.1 78.9 78.9 57.7 57.7 55.3 55.3 111 25.5 25.5 71.3 71.3 31.5 31.5 42 42 52 52 52 72 72 162 162 202 202 202 202 242 242 242 242 542 542 542 542 662 662 662 662 4.32 4.32 10.2 10.2 8.4 8.4 139 129 45.2 45.2 146 123 110 3.07 3.07 3.15 3.15 3.17 3.17 113 113 3.18 3.18 114 154 159 184 179 304 304 320 320 96.0 96.0 95.2 95.2 484 484 3.36 3.36 3.45 3.45 3.86 3.86 506 506 3.91 160 3.16 3.12 150 iments iments [Piper, [Piper, 1973]. 1973]. Based Basedon on the therelated relatedgeochemistries geochemistriesof of Mn and Mn and V V and and the the enrichment enrichment of of other other trace trace metals metals in in Mn Mn carcarbonate, the bonate, the Mn Mn carbonate carbonateovergrowth overgrowthwould would also alsobe bepredicted predicted to be enriched to enriched in vanadium. vanadium. To obtain V, itit is is essential to monitor To obtain reliable reliable foraminiferal foraminiferal V, essential to monitor Mn levels. Mn levels. Samples Samples with with Mn/Ca Mn/Ca > > 50 50 limol/mol !.tmot/moland and with with foramin*feral VV values values that that are are linearly foraminiferal linearly correlated correlatedwith with Mn/Ca, Mn/Ca, such suchas as those thosefrom from the theCaribbean Caribbeancores, cores,cannot cannotbe beinterpreted interpreted as indices of paleo-seawater vanadium levels. However, based as indices of paleo-seawatervanadiumlevels. However, based on measured Mn/Ca values values from from aa given on measured Mn/Ca given sample sample and and the the correlation observed correlation observed in the the Caribbean Caribbean cores cores where where the the Mn Mn carcar- bonate bonate phase phasecontrols controls the the V V content, content,we wecan canquantitatively quantitatively estimate the fraction of V from the Mn carbonate estimatethe fraction of V from the Mn carbonatephase. phase.The The estimated is 0.2 estimated V V content content of of the the Mn Mn carbonate carbonate is 0.2 and and 0.06 0.06 molar molar percent for for cores cores TT TI' 9l08-1GC percent 9108-1GCand andCP CP6001-4, 6001-4,respectively, respectively, based basedon on the the slope slopeof of the theregression regressionlines linesshown shownin in Figure Figure 2. 2. Although leaching briefly with acid (0.1 N HNO3) does Althoughleachingbriefly with acid (0.1 N HNO3) doesappear appear to to preferentially preferentiallydissolve dissolvethe-Mn the-Mn carbonate carbonatephase phaserelative relative to to the the calcite, calcite, removal removal of of the the Mn Mn carbonate carbonateis is incomplete, incomplete,and and losses associated with this modified cleaning lossesassociatedwith this modified cleaningprocedure procedureare are unacceptably high given unacceptablyhigh given the the required requiredsample samplesize. size. Eastern Equatorial Eastern Equatorial Atlantic Atlan½,ic Foraminiferal V/Ca V/Ca values valuesin in G. G. sacculifer sacculifer (355-425 tm) Foraminiferal (355-425 !.tm) from core core ENO66-17GGC EN066-17GGC are from arerelatively relativelyconstant constantin in the the upperuppermost 50 50 cm most cm (Figure (Figure 3; 3; Table Table 3) 3) and andare areconsistent consistentwith with the the global data set et al., al., 1996a]. In the the 50 global core-top core-top data set [Hastings [Hastingset 1996a]. In 50 to to 90-cm dramatically 90-cm interval, interval,V/Ca V/Ca increases increases dramaticallyto to105 105nmol/mol, nmol/mol, aa value value55 times timesgreater greaterthan thanthe thecore-top core-topvalue, value,and andthen thendoes does not not change changesignificantly. significantly. Mn/Ca Mn/Ca ratios ratios increase increaselinearly linearly with with depth depth to to values valuesof ofapproximately approximately200 200j.tmollmol !.tmol/molat at 200 200 cm. cm. Although both Mn/Ca Although both Mn/Ca and and V/Ca V/Ca increase increasewith with depth, depth,the the changes (Figure changesare are asynchronous asynchronous (Figure4). 4). 11Piston Piston core The dramatic dramatic increase increase in in foraminiferal foraminiferal vanadium vanadium from from 50 to to depths have been corrected by 25 core depths have been corrected byadding adding 25cm cmtotoaccount account The for 90 for loss lossof of core coretop. top. 90 cm cm is is due dueto topartial partialdissolution dissolutionof offoraminiferal foraminiferaltests testsfolfol- 300 300 ß I ' I ' I ' I ' I 600 6OO : (a) TT9108 ..., ....'"'• 250 25O ß I ' I ' I ' I ' I ' I (b) CP 6001 5OO 500 ' ....). ß . o E 200 200 0 400 400 0 300 3OO E o E 150 E '3 Q c1 100 lOO Q 200 200 50 5O 100 lOO 0 o 0o 0 100 100 200 200 300 500 300 400 400 500 600 600 V/Ca V/Ca(nmoVmol) (nmol/mol) • 0 0 .,.? ....... - ......+++ _•,"' I 50 50 , I , I , I , I 100 150 350 100 150 200 200 250 250 300 300 350 V/Ca V/Ca (nmol/mol) (nmol/mol) Figure TT91O8-1and and CP CP 6001-4. 6001-4. Calculated Calculated regressions Figure 2. 2. V/Ca V/Caplotted plottedversus versusMn/Ca Mn/Ca for forCaribbean Caribbeancores coresTT9108-1 regressions are intervals are shown shown by by the the dotted dotted lines. lines. (a) (a) Core Core TT9108T1'9l08are indicated indicatedby by the theheavy heavylines linesand and99% 99%confidence confidence intervalsare 1: calculated slope slope == 0.513, 1: calculated 0.513, y y intercept intercept= = -4.5, -4.5, r2=0.98, r2=0.98, and and n n == 18. 18.(b) (b) Core CoreCP CP6001-4: 6001-4:calculated calculatedslope slope= = 1.52, y intercept 1.52, y intercept= = -62.9, -62.9, r2=0.81, r2=0.81,and andnn == 23. 23. HASTINGS HF ET AL.: VANADIUM VANADIUM IN IN FORAMINIFERAL FORAMINIFERAL CALCITE 670 180 80 (%ø) (%) 44.5 4 3.5 3 Mn/Ca Mn/Ca(j.tmol/mol) (pmol/mol) G. sacculifer G. sacculifer 0 2.5 0 .._*_ E 0 9 a) .' + 3. . . . - ', 5 + - . . - - ---;. .. 200 2oo . :.-.. - - 250 25o (a) . C. C. wuellerstorfi wuellerstorfi 100 150 50 50 100 150 200 200 50 0 100 150 00 I_D . --+ - ++ . -I- -I- ................. xx . . x x o .- ,-' X 4. G. sacculifer sacculifer G. .................. ..+I- ,x + --'I. 150 15o I --x 50 50 100 lOO 200 200 I IC Q .c 100 100 ( V/Ca (nmol/mol) V/Ca (nmol/mol) V/Ca (nmoVmol) V/Ca (nmol/mol) :::::::::::::::::::::::::::::::::::::::::::::::::: ;-::::::::::::::::::::::::::::::::::::::: -II-H- . ++ - - X x - +4- + . . - ++ +::: .... :::::'. ........................ -s.- ::::::::: ............. :::::::::::::::::::::::: :.:,:,:,:.:,:.1.1,; ::::::::::::::::::::::: ++ ............... :1-:+;-.'.-,-...,,,,.,', - (b) :::::::::::::::::::::::::: (c) ) (d) - Figure 3. equatorial Atlantic at 3050 Figure 3. Eastern Eastern equatorial Atlanticcore coreEN066-17GGC EN066-17GGCat 3050 m. m. (a) (a) 6180 b]80 from from C. C. wuellerstorfi; wuellerstorfi;glacial glacial 6180 stages 1-6 are indicated on the right side of the depth profile and separated by dashed dashed lines. lines. (b) (b) Mn/Ca Mn/Ca and stages1-6 areindicatedon therightsideof the8•80 depthprofileandseparated by and (c) with depth species C. C. wuellerstoifi (c) V/Ca V/Ca variations variationswith depthin in cleaned cleanedG. G. sacculifer. sacculifer.(d) (d) V/Ca V/Ca in in cleaned cleanedbenthic benthicspecies wuellerstorfi from select from select intervals. intervals. lowing deposition. deposition. In In this this core, lowing core, mean mean V/Ca V/Ca values valuesfor for each each isotope stage stage show show aa clear on the the relative relative extent extent of of isotope clear dependence dependenceon dissolution, as estimated by the fragmentation data (Figure 5). dissolution,as estimatedby the fragmentationdata (Figure 5). Foraminiferal fragmentation fragmentation in in the Foraminiferal the >250-Jim >250-I.tm fraction, fraction, aa relireliable indicator indicator of of dissolution, is 6-7% 6-7% for for stage able dissolution,is stage 11 and and glacial glacial stage 2, 33and stage 2, increases increasesto to 13% 13%and and23% 23%for forstages stages and4,4,respecrespectively, and decreases to 10% for interglacial stage [Curry and and tively, and decreases to 10% for interglacialstage55 [Curry Lohmann, Lohmann,1986]. 1986]. Based Basedon on this thisindex, index,the thegreatest greatestdissolution dissolution would be be expected with would expectedin in stage stage4, 4, consistent consistent withfragmentation fragmentation data data from from adjacent adjacentcores coresat at the theSierra SierraLeone LeoneRise. Rise.Individual Individual foraminifera of aa given foraminiferaof givenspecies speciesin in the the85 85 to to90-cm 90-cminterval intervalare are visibly than those those at visibly more more dissolved dissolved than at or or above above50 50 cm cmwhen when viewed to viewed at at 40x 40x magnification, magnification, lending lending qualitative qualitative support support to this In the only the the 00 to this conclusion. conclusion.In the following following sections, sections,only to 5050- cm interval is considered from this this core this is is the cm interval is considered from core because because this the only section artifacts only sectionlargely largely free free of of postdepositional postdepositional artifactsrelated related to partial to partial dissolution dissolutionof of foraminifera foraminifera tests. tests. Since aa Mn-rich Since Mn-rich phase phasehas hasbeen beeninferred inferredtotoaffect affectsignifisignificantly the vanadium content of foraminifera even cantly the vanadium content of foraminifera even at at Mn/Ca Mn/Ca ratios below the V/Ca V/Ca values values from ratios below100 100j.LmolJmol, gmol/mol, the from core coreEN066EN066- 117GGC 7GGChave have been been corrected corrected for for the the amount amount of of vanadium vanadium expected to to be expected be in in the thecontaminant contaminantphase. phase.Based Basedon on the the slope slope of shown of the theregression regression shownin in Figure Figure2b 2bbetween betweenMn/Ca Mn/Ca and andV/Ca V/Ca in the in the Caribbean Caribbean core core CP CP 6001-4, 6001-4, 0.06 0.06 molar molar percent percent V V is is predicted to to be be in