Investigator Site File Index for Clinical Trials
advertisement
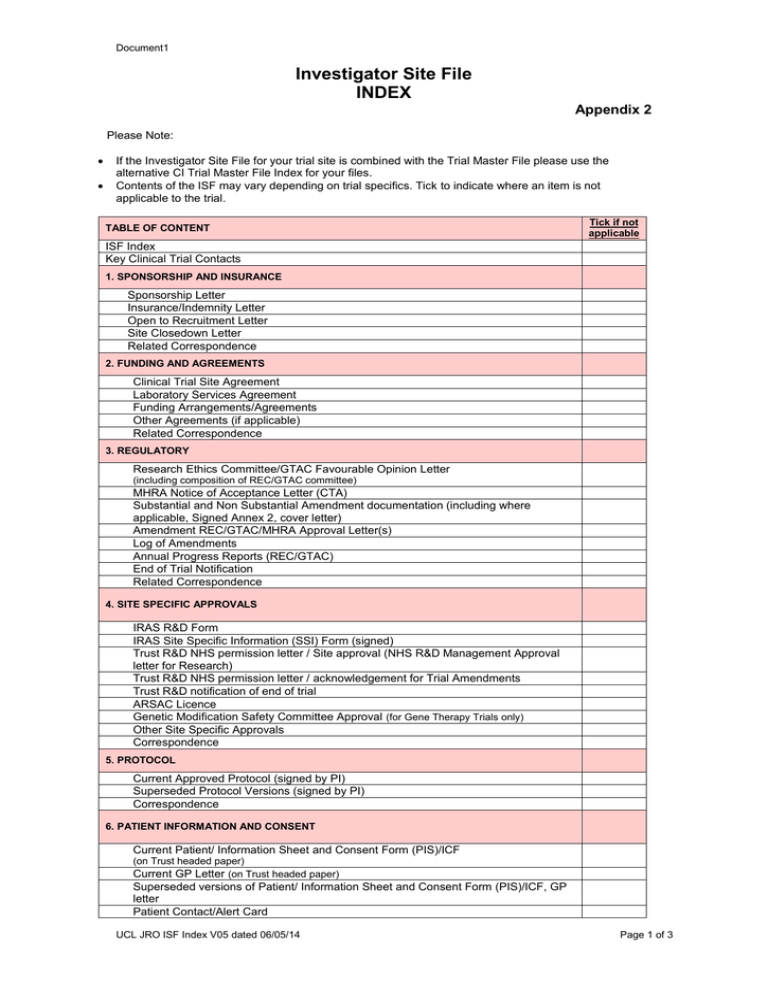
Document1 Investigator Site File INDEX Appendix 2 Please Note: If the Investigator Site File for your trial site is combined with the Trial Master File please use the alternative CI Trial Master File Index for your files. Contents of the ISF may vary depending on trial specifics. Tick to indicate where an item is not applicable to the trial. TABLE OF CONTENT Tick if not applicable ISF Index Key Clinical Trial Contacts 1. SPONSORSHIP AND INSURANCE Sponsorship Letter Insurance/Indemnity Letter Open to Recruitment Letter Site Closedown Letter Related Correspondence 2. FUNDING AND AGREEMENTS Clinical Trial Site Agreement Laboratory Services Agreement Funding Arrangements/Agreements Other Agreements (if applicable) Related Correspondence 3. REGULATORY Research Ethics Committee/GTAC Favourable Opinion Letter (including composition of REC/GTAC committee) MHRA Notice of Acceptance Letter (CTA) Substantial and Non Substantial Amendment documentation (including where applicable, Signed Annex 2, cover letter) Amendment REC/GTAC/MHRA Approval Letter(s) Log of Amendments Annual Progress Reports (REC/GTAC) End of Trial Notification Related Correspondence 4. SITE SPECIFIC APPROVALS IRAS R&D Form IRAS Site Specific Information (SSI) Form (signed) Trust R&D NHS permission letter / Site approval (NHS R&D Management Approval letter for Research) Trust R&D NHS permission letter / acknowledgement for Trial Amendments Trust R&D notification of end of trial ARSAC Licence Genetic Modification Safety Committee Approval (for Gene Therapy Trials only) Other Site Specific Approvals Correspondence 5. PROTOCOL Current Approved Protocol (signed by PI) Superseded Protocol Versions (signed by PI) Correspondence 6. PATIENT INFORMATION AND CONSENT Current Patient/ Information Sheet and Consent Form (PIS)/ICF (on Trust headed paper) Current GP Letter (on Trust headed paper) Superseded versions of Patient/ Information Sheet and Consent Form (PIS)/ICF, GP letter Patient Contact/Alert Card UCL JRO ISF Index V05 dated 06/05/14 Page 1 of 3 Document1 Original Signed Informed Consent Forms (per patient/donor) Other 7. INVESTIGATIONAL MEDICNAL PRODUCT (IMP) * Note maybe located in separate pharmacy/IMP file (if so file note location) Summary of drug arrangements/IMP Management Plan Current Investigator Brochure (IB) / Summary of Product Characteristics (SPC) Superseded Investigator Brochure (IB) / Summary of Product Characteristics (SPC) Sample of Approved Label Certified QP Release Statement Master Randomisation List Study Specific IMP Prescription Template Completed IMP Prescriptions IMP Shipment Records IMP Accountability Logs IMP Destruction Logs IMP Storage Temperature Logs Temperature Deviation Log IMP Recalls Other IMP Specific Forms / SOPs Correspondence 8. PATIENT / SUBJECT IDENTIFICATION / DATA COLLECTION Subject Identification and Code List Subject Screening Log Subject Enrolment, Withdrawal and Completion log Subject Randomisation Log Current Approved CRF Superseded versions of CRFs CRF Completion Instructions Template Quality of Life Questionnaires and Patient Diaries Superseded versions of Template Quality of Life Questionnaires and Patient Diaries Completed CRFs, Diary Cards and Quality of Life Questionnaires Data Management SOP Data Query Documentation Protocol Deviation/Violation Log Related Correspondence 9. STUDY SITE STAFF Staff Signature and Delegation of Tasks Log Staff CVs (signed and dated) Staff Training Records (e.g. Protocol, GCP, study specific training) (File note location if held separately) Related Correspondence 10. PHARMACOVIGILANCE Tick if not applicable Trial Specific SAE Reporting SOP Sample SAE form and Pregnancy form Completed Serious Adverse Events (SAEs) and e-SUSAR forms and logs Completed Pregnancy Reporting Form Notification of Urgent Safety Measures Emergency Un-blinding SOP (if applicable) Development Safety Update Reports (DSUR) Related Correspondence 11. MONITORING & AUDIT Trial Monitoring Visit Log Trial Site Initiation Report/letter/slides Pharmacy Initiation Summary letter Sponsor Monitoring Reports / Summary Letters Completed UK Compliance reports Audit Summary Report for Site Related Correspondence 12. LABORATORIES / SAMPLES UCL JRO ISF Index V05 dated 06/05/14 Page 2 of 3 Document1 Laboratory /Sample Processing Manual Local Laboratory Certification/Accreditation Local Laboratory Normal Ranges Record of retained samples Sample Shipment Records List of trial specific Labs used with contact details Other Related Correspondence 13. PROCEDURAL DOCUMENTS Trial Specific SOPs Sponsor SOPs 14. CORRESPONDENCE Emails/Letters Phone call logs Meeting Agendas/Minutes Newsletters UCL JRO ISF Index V05 dated 06/05/14 Page 3 of 3