predicted in the theMn Mn carbonate carbonatephase. phase.Sediments Sedimentsfrom from CP CP 6001-4 6001-4 are are more morerepresentative representativeof of the thepelagic pelagicconditions conditions encountered at the Sierra Leone Rise in the equatorial encounteredat the Sierra Leone Rise in the equatorialAtlantic; Atlantic; the the shallower shallowerwater waterdepth depthand andhigher higherproductivity productivityof of overlying overlying water water suggest suggestthat that sediments sedimentsfrom from TT9108-IGC TT9108-1GC are are more more reducing with reducing with higher higher pore pore water water[Mn] [Mn] than thanthose thosefrom fromeither either CP 6001-4 located in in the of the Caribbean Sea or or the the CP 6001-4 located the middle middle of Caribbean Sea equatorial Atlantic. Atlantic. The The correction equatorial correctionfor for Mn Mn carbonate carbonateassumes assumes that some that someMn Mn is is incorporated incorporatedinto into the theforaminiferal foraminiferaltest testwhen when it is it is originally originallyformed. formed.Based Basedon on the the average averageMn/Ca Mn/Ca value value of of 15 limol/mol 15 I.tmol/mol for for reductively reductivelycleaned cleanedplanktonic planktonicforaminifera foraminifera collected and the collectedin in sediment sedimenttraps traps[Boyle, [Boyle, 1983] 1983] and the range rangeof of core-top in this core-topMn Mn values valuesdetermined determinedin this work work (5-10 (5-10 imolJmol), !.tmol/mol), an for an average averagevalue valueof of Mn/Ca Mn/Ca = = 10 10 tmol/mol I.tmol/molhas hasbeen beenchosen chosen for lattice-bound overgrowth lattice-boundMn. Mn. The The Mn Mn carbonate carbonate overgrowthcorrection correction for for V/Ca V/Ca is is (V/Ca)corr ((Mn/Ca)m 10)xO.6 (V/ Ca)cor r =(V/Cm (V/ Ca)m - ((Mn/ Ca)m - 10)x0.6 (1) (1) where (V/Ca)m where (V/Ca)m and and(Mn/Ca)m (Mn/Ca)m refer refer to to measured measuredratios ratiosin in the the cleaned calcite inin units units of of nmol cleaned foraminiferal foraminiferal calcite nmol V/mol V/mol Ca Ca and and jimol I.tmol Mn/mol Mn/mol Ca, Ca, respectively. respectively.Corrections Corrections are are small: small: less less than than 15% 15% of of the theinitial initialvalues, values,except exceptfor forthe the50-cm 50-cminterval, interval, which which is is corrected correctedby by 30%. 30%. Unless Unlessstated statedotherwise, otherwise,all all V/Ca V/Ca data presented in the following sections are corrected data presentedin the following sectionsare correctedvalues. values. The The mean mean corrected correctedV/Ca V/Ca value value of of 21.6 21.6 (±2.8; (+2.8; n=14) n=14) nmollmol nmol/mol for for this this 35-kyr 35-kyr period period(Figure (Figure6) 6) compares compareswell well with with the themean mean global value of of V/Ca = 21.7 global core-top core-topvalue V/Ca = 21.7 (±3.6) (+3.6) for for G. G. sacculifer sacculifer [Hastings [Hastingset et al., al., 1996a]. 1996a]. The The trends trends in in the the vanadium vanadium measurements measurements for for benthic benthic species speciesC. C. wuellerstorfi wuellerstorfi(250-425 (250-425lIm) gm) are aresimilar similarto to the theplankplank- tonic tonic profile, profile, with with aa constant constantV/Ca V/Ca value valueof of109 109nmol/mol nmol/mol (±10%) in the top 30 cm and a significant increase (+10%) in the top 30 cm and a significantincreaseat at 90 90 cm cm (Figure con(Figure 3d; 3d; Table Table 4). 4). The Theexisting existingbenthic benthicmeasurements measurements confirm species. firm the thetrend trendobtained obtainedfrom fromthe theplanktonic planktonic species. 671 671 HASTINGS Er AL: VANADIUM IN CALCITE HASTINGS ET AL.: VANADIUM INFORAMINIFERAL FORAMINIFERAL CALCITE Table for From Equatorial Atlantic Table3. 3.V/Ca, V/Ca,Mn/Ca, Mn/Ca,and andMg/Ca Mg/CaValues Values forG. G.sacculifer sacculifer FromEastern Eastern Equatorial AtlanticCore CoreEN066-17GGC EN066-17GGC Depth, Depth, cm cm Age, Age, ka ka V/Ca, V/Ca, nmol/mol nmol/mol (V/Ca)co,' 1 (V/Ca)corr 22.1 22.1 (31.0) (31.0) 21.6 21.6 20.0 24.8 1 0.8 22.1 1 3.5 3.5 3.5 3.5 0.8 0.8 (31.0) (31.0) 21.6 21.6 20.0 1 10 2.9 2.9 8.3 10 8.3 8.3 20 16.0 20 16.0 24 24 30 30 37 37 50 70 70 70 70 90 90 90 90 110 130 130 130 150 150 170 190 190 200 200 200 200 210 210 210 210 220 220 220 220 230 230 230 230 240 240 240 240 250 250 250 250 18.5 18.5 22.4 22.4 22.4 22.4 26.9 26.9 34.8 46.7 46.7 46.7 58.6 58.6 58.6 58.6 69.0 69.0 24.8 17.8 17.8 142 142 28.2 20.9 51.5 41.3 76.6 76.6 68.9 71.8 62.2 62.2 61.8 62.7 62.7 48.5 48.5 66.1 -2.0 -2.0 -8.5 -8.5 32.3 32.3 4.6 4.6 43.4 43.4 44.9 44.9 93.5 93.5 21.0 21.0 107 6.1 140 140 (347) 103 --17.2 19.6 106 19.1 122 122 11.4 123 123 59.5 105 97.1 103 108 110 97.3 97.3 97.3 111.9 111.9 126.5 126.5 122 110 113 86.0 86.0 83.5 135 121 145 145 136 136 199 199 98.0 98.0 103 99.5 99.5 19.9 19.9 19.4 3.74 3.74 3.66 3.66 3.66 3.73 3.82 3.64 3.37 3.48 3.48 3.11 3.10 3.22 3.12 2.93 2.93 2.99 2.91 3.03 3.03 3.00 3.00 3.09 3.09 3.21 3.51 3.49 3.49 3.48 3.48 3.70 3.70 3.76 3.76 3.75 3.75 3.73 3.73 3.93 3.93 3.88 3.88 3.52 3.52 3.38 3.38 3.57 3.57 3.44 3.44 3.45 3.45 3.73 3.73 3.64 3.66 3.66 3.68 3.68 3.66 3.66 3.78 3.78 7.5 ;; 11.1 11.1 • 10.3 10,3 33.1 33.1 30.5 69.8 18.5 18.5 Mg/Ca, MgtCa, minol/mol mmol/mol 2.3 2.3 2.3 2.3 8.0 8.0 8.9 6.4 6A •'i. 23.3 23.3 24.7 24.7 20.3 20.3 21.1 21.1 82.7 147.0 147.0 147.0 147.0 153.8 153.8 153.8 160.6 160.6 160.6 160.6 167.6 167.6 167.6 167.6 17.8 17.8 Mn/Ca, Mn/Ca, iniol/mol gmol/mol 24.0 24.9 22.4 23.0 21.2 22.8 24.1 82.7 82.7 126. 126.5 133.4 133.4 133.4 133.4 140.2 140.2 140.2 140.2 nmol/mol nmol/mol 13.6 13.6 13.1 13,1 14.5 14.5 14.8 14.8 17.9 18.2 18.2 26.0 26.0 30.8 30.8 30.6 30.6 44.7 44.7 43.9 43.9 47.3 67.1 67.1 70.6 70.6 85.3 85.3 87.9 87.9 68.4 68.4 123 128 143 160 160 141 127 146 146 166 166 120 120 117 117 , , Foraininiferal V of Foraminiferal V values valuesbelow below50 50cm cmare arenot notreliable reliabledue dueto topartial partialdissolution dissolution oftests. tests. = -_((Mn/Ca) -- 10) text). 1(V/Ca)0 (V/Ca)corr _ (V/Ca)measd (V/Ca)measured ((Mn/Ca) 10)xx 0.6 0.6(see (see text). Since of VV in in sediments is 44 orders Since the the concentration concentration of sediments is orders of of magnitude calcite, magnitudehigher higherthan thanthat thatin inforaminiferal foraminiferal calcite,the thepotenpotenfor exchange between the two phases tial exists exists for exchange between the two phases if if foraminiferal is not lattice foraminiferal vanadium vanadium is lattice bound. In sediments sediments from from core core EN066-17GGC, EN066-17GGC, the the bulk bulk V V content contentisisrelatively relativelyconstant constant with with depth depth (Table (Table 5) 5) and andshows showsno nocorrelation correlationwith with foraminiferal foraminiferalvanadium vanadium(Figure (Figure7), 7), which whichvaries variesconsiderably considerably downcore. downcore.This This suggests suggeststhat thatthe thevanadium vanadiumin inforaminiferal foraminiferal calcite calcite is is aa closed closedsystem systemwith with respect respectto tobulk bulksedimentary sedimentaryV V and does not exchange over the 1 60-kyr period represented by and doesnot exchangeover the 160-kyrperiodrepresented by the the core. core. Sensitivity SensitivityAnalysis Analysis A based on A simple simpletime-dependent time-dependentmodel model based on the the marine marinemass mass balance balancefor for vanadium vanadiumis is used usedto toevaluate evaluatethe thesensitivity sensitivityof ofdisdissolved V V to solved to changes changesin in the theareal arealextent extentof of anoxic anoxicand andsuboxic suboxic sediments over time, time, as as well and sedimentsover well as as the the other otherimportant importantsource sourceand sink sink terms. terms. 9C •C R +A50F0 A0F0 AaFa H Vo'•- ==JR +Aso Fso - Ao Fo- Aa Fa- JH V0 (2) where is the the ocean of whereV0 Vo is oceanvolume; volume;C C is isthe theconcentration concentration ofvanavana- 672 67 2 HASTINGS ET FT AL.: VANADIUM HASTINGS VANADIUM IN IN FORAMINIFERAL FORAMINIFERAL CALCITE CALCITE 200 V/Ca (nmoVmol) V/Ca (nmol/mol) 0 0 0 150 150 ß -i- .,i- E 100 lOO 20 20 10 10 ' ' ' ' I 4, 4, 10 10 40 40 30 30 I •,• -i- 50 ++ + + + .4 0 20 w20 D) ß 0 0 (1 + ++ I 50 5O i i 100 1O0 i I 0 +4-;o i 150 + 0o 4- + 0 30 30 V/Ca V/Ca(nmol/mol) (nmol/mol) Figure 4. V/Ca versus Figure 4. Foraminiferal Foraminiferal V/Ca versus Mn/Ca Mn/Ca for for eastern eastern equatorial Atlantic equatorial Atlantic core core EN066-17GGC. EN066-17GGC. + 0 40 40 (0 dium F0, flux diumin in seawater; seawater; Fo,Fa, Fa,and andF50 Fsoare areequal equalto tothe thevanadium vanadium flux from sediments, respectively; A0, fromoxic, oxic,anoxic anoxicand andsuboxic suboxic sediments, respectively; Ao, Aa, and A50 are equal to the areal extent of oxic, anoxic, Aa, andAso are equalto the areal extentof oxic, anoxic,and and suboxic sediments; R is is the the fiveddne nverine flux flux of of vanadium; vanadium;and andJH H suboxic sediments; JR is removal Fe oxides. is removalby by hydrothermally hydrothermallyproduced producedFe oxides.An An instaninstantaneous change change is is assumed at tt == 00 using seawater taneous assumedat usingthe thepresent present seawater value of 36 nM. Removal to anoxic and oxic sediments and valueof 36 nM. Removalto anoxicandoxicsediments andby by hydrothermally derived derived Fe oxyhydroxides oxyhydroxides is modeled modeled as as a firstfirsthydrothermally 25 25 I ' I ' I carbonate phase plotted versus calendar age carbonate phase plotted versus calendar agein inthe theuppermost uppermost 50 equatorial Atlantic EN066-17GGC. 50 cm cmof ofeastern eastern equatorial Atlanticcore core EN066-17GGC. Corrected V/Ca V/Ca values values (see (see text) by Corrected text)are areindicated indicated byplus plussigns, signs, and values by anduncorrected uncorrected valuesare areindicated indicated by open opencircles, circles,for forcomcomparison. Vertical line the coreparison. Verticaldashed dashed lineindicates indicates theglobal globalmean mean coretop determined earlier et topvalue valueof of21.7 21.7nmol/mol nmol/mol determined earlier[Hastings [Hastings etal., al., 1996b]. 1996b]. sented sentedin in the theappendix. appendix. The sensitivity of to changes changes in source The sensitivity of[V]5 [V]swto sourceand andsink sinkterms terms is in A0, is illustrated illustrated in Figure Figure8. 8.Increasing Increasing Aa, the theareal arealextent extentof of anoxic sediments, sediments, by by a factor anoxic factorof 10 10 from from 0.3% to to 3% 3% of of ocean ocean 15 cn. O5 o$ c•,w V/Ca a'• Figure 6. Figure 6. V/Ca V/Ca values values corrected correctedfor vanadium vanadiumin the theMn Mn order A description of the orderreaction. reaction. A complete complete description of themodel modelis isprepre- 20 20 .2 - aE 2E OD) oo) V/Ca corrected corrected V/Ca V/C , sediments results results in in aa 12-nM sediments 12-nM or or 32% 32% decrease decreasein in vanadium vanadium © concentration after 50 50 kyr, kyr, or concentration after or about abouthalf halfaaglacial glacialcycle. cycle. Increasing A50, the area area of of suboxic sediments, by aa factor Increasing Aso,the suboxic sediments, by factorof of 55 from fromthe thecurrent currentestimated estimatedvalue valueof 2.5% 2.5% to to 12.5% 12.5%of of the theocean ocean bottom increase bottomresults resultsin in aacorresponding corresponding increaseof 22 22 nM nM or or62% 62% after after50 50 kyr. kyr. 10 10 LU o 5 5 0 ß 0 I , 20 20 I 40 , Table 4. Mn/Ca from Table 4. V/Ca V/Caand and Mn/CaValues Valuesfor forC. C.wuel!erstorfl wuellerstiirfi from I 60 6o 80 V/Cacorr (nmol/mol) V/Cacorr (nmol/mol) Figure 5. V/Ca Figure 5. Mean Meancorrected corrected V/Ca for for isotope isotopestages stages1, 1, 2, 2, 3, 3, and 44 shown fragmentation in and shownas asaafunction functionof offoraminiferal foraminiferal fragmentation in eastern equatorial eastern equatorialAtlantic Atlanticcore coreEN066-17GGC. EN066-17GGC. Each Eachisotope isotope stage number stage is is indicated indicatedby bythe thecorresponding corresponding numberinside insidethe the open circle. circle. Error Error bar bar for the actual range of of open for stage stage33 indicates indicatesthe actualrange foraminiferal V/Ca V/Ca values values for for that foraminiferal thatinterval, interval.Increasing IncreasingV/Ca V/Ca with increasing is consistent with increasingfragmentation fragmentationis consistentwith with earlier earlier data, data, indicating that that foraminiferal V is is sensitive indicating foraminiferal V sensitiveto to partial partialdissodisso- lution of of tests. lution tests. Eastern Equatorial 1 7GGC Eastern EquatorialAtlantic AtlanticCore CoreEN066EN066-17GGC Sample SampleDepth. Depth, cm cm 2-6 2-6 6-11 6-11 25-30 25-30 30-35 30-35 86-90 86-90 V/Ca, V/Ca, nmol/mol nmol/mol 119 Mn/Ca, Mn/Ca, Itmol/mol gmol/mol 11.1 11.1 110 110 14.8 14.8 98 32.0 (243) (243) 104 104 57.8 202 673 673 HASTINGS EF AL.: AL.: VANADIUM CALCITh HASTINGS ET VANADIUM IN IN FORAMINIFERAL FORAMINIFERAL CALCITE Table Bulk Metal Table 5. 5. Bulk Metal Concentrations Concentrationsfor for Equatorial EquatorialAtlantic Atlantic Core EN0661 7GGC EN066-17GGC Depth, Mn, Depth, CaCO3,' CaCO3,1Mn, % cm ppm cm % ppm 10 40 75 53 53 % % V, V, ppm ppm Al, AI, 516 516 0.86 0.86 34.6 34.6 2.11 546 546 2.34 2.34 77.0 77.0 4.44 4.44 measurable change change within within this almeasurable this interval. interval.Postdepositional Postdepositional alterations including terations including partial partial dissolution dissolutionand and Mn Mn carbonate-overcarbonate-•vergrowth are are not not important in this growth important in this interval, interval, as as reflected reflected in in the the Fe, Fe, % 70 58 58 411 2.06 2.06 61.7 61.7 4.27 4.27 100 36 36 299 299 1.30 1.30 43.8 43.8 3.05 3.05 130 130 67 3.14 3.14 89.5 89.5 5.33 5.33 160 160 56 56 58 276 276 370 370 317 317 287 287 342 342 190 220 220 250 250 45 38 38 value = 10) di value of of 0.3% 0.3% to to 3% 3% (relative.change (relative•ehange = 10) and and the the extent extento• suboxic did not not change = 1), suboxicsediments sedimentsdid change(relative (relative change change= 1), the the vanadium concentration of of seawater vanadium concentration seawaterwould would be be expected expectedto to decrease by 30% over 35 kyr, as indicated by the in decreaseby 30% over 35 kyr, as indicatedby the asterisk asteriskin --- 70.0 70.0 --- 1.77 54.2 3.47 3.47 2.16 2.16 76.1 4.60 4.60 -- 86.5 86.5 -- 1I Percent Percent carbonate carbonate data from Curry and Lohmann [1986]. data from Curry and Lohmann [1986]. Because the the riverine Because riverine input input and andhydrothermal hydrothermalactivity activity are are major terms major termsin in the themarine marinevanadium vanadiumcycle, cycle,possible possibletemporal temporal variability in in these variability these processes processesmust must also also be beconsidered. considered. Changes in chemical erosion and riverine input Changesin chemical erosion and riverine inputover overglacialglacialinterglacial cycles cycles are interglacial are not not well well constrained. constrained.Recent Recentcalculacalculations based tions basedon on changes changesin in global globalbicarbonate bicarbonatefluxes fluxesestimate estimate that chemical chemical erosion erosion at at 18 that 18 ka ka B.P. B.P. was wasonly only 20% 20% greater greaterthan than present-day values values [Gibbs present-day [Gibbsand and Kump, Kump, 1994]. 1994]. A A 20% 20% increase increase by only only 22 nM, in chemical erosion would wouldincrease increase[V]sw []5 by in chemicalerosion nM, or or 6%, relative 6%, relative to to present presentconditions. conditions.Doubling Doubling the the riverine riverine input, as as predicted by Froelich Froelich et input, predictedby et al. al. [1992], [1992], would wouldresult resultin in aa 35% increase in V (12.5 nM) over 50 kyr, clearly a significant 35% increasein V (12.5 nM) over 50 kyr, clearly a significant change (Figure (Figure 8). 8). Riverine Riverine flux flux is is assumed to be be constant in change assumedto constantin the interpretation the interpretationof of the the foraminiferal foraminiferaldata. data. Change is one of Changein in the the rate rateof of seafloor seafloorspreading spreadingis one indicator indicatorof variations in in metalliferous variations metalliferoussediment sedimentproduction productionat at hydrotherhydrothermal spreading mal spreadingcenters. centers.Precise Preciseestimates estimatesof of seafloor seafloorspreading spreading rates indicate rates indicate that that plate plate motions motionshave have been beennearly nearly constant constant (±3%) over the the past (_+3%) over past 5 5 million million years years [Wilson, [Wilson, 1993]. 1993]. Plate Plate velocities averaged averaged over over shorter shorter timescales timescales of of several velocities several to to aa dozen years years agree agree within within _+5% ±5% to to those over the the past dozen those over past 33 Myr Myr [Gordon, [Gordon, 1993]. 1993]. Consequently, Consequently,removal removal by by hydrothermally hydrothermally produced Fe Fe and produced and Mn Mn rich rich oxides oxidesis is assumed assumedto to be beconstant. constant. Figure 9. Figure 9. From 35 From 35 kyr kyr B.P. B.P. to tothe thepresent, present,corrected correctedV/Ca V/Ca values valuesare are constant in constant in equatorial equatorialAtlantic Atlanticcore coreEN066-17GGC. EN066-17GGC.Analysis Analysis of benthic of benthicspecies speciesC. C. wuellerstorfi wuellerstorficonfirms confirmsthe the result resultof of no no foraminiferal fragmentation fragmentation data, data, which which indicate indicate no no change foraminiferal changein in dissolution intensity. intensity. Mn/Ca Mn/Ca levels levels are are low low (<30 (<30 •tmol/mol), imoI/mol), dissolution and correlation correlation between between foraminiferal Mn and uncorrected V V is and foraminiferal Mn poor that a a poorover overthis thisperiod period(r2 (r2 == 0.4; 0.4;Figure Figure4), 4), demonstrating demonstrating that Mn carbonate overgrowth phase is not important. Mn carbonate overgrowth phase is not important. Foraminiferal Foraminiferal vanadium vanadium data indicate that that there there was was no no measurmeasurable in [V]5. the in ablechange changein [V]sw.Consequently, Consequently, thechanges changes in the theextent extent of reducing by the of reducingsediments sedimentspredicted predictedby themodel modelare areconstrained constrained by the of the ±12%, by the standard standarddeviation deviation.of themeasurement, measurement, _+12%,which whichis is indicated by by the the stippled region in in Figure indicated stippledregion Figure9. 9. The The model modelresults results indicate indicatethat that assuming assumingno no change changein in the thearea areaof of suboxic suboxicsedisediments, the areal extent of anoxic sediments did not by ments,the arealextentof anoxicsediments did notincrease increase by more morethan thanfivefold, fivefold, or or 1.5% 1.5% of of the theocean oceanfloor, floor,over overthe thepast past 35 kyr. kyr. This This is arrows 35 is shown shownby by the theannotated annotated arrowsat atyy == 11and andxx == 5. 5. Alternatively, Alternatively, given given aa constant constantarea areaof ofanoxic anoxicsediments, sediments, suboxic are by suboxicsediments sediments arepredicted predictednot notto tohave havedecreased decreased bymore more than 0.5 times than 0.5 times or or enlarged enlargedby by more morethan than1.5 1.5 times timesthe the current current value. Assumingaa current current value value of of 2.5% [Hastings and value. Assuming 2.5% [Hastings and Emerson, 1995] Emerson, 1995] this this corresponds correspondsto to 1.3-3.5% 1.3-3.5% of of total totalocean ocean sediments. Simultaneous increases in in both sediments. Simultaneous increases bothvariables variablesmight mightbe be expected. For For example, example, aa fivefold increase in in the the area expected. fivefold increase area of of anoxic sediments constrains the the change in suboxic anoxic sedimentsconstrains changein suboxicsediments sediments from approximately approximately 1I to from to 22 times timesthe thecurrent currentvalue. value. Since in sedSincereal-world real-worldchanges changes in the theareal arealextent extentof ofreducing reducing sed- iments are are not another iments not likely likely to to be beinstantaneous, instantaneous, anothermodel model was was 100 + -I- 80 80 + + + E60 + 60 Discussion Discussion To interpret data, the To interpret the the foraminiferal foraminiferal data, the model model and and V V mass mass balance presented presented earlier earlier are are used used to changes in in the balance to constrain constrainchanges the area area of of reducing reducingsediments. sediments.The The change changein in the the vanadium vanadiumconconcentration of seawater that would be predicted 35 kyr kyr after centrationof seawaterthat would be predicted35 after an an instantaneous change instantaneous changein in the theareal arealextent extentof ofanoxic anoxicand andsuboxic suboxic sediments is is illustrated illustrated in in Figure sediments Figure 9. 9. Since Sincethe the changes changesin in subsuboxic and oxic and anoxic anoxicsediments sedimentsare arelikely likely to to be becorrelated, correlated,different different combinations of of changes 1, combinations changesare are shown. shown.The The modem modernvalue valueatatx= x=l, y=l y=l isisindicated indicatedby byaasolid solidsquare. square.Using Usingthe themodem modernvalues values estimated for for the the extent estimated extent of of anoxic anoxic and and suboxic suboxic sediments sediments of of 0.3% 0.3% and and2.5%, 2.5%, respectively, respectively,the thepercent percentchanges changesin in seawater seawater V V concentration concentrationare are given given in in this thiscontour contourplot. plot. For For example, example,if if the area area of of anoxic anoxic sediments sediments increased increased tenfold tenfold from from the the current current +,,I- + + 40 + 20 20 0 ß 0 0 , ß i ! 50 50 i i i i I i 100 • 100 i i i 150 150 V/Ca (nmol/mol) V/Ca (nmol/mol) Figure 7. Figure 7. Solid Solidphase phasevanadium vanadiumversus versusforaminiferal foraminiferalvanavanadium in in eastem dium easternequatorial equatorialAtlantic Atlanticcore coreEN066-17GQC. EN066-17GGC. HASTINGS Er AL.: AL.: VANADIUM HASTINGSET VANADIUMIN INFORAMINIFERAL FORAMINIFERALCALCITE CALCITE 674 200 200 Asuboxic A suboxic x5 x5 150 150- 100 100a) 0 5050- - J river J river x2 x2 -T x *.- x Aanoxjc A anoxic x5 x5 AanoxjcXlO A anoxic x I 0 50 -50 00 40 20 60 80 100 100 time time (kyr) (kyr) in the concentrationinin seawater seawateras as aa function function of of time Figure Figure 8. 8. Percent Percentchange changein the vanadium vanadiumconcentration time after after an an instantaneous fivefold and aafiveinstantaneous fivefold(crosses) (crosses) andtenfold tenfold(triangles) (triangles)increase increasein in the thearea! arealextent extentof ofanoxic anoxicsediments, sediments, fivefold (circles), fold increase increasein in suboxic suboxicsediments sediments (circles),and andaadoubling doublingof ofriverine riverineinput input(squares). (squares). created using totoilluscreated usingaa variable variableinput. input.This Thismodel modelisispresented presented illustrate the ininthe trate the effect effectof ofgradual gradualchanges changes thearea! arealextent extentofofsubsuboxic sediments. oxic sediments.Since Since changes changesin in the the areal arealextent extentof ofanoxic anoxic sediments result in in relatively sedimentsresult relatively minor minor changes changesin in [V], [V]sw comcompared with pared with suboxic suboxicsediments, sediments,Aa Aa is isassumed assumedto to be beconstant. constant. Changes in the Changesin the area areaof of suboxic suboxicsediments sedimentsare areproportional proportionalto to 10 ' 9 9• observed changes in in the 6180 observed changes thestacked stacked •5180SPECMAP SPECMAPrecord record [Martinson er al., [Martinson et al., 19871. 1987]. Two Two different different solutions solutionsare areprepresented, given an an increase in the sented,given increasein the areal areal extent extentof of suboxic suboxicsedisedi- 8 • ments by by aa factor ments factor of of 2x 2x and and4x 4x during duringthe thelast lastglacial glacialmaximum maximum (LGM) (Figure 1 Oa). The value for A50 at other times is (LGM) (Figure 10a). The value for Aso at othertimes is scaled scaled to the of to the curve curveshown shownin in Figure FigurelOb. 10b.Concentrations Concentrations of vanadium vanadium in seawater by stepping in time in seawaterwere were calculated calculatedby steppingforward forward in time using using the the model model described describedearlier earlier but but reassigning teassigningthe the initial initial condicondition tion every every 66 kyr. kyr. Foraminiferal V/Ca V/Ca values values limit limit the Foraminiferal the changes changesof of [V] [V]swwithin within the the last last35 35 kyr kyr to toless lessthan than±12%, +12%,or orabout about±4 +4nM, nM, equivalent equivalent to to aa maximum maximumglacial-interglacial glacial-interglacialdifference differenceof of 24%. 24%. For Forthe the 7 6 5 4 • +50% 3 scenario in which scenario in which suboxic suboxic sediments sediments are are doubled, doubled, the the variability variability reflected reflectedin in model-derived model-derived[V]5 [V]sw is is 33 nM nM (8%), (8%), 0 1 I 23 45 2 3 4 5 6 7 7 8 9 10 10 Relative change in in extent Relative change extent of of anoxic anoxicsediments sediments Figure 9. Figure 9. Percent Percentchange changein in the thevanadium vanadiumconcentration concentrationof of seawater predicted 35 change seawaterpredicted 35 kyr kyr after afteran aninstantaneous instantaneous changein in the the areal areal extent extent of of anoxic anoxic and and suboxic suboxic sediments. sediments. Units Units for for the the x x and y axes and y axesare arechanges changesin in their theirareal arealextent extentcompared comparedwith with the the modern modern values values of of 0.3% 0.3% and and2.5% 2.5%respectively. respectively.The Themodern modern value at x=l, x=I, y=l value at y=l isisindicated indicatedby bythe thesolid solidsquare. square.The The stippled stippled region corresponds to the region correspondsto theprecision precisionof of the theV/Ca V/Cameasurement measurement (±12%), whichisisused usedas asaa proxy proxyfor for[V]sw. [V]. Changes (+12%), which Changesin in the the areal over the the past areal extent extent of of reducing reducingsediments sedimentsover past 35 35 kyr kyr are are constrained to this constrainedto thisstippled stippledgray grayregion. region. which is within Aso is which is within this thismargin marginof of error. error.For For the thecase casewhere whereAso is changes by 99 expanded by a factor of 4, model-derived [V] expandedby a factor of 4, model-derived[V]sw changesby nM, or the last nM, or 25%, 25%, during during the last 35 35 kyr, kyr, which which is is larger largerthan thanthe the measurement error. error. Given Given aa variable input for measurement variable input for Aso Aso based basedon on changes in 6180, VV data constrain the changes •5180,the theforaminiferal foraminiferal data constrain theinincrease in crease in the area! areal extent extent of of suboxic suboxic sediments sediments to to no no more more than than aa factor factorof of 4, 4, equivalent equivalentto to10% 10%of oftotal totalocean oceansediments. sediments. Previous reconstructions of sediment Previous reconstructions of sediment redox redox conditions conditions difdiffer in of Cd fer in their their conclusions. conclusions. Based Based on on enrichments enrichments of Cd and and U U in in glacial Subantarctic glacial Subantarcticsediments, sediments,Rosenthal Rosenthaler etal. al.[1995] [1995]sugsuggest that gest that higher higherglacial glacialproductivity productivitymight mighthave haveincreased increasedthe the areal extent to 16% areal extent of of reducing reducing sediments sediments to 16% during during the the last last glacial maximum, twice the the current glacial maximum, twice currentvalue valueof of 8%. 8%. Other Otherrecords records from the the Sea of Japan Japan [Piper [Piper and from Sea of and Issacs, Issacs,1995] 1995] and andthe the southsouth- 675 675 HASTINGS Er AL.: HASTINGSET AL.:VANADIUM VANADIUMIN INFORAMINIFERAL FORAMINIFERALCALCITE CALCITE (a) (a) 60 60 A. x 50 50- - U) ci) x x Aso= 4x 4x at at LGM LGM Aso= 0O Aso= 2x 2x at at LGM LGM Aso= F 40 •' 40 X)cxx 30 30- - : : XXx x : : : x ß :... : : -C C) x 20 20- - *0 00 10 - ß : 0 000.0* ox o o o : ß : o o x x :X X X X X X 0ø',000090 000*00 0 40 20 0 0 60 60 80 80 100 100 120 120 time (kyrBP) time (kyr BP) (b) I 1 I I I fF I ---I; C 0 C-) C = U- 0.5 0.5 4' 0 , 0 0 II 20 20 , I I , 40 40 I 60 60 , I 80 80 , I 100 1 O0 , I 120 120 time (kyr time (kyrBP) BP) Figure changes for for the the areal areal extent of subFigure 10. 10. (a) (a) Percent Percentchange changein in[V]5 [V]sw relative relativeto to 36 36 nM, nM, given given gradual gradualchanges extentof suboxic by and at the oxic sediments. sediments.A0 Asoisisincreased increased byaafactor factorof of22(crosses) (crosses) and 44 (squares) (squares)at the last lastglacial glacialmaximum maximumand andis is scaled for scaledto to the theoxygen oxygenisotope isotopestratigraphy stratigraphy for the theother othertimes timesusing usingthe thefunction functionplotted plottedin in Figure FigurelOb. 10b. eastern Indian eastern Indian Ocean Ocean [McCorkle [McCorkle et et al., al., 1994] 1994] also also suggest suggest more reducing more reducingconditions conditionsin in glacial glacial sediments. sediments.The The observed observed ical ical and and chemical chemical alteration alteration of of the the tracers tracers used used to to make make the the estimates. estimates. increase in export increasein exportproduction productionin in the theglacial glacialSouthern SouthernOcean, Ocean, based on several radionuclide proxies and coincident with an basedon severalradionuclideproxiesand coincidentwith an 35 kyr, kyr, global 35 global changes changesin in the theareal arealextent extentofofsuboxic suboxicsedisedi- increase in in lithogenic increase lithogeniciron iron input inputfrom fromwind-blown wind-blowndust dust[Kumar [Kurnar be expected to et al., 1995], et al., 1995], would would be expectedto to drive drive glacial glacial sediments sedimentsto more By contrast, more reducing reducingconditions. conditions.By contrast,the the existence existenceof of aa Mn Mn spike with aa pulse spike in in Panama Panamabasin basinsediments sedimentscoincident coincidentwith pulsein in organic organiccarbon carbonis is evidence evidencethat thatoverlying overlyingwaters watersin in the theeastern eastern equatorial Pacific could could not not be equatorialPacific be suboxic suboxicand andmust musthave havebeen been [Yang et al., 1995] since the mechanism to preserve oxic oxic [Yang et al., 1995] since the mechanismto preservethe the Mn Mn spike spikerequires requiresan an oxic oxic cap cap [Calvert [Calvertand andPederson, Pederson,1996]. 1996]. These eastern These easternequatorial equatorialPacific Pacificsediments sedimentsare areparticularly particularlysensensitive sitive to to potential potentialchanges changessince sinceaa pulse pulsein inorganic organiccarbon carbonrerelated 1.a.tedto to higher higherproductivity productivityseen seenduring during the the last last glacial glacialwould would be expected be expectedto to drive drive them themto to aamore morereduced reducedstate. state.These Theseestiestimates matesof of sediment sedimentredox redoxconditions conditionsare aretypically typicallyqualitative, qualitative, restricted restrictedto to local local environments, environments,and andsubject subjectto topossible possiblephysphys- The The foraminiferal foraminiferalvanadium vanadiumdata data indicate indicatethat that over over the thepast past ments and and sediments by anoxic ments sediments overlain overlain by anoxic bottom bottom water water were were minimal. If our minimal. If our understanding understandingand and assumptions assumptionsabout about the the V V mass massbalance balanceare are correct, correct,this thisexcludes excludesthe thepolar polarnutrient nutrientmodmodels els as asdescribed describedby by simple simplebox box model modelsimulations simulations[Knox [Knox and and 1984; Sarmiento McElroy, 1984; 1984; McElroy, Sarrniento and and Toggweiler, Toggweiler, 1984; Siegenthaler and Wenk, Siegenthalerand Wenk,1984] 1984] as as aa viable viableexplanation explanationfor for reduced pCO2 during reducedatmospheric atmosphericpCO2 duringthe the last lastglacial glacial period. period.The The nutrient global nutrientdepletion depletionmodel model using usingaa three-dimensional three-dimensional globalcircirculation model is is also culation model also not not viable, viable, as as it it results resultsin in bottom bottom water water oxygen depletion of of 40 I.tM 02 02 [Sar,niento oxygen depletion 40 IxM [Sarrnientoand andOrr, Orr,1991], 1991], leading Indian leading to to anoxic anoxic sediments sedimentsin in the thesouthwestern southwestern Indian Ocean. Ocean. Although difficult to to quantify, quantify, an an increase in the the areal Although difficult increasein areal extent extent of of suboxic suboxicsediments sedimentswould wouldbe be expected expectedto toaccompany accompanyan anexexpansion pansionin in anoxic anoxic sediments. sediments.Both Both of of these thesechanges changesare are large large 676 676 HASTINGS ET EF AL.: AL: VANADIUM CALCiTE HASTINGS VANADIUM IN IN FORAMINIFERAL FORAMINIFERAL CALC• enough enoughto to be bereflected reflectedin inthe thevanadium vanadiumforaminiferal foraminiferalrecord, record, yet change yet there thereis isno nodetectable detectable changein inthe theV/Ca V/Cavalues. values. Other mechanisms mechanisms to to explain pCO22 during the last last Other explain lowered loweredpCO duringthe glacial maximum rely on alkalinity changes in the ocean. The glacial maximum rely on alkalinity changesin the ocean.The polar 1989] polar alkalinity alkalinity hypothesis hypothesis [Broecker [Broecker and and Peng, Peng, 1989] increases increases the the alkalinity alkalinity of of glacial glacialage ageCircumpolar CircumpolarDeep Deep Water, Water, which which results resultsin in an analkalinity alkalinity increase increasein in Antarctic Antarcticsursurface waters reduced pCO2. face watersand andconsequently consequently reducedatmospheric atmospheric pCO 2.An An increase increase in in the theorganic organiccarbon/CaCO3 carbon/CaCO3ratio ratioof ofmaterial material changes in in deep by changes deepwater water oxygen oxygenlevels levelsand andcan canbe beconstrained constrained by the data the dataset setpresented presentedhere. here.Polar Polarnutrient nutrientdepletion depletionscenarios scenarios require significant oxygen reductions reductions in in bottom require significant oxygen bottom waters waters that that would have have led would led to an an increase increase in in the the areal areal extent extent of of anoxic anoxic sedsed- iments and iments and suboxic suboxicsediments sedimentsin in parts partsof of the theworld word ocean. ocean.The The foraminiferal vanadium vanadium data data indicate foraminiferal indicatesuch suchchanges changeshave havenot not occurred over the based occurredover the past past35 35 kyr. kyr. Those Thosescenarios scenarios basedon onalkaalkalinity changes in the little or or no linity changesin the global globalocean, ocean,which which require requirelittle no change with changein in deep deepwater wateroxygen, oxygen,are areconsistent consistent with the theconstant constant V/Ca V/Ca values valuesobserved observedin in this thisstudy. study. reaching reachingthe the sediments sedimentswould wouldincrease increaseorganic-carbon-driven organic-carbon-driven dissolution and drive up the [CO3=] dedissolutionand drive up the [CO3=]with witha acorresponding corresponding decrease pCO2 creasein in atmospheric atmospheric pCO2 [Archer [Archerand andMaier-Reimer, Maier-Reimer,1994]. 1994]. Appendix Appendix The [Opdyke The coral coralreef reefhypothesis hypothesis [Opdykeand andWalker, Walker,1992] 1992]relies re}ieson on decreased The model in decreasedcoral coral growth growth in in shallow shallowsurface surfacewaters watersduring during glacial glacial The model for for sensitivity sensitivityof of the thevanadium vanadiumconcentration concentration in times with a corresponding shift of CaCO3 deposition to the seawater to changes in the redox state of sediments is based times with a correspondingshift of CaCO3 depositionto the seawaterto changesin the redox stateof sedimentsis basedon on deep the oceanic the oceanic mass balance balance for for vanadium. vanadium. deepsea. sea.These Thesemodels modelsrequire requireless lessor or no no deep deepwater wateroxygen oxygen reduction, reduction,no no additional additionalanoxic anoxicsediments, sediments,and andno no significant significant increases in the increases in the areal areal extent extent of of suboxic suboxic sediments. sediments. The The foraminiferal vanadium vanadium data data presented in this foraminiferal presentedin this work work support support explanations explanationsthat that require requireminimal minimal excursions excursionsin in the theareal arealexextent tent of of anoxic anoxicor or suboxic suboxicsediments sedimentsover over the the past past35 35 kyr. kyr. Conclusions Conclusions Downcore profiles of of foraminiferal in the Downcore profiles foraminiferal vanadium vanadium in the Caribbean Caribbeanand and Atlantic Atlantic basins basinsreveal reveal the the utility utility of of V/Ca V/Ca ratios ratios as a paleotracer of seawater vanadium. Postdepositional efas a paleotracerof seawatervanadium.Postdepositional effects, fects,including includingpartial partialdissolution dissolutionand andprecipitation precipitationof of an anininferred ferredMn Mn carbonate carbonateovergrowth overgrowthphase, phase,can cansignificantly significantlyalter alter the in theoriginal originalV/Ca V/Ca values. values.Such Sucheffects effectsmust mustbe beconsidered considered in all all efforts efforts to to interpret interpret the the data. data.The Theadvantage advantageof of examining examiningaa conservative tracer such conservative tracer such as as vanadium vanadium is is that thatonly only one oneacceptacceptable able core core is is required requiredto to yield yield aaglobal globalrecord recordof ofchanges changesin in vanadium The top 35 vanadiumconcentrations. concentrations. The top 50 50 cm cm(representing (representing 35 kyr) kyr) of such a core in the eastern equatorial Atlantic yields of such a core in the easternequatorialAtlantic yieldssuch suchaa record. record. Using Using foraminiferal foraminiferal V V data data from from the theCaribbean Caribbeanwhere wherethe the Mn phase controls controls the the VV content of the the tests, Mn carbonate carbonate phase content of tests, aa minor was applied applied to to the the top minor correction correctionwas top 50 50 cm cm of of the theeastern eastern equatorial to for equatorialAtlantic Atlantic V V measurements measurements to compensate compensate for the the V V in in this contaminant phase. The this contaminantphase. The corrected correctedV/Ca V/Ca profile profile for for this this interval reveals interval revealsno no change, change,indicating indicatingthat that within within the the error errorof of the the measurement measurement (±12%), (_+12%), seawater seawater vanadium vanadium levels levels have have not not changed over the changedover the past past35 35 kyr. kyr. Applying Applying an an oceanic oceanicmass massbalbalance ance for for vanadium vanadiumand andaatime-dependent time-dependentmodel, model,this thisconstant constant value constrains the changes in the areal extent of value constrainsthe changesin the areal extent of anoxic anoxicand and suboxic suboxicsediments. sediments.Assuming Assumingno no change changein in the thearea areaof ofanoxic anoxic sediments, the area sediments,the area of of suboxic suboxicsediments sedimentsis is predicted predictednot not to to have have changed changedby by more more than than0.5 0.5 to to1.5 1.5times timesthe thecurrent currentvalue, value, which to 1.3-3.5% of the which corresponds corresponds to 1.3-3.5% of the total total area areaof of ocean oceansedsediments. This This is is the iments. the first first effort effort to to quantify quantifythe the global global change changein in suboxic over late suboxic sediments sediments over late Pleistocene Pleistocene climate climate cycles. cycles. Alternatively, assuming Alternatively, assumingaa constant constantarea areaof ofsuboxic suboxicsediments, sediments, the areal the areal extent extentof of anoxic anoxicsediments sedimentsdid did not notincrease increaseby by more more than than five-fold, five-fold, or or 1.5% 1.5% of of the theocean oceanfloor floorover overthe thepast past35 35kyr. kyr. Using Using aa numerical numericalmodel model where where changes changesin in the the extent extentof of subsubi8O, the oxic are to in oxicsediments sediments areproportional proportional to changes changes in •5•O, the increase in in suboxic increase suboxic sediments sediments is constrained constrained to no more more than than aa factor of of 4, factor 4, equivalent equivalentto to 10% 10% of of total totalocean oceansediments. sediments. Model simulations presented to to explain in atmoModel simulations presented explain changes changesin atmospheric pCO2 during the glacial period differ in their predicted sphericpCO2 duringthe glacial perioddiffer in their predicted 3C + A50F50 - A0F0 - Aa Fa - -'H = Vø•tt = JR+ asoFso - aoFo - aaFa - JH V0 where V0 where Vo is is the the ocean oceanvolume; volume; C C is is the theconcentration concentrationof of disdissolved vanadium vanadium in in seawater; F0, solved seawater; Fo,Fa, Fa,and andF50 Fsoare arethe thevanadium vanadium flux from oxic, anoxic and suboxic sediments, respectively; flux from oxic, anoxicand suboxicsediments, respectively; A0, Aa, A50 are are equal equal to to the A o, A a, and andAso theareal arealextent extentof ofoxic, oxic,anoxic, anoxic, and suboxic R is is the the riverine and suboxicsediments; sediments'JR riverineflux flux of of vanadium; vanadium;and and H is is removal produced iron JH removalby by hydrothermally hydrothermally produced ironoxides. oxides. Sinks are (C): Sinks are proportional proportionalto to vanadium vanadiumconcentration concentration (C): F0 'h =eC F o ==j3C; tiC' Fa Fa = = aC aC. Jh = eC.Here, Here,B, 13,a, Ix,and and care œaresolved solved for using values, C C=o are for usingpresent-day present-dayvalues, t=o== 36 36nM. nM.Constants Constants are V0 = 1.37 cm3; A ==3.61 xx1018 cm2; Vo = 1.37xx1024 1024 cm3'A 3.61 1018 cm2'.1,. Jr==5.4 5.4Xx108 108 mol F0 nmol cm2 Assumed present day molyrt; yr-l'and and Fo==0.12 0.12 nmol cm-2yr* yr-1. Assumed present day values are = 2.5% 2.5% A A and and A Aaa = = 0.3% values areA50 Aso= 0.3%A. A. Solution is Solution is C= --+ A X exp (-7t) 7 where where R+A,F5 X=C1_0_!. = Ao13+Aaa+e R+AsoFso Aofi +V0 Aaa +e X Ct= 0 . V0 - 7r Vo Vo Acknowledgments. sediment cores from Acknowledgments. Ocean Ocean sediment cores fromthe theSierra SierraLeone Leone Rise were graciously graciouslyprovided providedby by Bill the Rise (EN066-17GGC) (EN066-17GGC) were Bill Curry Curry and andthe Seafloor Institution. SeafloorSamples SamplesLab Lab of of the theWoods WoodsHole HoleOceanographic Oceanographic Institution. Caribbean 108) was was kindly kindly provided provided by by the Caribbeancore core(TT-9 (TT-9108) the Oregon OregonState State University core core library University library and and funded fundedby by NSF NSF grant grant0CE94-02298. OCE94-02298. Samples -4 were Samplesfrom fromCaribbean Caribbeancore coreCP600I CP6001-4 werecheerfully cheerfullysupplied suppliedby by Larry who stable isotope values and LarryPeterson, Peterson, whoalso alsoprovided provided stable isotope values andbackground background information for for the information the core. core. Jack Jack Fassett Fassett at at National National Institute Institute of of Standards Standards and Technology kindly the enriched 50V isotope spike spike necesnecesand Technology kindlyprovided provided the enriched 50Visotope sary for sary for this thiswork. work.Louis LouisLu Luand andTooba ToobaDurrani Durranihelped helpedpick pickmany, many, many of the Critical reviews reviews by by Steve Lea, many theforarninifera. foraminifera.Critical SteveCalvert, Calvert,David DavidLea, Harry Elderfield, Elderfield, Yair Yair Rosenthal, Rosenthal,Ross RossHeath, Heath, Ann Ann Russell, Russell,and and Tamara Tamara Nameroff Support for Nameroffgreatly greatlyimproved improvedthis thismanuscript. manuscript. Support forthis thiswork workwas was provided 16316 and 165providedby byNSF NSFgrant grantOCE-91 OCE-9116316 andby byACS-PRF ACS-PRFgrant grant25 25165AC8 A C8 to to S. S.Emerson. Emerson.Kristin Kristin Orians Oriansand andthe theUniversity Universityof of British British Columbia provided provided support of Columbia supportduring duringthe thepreparation preparation ofthis thismanuscript. manuscript. This contribution This is is University Universityof of Washington WashingtonSchool Schoolof ofOceanography Oceanography contribution 2151. 2151. References References Archer, sedimentary Archer, D., D., and andE. E.Maier-Reimer, Maier-Reimer,Effect Effectof ofdeep-sea deep-sea sedimentary calcite on CO2 Nature, 367, calcitepreservation preservation onatmospheric atmospheric CO2 concentration, concentration, Nature, 367, 260-263, 260-263, 1994. 1994. HASTINGS ET EF AL.: VANADIUM HASTINGS VANADIUM IN IN FORAMINIFERAL FORAMINIFFJLa, L CALCITE Bard, E., B. Hainelin, Hamelin, R. G. Fairbanks, Fairbanks,and and A. Zindler, Calibration Calibration of the the C-14 C-14 timescale timescaleover overthe thepast past30,000 30,000years yearsusing usingmass massspectrometric spectrometric U-Th ages from corals, Nature, Nature, 345, 345,405-410, U-Th ages from Barbados Barbadoscorals, 405-410,1990. 1990. Bard, and 230Th-234U Bard,E. E. M., M., M. M. Arnold, Arnold,R. R.G. G.Fairbanks, Fairbanks, andB. B.Hasnein, Hamelin, 230Th-234U and ages obtained obtained by by mass on corals, corals, Radiocarbon, Radiocarbon, and14C 14C ages massspectrometry spectrometry on 35, 191-199, 1993. 1993. Barnola, J. M., Barnola,J. M., D. D. Raynaud, Raynaud,V. V. S. S. Korotkevich, Korotkevich,and andC. C. Lorius, Lorius,Vostok Vostok ice core provides CO2. Nature, ice coreprovides160,000 160,000year yearrecord recordof of atmospheric atmospheric CO2, Nature, 329, 408-413, 329, 408-413, 1987. 1987. Bender, M., The The Dole the last Bender,M., Dole effect effect and andits its variations variationsduring duringthe last 130,000 130,000 677 677 water water by by isotope isotopedilution dilutioninductively inductivelycoupled coupledplasma plasmamass mass spectrometly with vaporization, Anal. spectrometry withelectrothermal electrothermal vaporization, Anal.Chem., Chem.,68, 68, 371371378, 378, 1996b. 1996b. Holland, H. H. D., D., The Atmosphere and and Oceans, Holland, The Chemical ChemicalEvolution Evolutionof of the theAtmosphere Oceans, 582 N. J., 1984. 582 pp., pp., Princeton PrincetonUniv. Univ. Press, Press,Princeton, Princeton, N.J., 1984. Imbrie, J., J., J. I. D. A. McIntyre, A. C. C. Mix, Mix, J. J. J. J. Imbrie, D. Hays, Hays,D. D. G. G. Martinson, Martinson,A. Mcintyre, A. Morley, J. Shackleton, The Morley, N. N. G. G. Pisias, Pisias,W. W. L. L. Prell, Prell,and andN. N.J. Shackleton, Theorbital orbital theory climate: of theoryof of Pleistocene Pleistocene climate:Support Supportfrom fromaa revised revisedchronology chronology of the 6180 in and part 1,1,edited themarine marine õ180record, record, inMilankovitch Milankovitch andClimate, Climate, part edited by D. Reidel, Reidel, Norwell, by A. A. L. L. Berger, Berger,pp. pp. 269-305, 269-305,D. Norwell,Mass., Mass.,1984. 1984. years as as measured in the Imbrie, J., J., et.al, et.al, On On the and years measuredin the Vostok Vostok ice ice core, core, Global Global Biogeochem. Biogeochem. Imbrie, thestructure structure andorigin originof of major majorglaciation glaciationcycles, cycles, Cycles, 8, 363-376, 1. Linear Linear responses responses to to Milankovitch Milankovitch forcing, forcing,Paleoceanography, Paleoceanography, 7, 7, Cycles,8, 363-376, 1994. 1994. 1. Boyle, E. E. A., on foraminifera tests, 701-738, 701-738, 1992. 1992. Boyle, A., Manganese Manganesecarbonate carbonateovergrowths overgrowthson foraminiferatests, Geochim. Cosmochim. Acta, 47, 47, 1815-1819, Jouzel, Geochim. Cosmochim.Acta, 1815-1819, 1983. 1983. Jouzel,J., J., et.al., et.al.,Extending Extendingthe theVostok Vostokice-core ice-corerecord recordof ofpaleoclimate paleoclimate Boyle, E. E. A., of Atlantic Atlantic and and Pacific Pacific to the the penultimate penultimate glacial glacial period, period, Nature, Nature, 364, 364,407-412, Boyle, A., and and L. L. D. D. Keigwin, Keigwin, Comparison Comparisonof to 407-412,1993. 1993. paleochemical records records for for the the last Kadko, D., An of scavenging within paleochemical last215,000 215,000 years: years:Changes Changesin in deep deep Kadko,D., An assessment assessment of the theeffect effectof ofchemical chemical scavenging within ocean circulation circulation and and chemical chemical inventories, inventories,Earth EarthPlanet. Planet. Sci. Sd. Lett., Lett., submarine hydrothermal plumes plumes upon upon ocean ocean geochemistry, geochemistry, Earth Earth ocean submarinehydrothermal 76, Planet. Sci. 76, 135-150, 135-150, 1985/86. 1985/86. Planet. Sci. Lett., 120. 120, 361-374, 1993. 1993. Breit, G. G. N., N., and in carbonaceous carbonaceous Keigwin, L. D., Breit, andR. R. B. B. Wanty, Wanty,Vanadium Vanadiumaccumulation accumulation in Keigwin, L. D., and and G. G. A. A. Jones, Jones,Western WesternNorth NorthAtlantic Atlanticevidence evidencefor for rocks: millenial-scale changes changes .in in ocean and J. Geophys. rocks' A A review review of ofgeochemical geochemicalcontrols controlsduring duringdeposition depositionand and millenial-scale oceancirculation circulation andclimate, climate,J. Geophys. diagenesis, C/rem. Res, 1994. Res,99, 99,12,397-12,410, 12,397-12,410, 1994. diagenesis, Chem.Geol., Geol.,91, 91, 83-97, 83-97,1991. 1991. Broecker, W. The of to Klinkhammer, G. G. P., P., and and M. M. R. R. Palmer, in the the oceans: oceans: Where Where itit Palmer,Uranium Uraniumin Broecker, W.S., S.,and andT. T.H. H.Peng, Peng, Thecause cause ofthe theglacial glacial tointerglacial interglacial Klinkhammer, atmospheric Global goes Acta, atmosphericCO2 CO2 change: change'A A polar polaralkalinity alkalinityhypothesis, hypothesis,Global goesand andwhy, why,Geochim.et Geochim.etCosmochim. Cosmochim. Acta,55, 55, 1799-1806, 1799-1806,1991. 1991. Knox, F., F., and and M. M. McElroy, in atmospheric CO2: Influence Influence of of Biogeochem. Cycles, 3, 215-239, Knox, McElroy,Changes Changesin atmospheric CO2: Biogeochem. Cycles,3, 215-239,1989. 1989. Calvert, Geochemistry of biota.at I. Res., 1984. Calvert, S. S. E., E., and andT. T. F. F.Pedersen, Pedersen, Geochemistry of Recent Recentoxic oxic and and biotaathigh highlatitudes, latitudes, J.Geophys. Geophys. Res.,89, 89,4629-4637, 4629-4637, 1984. anoxic for the Kumar, Kumar, N., R. F. Anderson, Anderson,R. R. A. A. Mortlock, Mortlock,P. P.N. N. Froelich, Froelich,P. P.Kubik, Kubik,B. B. anoxic marine marine sediments: sediments:Implications Implicationsfor the geological geologicalrecord, record, Mar. Dittrich-Hannen, and biological and Mar. Geol., Geol., 113, 67-88, 67-88, 1993. 1993. Dittrich-Hannen, andM. M. Suter, Suter,Increased Increased biologicalproductivity productivity and Calvert, S. Sedimentary geochemistry ofofmanexport production Calvert, S.E., E.,and andT.T.F.F.Pederson, Pederson, Sedimentary geochemistry manexport productionin in the theglacial glacialSouthern SouthernOcean, Ocean,Nature, Nature,378, 378,675675680, 1995. ganese: implications of 1995. ganese' implicationsfor for the theenvironment environment of formation formationof ofmanganifmanganiferous black shales, erous shales,Econ. Econ. Geol.,91, Geol.,91, 36-50, 36-50, 1996. 1996. Legeleux, F., J.-L. Legeleux,F., J.-L. Reyss, Reyss,P. P. Bonte, Bonte,and andC. C.Organo, Organo,Concomitant ConcomitantenenCollier, vanadium richments and arsenic Collier,R. R. W., W., Particulate Particulateand anddissolved dissolved vanadiumin in the theNorth NorthPacific Pacific richments of of uranium, uranium, molybdenum molybdenum and arsenic in in suboxic suboxic Ocean, Nature, 309, 441-444, 1984. Ocean, Nature, 309, 441-444, 1984. continental margin OceanoL continental marginsediments, sediments, Oceanol.Acta, Acta,17, 17,417-429, 417-429,1994. 1994. Colodner, D., R. Rhenium -redox redux, Eos H. P., P., G. G. A. Longerich, H. A. Jenner, Jenner, B. B. J. J. Fryer, Fryer, and and S. S. E. E. Jackson, Jackson, Colodner, D.,R. R.An, An,and and R.F.F.Anderson, Anderson, RheniUmredon.redux, Eos Longerich, Trans. Trans. AGU, AGU, 75, 397, 1994. 1994. Inductively spectrometric analysis Inductivelycoupled coupledplasma-mass plasma-mass spectrometric analysisof of geologigeologiCronan, D. S., Manganese nodules and other ferromanganese oxide decal A based Chem. Cronan, D. S.,Manganese nodules andother ferrømanganese oxidedecal samples: samples: A critical criticalevaluation evaluation basedon oncase casestudies, studies, Chem. posits, in Chemical Oceanography, vol. 5, edited by J. Riley and R. Geol., 18, 1990. posits,in Chemical Oceanography,vol. 5, editedby•J. Riley andR. Geol., 83, 83, 105-1 105-118, 1990. Chester, pp. pp. 217-263, San Lynn, C., and of Chester, 217-263,Academic, Academic, SanDiego, Diego,Calif., Calif.,1976. 1976: Lynn, D. D.C., andE. E. Bonatti, Bonatti,Mobility Mobility of of manganese manganesein in diagenesis diagenesisof Crusius, J., T. and Rhenium and molybdedeep-sea Mar. Geol., Crusius, J.,S. S.Calvert, Calvert, T.Pedersen Pedersen andD. D.Sage, Sage, RheniUm and.molybdedeep-seasediments, sediments,Mar. Geol.,3, 3, 457-474, 457-474, 1965. 1965. num enrichments in sediments Martin, Fitzwater, and and R. R. Gordon, Gordon, Iron hon in waters, Nature, Nature, num enrichments in sedimentsas as indicators indicatorsof of oxic, oxic, suboxic, suboxic,and and Martin, J., J., S. S. Fitzwater, in Antarctic Antarctic waters, anoxic conditions conditions of of deposition, Earth Planer. Sd. Lett., 345, 156-158, anoxic deposition, Earth Planet•Sci: Lett.,in inpress, press, 156-158, 1990. 1990. 1996. 1996. Martinson, D. G., G., N. Martinson,D. N. G. G. Pisias, Pisias,J. J. D. D. Hays, Hays,J. J.Imbrie, Imbrie,T. T. C. C. Moore, Moore, and and Curry, W. W. B., carbonate sedimenCurry, B., and andG. G. P. P.Lohmann, Lohmann,Late LateQuaternary Quaternary carbonate sedimenN. J. Shackleton, N.J. Shackleton,Age Age dating datingand andthe theorbital orbitaltheory theoryof of the theice iceages: ages: tation at at the the Sierra Mar. Geol., tation Sierra Leone Leone Rise, Rise, Mar. Geol., 70, 223-250, 1986. 1986. Development of aa high-resolution 00 to Developmentof high-resolution to300,000 300,000year yearchronostratigchronostratigCurry, W. W. B., B., and past particle particle fluxes fluxesin Curry, andG. G. P. P. Lohmann, Lohmann,Reconstructing Reconstructing past in raphy, Res.,27, raphy, Quat. Quat. Res., 27, 1-29, 1-29, 1987. 1987. the tropical tropical Atlantic Atlantic Ocean, Ocean, Paleoceanography, Paleoceanography, 5, 487-505, 1990. the 5,.'48:7-505,• I990. McCorkle, D. C., H. Glacial-Holocene McCorkle,D.C., H. H. 'H.Veeh, Veeh,and andD. D.T.T.Heggie, Heggie, Glacial-Holocene Dean, W. W. E., E., Changes in Dean, Changesin in redox redoxconditions conditions in deep-sea deep-seasediments sedimentsof of the the paleoproductivity off off western paleoproductivity westernAustralia: Australia: A A comparision comparisionof of proxy proxy subarctic North North Pacific subarctic Pacificocean: ocean:Possible Possibleevidence evidencefor for the the presence presenceof of records, in in Carbon on records, CarbonCycling Cyclingin in the the Glacial Glacial Ocean: Ocean:Constraints Constraints onthe the North Pacific deep water, Paleoceanography, 4, 639-653, 1989. North Pacificdeepwater,Paleoceanography, 4, 639-653, 1989. Ocean's Ocean'sRole Role in in Global GlobalChange, Change,edited editedby byR. R.Zahn, Zahn,M. M. Kaminski, Kaminski,L. L. Dubois, L. L. G., G., and W. L. Prell, Effects of carbonate dissolution on the and L. Prell, Effects of carbonatedissolution on the Labeyrie, and ASISer., SerA, Labeyrie, andT. T. Pederson, Pederson,NATO NATO ASI Ser., Ser A, 17, 17, 443-479, 443-479, 1994. 1994. radiocarbon age age structure radiocarbon structureof of sediment sedimentmixed mixedlayers, layers,Deep DeepSea SeaRes., Res.., McLaren, J., D. McLaren, J., D. Beauchemin, Beauchemin, and and S. S. Berman, Berman, Determination Determination of of trace trace 35, 1875-1885, 35, 1875-1885, 1988. 1988. metals in in marine maxinesediments sedimentsby by inductively inductivelycoupled coupledplasma plasmamass mass Emerson, S. S. S. Huested, Ocean anoxia and the Emerson, S.R., R.,and and S.S. Huested, Ocean anoxia and theconcentrations concentrationsmetals spectrometry, I. spectrometry, J.Anal. Anal.At. At. Spectrom., Spectrom.,2, 2, 277-281, 277-281,1987. 1987. of molybdenum molybdenum and and vanadium Mar. C/rem., 34, 177-196, of vanadiumin in seawater, seawater,Mar. Chem.,34, 177-196, Middelburg, J., G. G. J. de Lange, and C. H. van Middelburg, J., J. de Lange, and C. H. vander derWeijden, Weijden,Manganese Manganese 1991. 1991. solubility solubilitycontrol controlin in marine marinepore porewaters, waters,Geochim. Geochim.Cosmochim. Cosmochim.Acta, Acta, Froelich, P. R. S. W. A. Froelich, P.N., N.,V. V. Blanc, Blanc, R.A. A.Mortlock, Mortlock, S.N. N.Chillrud, ChillrUd, W.Dunstan, Dunstan, A. 51, 759-763, 1987. 51,759-763, 1987. Udomkit, and T.-H. T.-H. Peng, Peng, River River fluxes fluxes of of dissolved dissolved silica silica to to the the Udomkit, and Opdyke, B. N., N., and Opdyke,B. and J. J. C. C. G. G. Walker, Walker, Return Returnof of the the coral coralreef reef hypothesis: hypothesis: ocean ocean were were higher higher during during glacials: glacials: Ge/Si Ge/Si in in diatoms, diatoms,rivers, rivers, and and Basin to shell partitioning of CaCO3 and its effect on atmospheric Basin to shelf partitioning of CaCO 3 and its effect on atmospheric oceans., Paleoceanography, 7, 739-767, 1992. oceans.,Paleoceanography,7, 739-767, 1992. CO2. Geology, 20, 20, 733-736, CO 2, Geology, 733-736,1992. 1992. Gibbs, Global chemical erosion the Gibbs,M. M. T., T., and andL. L. R. R.Kump, Kump, Global chemical erosio n during during thelast last Pedersen, T. F., and N. B. of carPedersen, T. F., and N. B.Price, Price,The Thegeochemistry geochemistry ofmanganese manganese carglacial maximum glacial maximumand andthe thepresent: present:Sensitivity Sensitivityto to changes changesin in,lithology lithology bonate in Panama bonate PanamaBasin Basinsediments, sediments,Geochim. Geochim.Cosmochim. Cosmochim.Acta, Acta, 46, and Paleoceanography, 9, andhydrology, hydrology,Paleoceanography, 9,529-543, 529-543,1994. 1994. 59-68, 1982. 1982. Gordon, R. R. G., G., Orbital dates and rates, Nature, 364, Gordon, Orbital dates andsteady steady rates, Nature, 364,760-761, 760-761, Pedersen, T. F., F., M. J. S. Pedersen, T. M. Pickering, Pickering,J. S. Vogel, Vogel, J. J. N. N. Southon, Southon,and and D. D. E. E. 1993. 1993. Nelson, The of cycles .Nelson, The response response of benthic benthicforaminifera foraminiferato toproductivity productivity cycles Hastings, D. W., balance and Hastings,D. W., Vanadium Vanadiumin in the theocean: ocean:A A marine marinemass mass-balance and in constraints in the theeastern easternequatorial equatorialPacific: Pacific:Faunal Faunaland andgeochemical geochemical constraints paleoseawater record, record, Ph.D. Wash., Seattle, paleoseawater Ph.D. dissertation, dissertation,Univ. Univ. of of:Wash., Seattle, on bottom water water oxygen oxygen levels, levels, Paleoceanography, Paleoceanography, 3, 3, 157on glacial glacial bottom 1571994. 1994. 168, 1988. Hastings, D., and S. The marine geochemistry of Hastings, Di, and S.Emerson, Emerson, The marine geochemistry ofvanadium, vanadium, 168, Peterson, L. C., of Eos Trans. Trans. AGU, AGU, 76, 303, 1995. 1995. Peterson,L. C., Late LateQuaternary Quaternarypaleoceanography paleoceanography of the themiddepth middepth Atlantic: Proxy Proxy records records from from the the deep Hastings, D. W., W., S. J. Erez, inAtlantic: deepCaribbean CaribbeanSea, Sea, Eos Eos Trans. Trans. Hastings,D. S. Emerson, Emerson,J. Erez, and andB. B. K. K. Nelson, Nelson,Vanadium VanadiuminAGU, corporation in in foraininiferal calcite as as aa paleotracer AGU, 71, 71, 1382-1383, 1382-1383, 1990. 1990. corporation foraminiferal calcite paleotracerfor for seawater seawater vanadium Acta, in in press, Piper, sediments from vanadium concentrations, concentrations,Geochim. Geochim. Cosmochim; Cosmochim.:Acta, press, Piper,D. D. Z., Z., Origin Originof ofmetalliferous metalliferous sediments fromthe theEast EastPacific Pacific 1996a. 1996a. Rise.; Earth Planet. Planet. Sci. Sd. Lelt., Rise.;•Earth Lett., 19,75-82, 19, 75-82, 1973. 1973. Hastings, D. W., and B. Determiiiation of Piper, D. D. Z., Minor in sediments Hastings,D. W., S. S. E. E.Emerson, Emerson,'and B.Nelson, Nelson,i:Dete'rmination of Piper, Z., and andC. C. M. M. Isaacs, Isaacs, Minorelements elements inQuaternary Quaternary sediments picogram quantities quantities of from AArecord ofofsurface-water productivity and picogram of vanadium vanadiumin in foraminiferal foraminiferalcalcite calciteand andseaseafromthe theSea Seaof ofJapan: Japan: record surface-water productivity and 678 67 8 HASTINGS Er AL.: HASTINGS ET AL.: VANADIUM VANADIUM IN IN FORAMIMFERAL FORAMINIFERAL CALCITE CALCITE intermediate intermediate water water redox redox conditions, conditions,Geol. Geol.Soc. Soc.Am. Am. Bull., Bull., 107, 107, 545467, 1995. 1995. Reimers, C. McCorkle, Reimers, C. C. E., E., R. R. A. A. Jahnke, Jahnke,and andD. D.C. McCorkle, Carbon Carbonfluxes fluxes and and burial burial rates ratesover over the the continental continentalslope slopeand and rise rise off off central centralCalifornia California with with implications implicationsfor for the theglobal globalcarbon carboncycle, cycle,Global GlobalBiogeochem. Biogeochern. Cycles, 6, 6, 199-224, Cycles, 199-224, 1992. 1992. Rosenthal, Rosenthal,Y., Y., E. E. A. A. Boyle, Boyle,L. L. Labeyrie, Labeyrie,and andD. D. Oppo, Oppo,Glacial Glacialenrichenrichments sediments: A mentsof of authigenic authigenicCd Cd and andU U in inSubantarctic Subantarctic sediments: A climatic climatic control control on on the the elements' elements'oceanic oceanicbudget?, budget?,Paleoceanography, Paleoceanography,10, 10, 395-413, 395-413, 1995. 1995. Rudnicki, M. D., and Rudnicki, M.D., andH. H. Elderfield, Elderfield, A A chemical chemicalmodel modelof of the thebuoyant buoyant and andneutrally neutrallybuoyant buoyantplume plume above abovethe the TAG TAG vent vent field, field, 26N, 26N, MidMidAtlantic Acta, AtlanticRidge., Ridge.,Geochim. Geochirn.Cosnwchim. Cosrnochirn. Acta,57, 57, 2939-2958, 2939-2958,1993. 1993. Russell, A. D., S. Russell, A.D., S. Emerson, Emerson, B. B. K. K. Nelson, Nelson, J. J. Erez, Erez, and andD. D. W. W. Lea, Lea, Uranium calcite Uranium in in foraininiferal foraminiferal calcite as as a a recorder recorder of of seawater seawater uranium uranium concentrations, concentrations,Geochim. Geochirn.Cosmochim. Cosrnochirn.Acta, Acta, 58, 58, 671-681, 671-681, 1994. 1994. Russell, A. D., S. A. C. The use use of of Russell, A.D., S. Emerson, Emerson, A. C. Mix, Mix, and and L. L. C. C. Peterson, Peterson, The foraminiferal U/Ca U/Ca as as an an indicator foraminiferal indicatorof of changes changesin in seawater seawateruranium uranium content, in content,Paleoceanography, Paleoceanography, inpress, press,1996. 1996. Sarkar, and Sarkar,A., A., S. S. K. K. Bhattacharya, Bhattacharya, andM. M. M. M. Sarin, Satin,Geochemical Geochemicalevidence evidence for for anoxic anoxicdeep deepwater waterin in the theArabian ArabianSea Seaduring duringthe thelast lastglaciation, glaciation, Geochim. Cosmochim. Geochirn. Cosrnochirn.Acta, 57, 1009-1016, 1009-1016, 1993. 1993. Sarmiento, J. J. L., L., and and J. of the the imimSarmiento, J. C. C. Orr, Orr, Three-dimensional Three-dimensional simulations simulationsof pact Ocean CO2 pactof of Southern Southern Oceannutrient nutrientdepletion depletionon onatmospheric atmospheric CO2 and and ocean oceanchemistry, chemistry,Limnol. Lirnnol.Oceanogr., Oceanogr.,38, 38, 1928-1950, 1928-1950,1991. 1991. Sarmiento, J. J. L., L., and and R. R. Toggweiler, A new new model model for for the the role role of of the the Sarmiento, Toggweiler, A oceans in pCO2, Nature, 308, oceans in determining determiningatmospheric atmospheric pCO2, Nature, 308, 621-624, 621-624, 1984. 1984. Seralathan, P., P., and and vanadium in sediSeralathan, and M. M. Hartmann, Hartmann,Molybdenum Molybdenumand vanadiumin sediment cores and their ment cores from from the the NW-African NW-African continental continental margin margin and their relations to relations to climatic climatic and andenvironmental environmental conditions, conditions, Meteor Meteor Forschungsergeb., Reihe Forschungsergeb., ReiheC, C, 40, 40, 1-17, 1-17,1986. 1986. Shiller, A.M., A. M., and Shiller, andE. E. A. A. Boyle, Boyle,Dissolved Dissolvedvanadium vanadiumin in rivers riversand andestuestuaries, 86,214-224, aries, Earth Earth Planet. Planet. Sci. Sci. Lett., Lett., 86, 214-224, 1987. 1987. Shimmield, G. G. B., B., and Shimmield, and N. N. B. B. Price, Price,The Thebehavior behaviorof of molybdenum molybdenumand and manganese during early early sediment manganese during sediment diagenesisOffshore diagenesis-OffshoreBaja Baja California, Mar. Mar. Chem., California, Chern.,19, 19, 261-280, 261-280, 1986. 1986. Siegenthaler, U., and and 1. CO2 and Siegenthaler, U., T. Wenk, Wenk,Rapid Rapidatmospheric atmospheric CO2 variations variationsand ocean circulation, Nature, 308, ocean circulation, Nature, 308, 624-625, 624-625, 1984. 1984. Thomson, J., N. N. C. C. Higgs, Higgs, T. T. R. G. J. J. de Thomson, J., R. S. S. Wilson, Wilson, I. I. W. W. Croudace, Croudace,G. de Lange, and Lange, and P. P. J. J. M. M. van vanSantvoort, Santvoort,Redistribution Redistributionand andgeochemical geochemical behavior of of redox-sensitive elements around around S1, SI, the behavior redox-sensitive elements the most most recent recent eastern Mediterranean 59, eastern Mediterraneansapropel, sapropel,Geochim. Geochirn.Cosmochim. Cosrnochirn.Acta, Acta, 59, 3487-3501, 1995. 3487-3501, 1995. Thunell, R. C., in the Thunell, R. C., Carbonate Carbonatedissolution dissolutionand and abyssal abyssalhydrography hydrographyin the Atlantic Ocean, Mar. Geol., Atlantic Ocean, Mar. Geol., 47, 47, 165-180, 165-180, 1982. 1982. Trefry, precipitates in Trefry, J. J. H., H., and andS. S. Metz, Metz, Role Roleof of hydrothennal hydrothermal precipitates in the thegeogeo- chemical 1-533, 1989. chemicalcycling cyclingof of vanadium, vanadium,Nature, Nature,342, 342,53 531-533, 1989. Warren, B. B. A., of the Warren, A., Deep Deep circulation circulation of the world world ocean, ocean,in in Evolution Evolution of of Physical Oceanography, edited Physical Oceanography, editedby byB. B.A. A. Warren Warrenand andC. C. Wunsch, Wunsch,pp. pp. 6-41, MIT Press, Press, Cambridge, Mass., 6-41, MIT Cambridge, Mass.,1981. 1981. Wilson, calibration Wilson, D. D. S., S., Confirmation Confirmationof of the theastronomical astronomical calibrationof of the themagmagnetic netic polarity polaritytimescale timescalefrom from sea-floor sea-floorspreading spreadingrates, rates,Nature, Nature,364, 364, 788-790, 1993. 788-790, 1993. Yang, Pedersen, and Yang, Y.-L., Y.-L., H. H. Elderfield, Elderfield, T. Pedersen, and M. M. Ivanovich, Ivanovich, Geochemical record of of the the Panama Geochemicalrecord Panamabasin basin during during the the last lastglacial glacial maximum maximumcarbon carbonevent eventshows showsthat thatthe theglacial glacialocean oceanwas wasnot notsuboxic, suboxic, Geology,23, Geology,23,1115-1118, 1115-1118,1995. 1995. S. WB-10, S. R. R. Emerson, Emerson,School Schoolof ofOceanography, Oceanography, WB-10, University Universityof of Washington, Seattle, Washington, Seattle,WA WA 98195. 98195.(email: (email:emerson@u.washington.edu) emerson@u.washington.edu) D. D. W. W. Hastings, Hastings, Department Department of of Earth Earth and andOcean OceanSciences, Sciences, University of of British British Columbia, Columbia, Vancouver, Vancouver, British British Columbia, Columbia, Canada, University Canada, V6T 1Z4. V6T 1ZA.(email: (email:hastings@ocgy.ubc.ca) hastings@ocgy.ubc.ca) A. Oregon A. C. C. Mix, Mix, College Collegeof of Oceanography, Oceanography, OregonState StateUniversity, University, Corvallis, OR 97331 (email: (email: mix@oce.orst.edu) mix@oce.orst.edu) Corvallis, (Received (ReceivedJanuary January6, 6, 1996; 1996;revised revisedJune June18, 18,1996; 1996; accepted acceptedJune June27, 27, 1996.) 1996.)