"
advertisement

" An introduction to capillary electrophoresis (CE), its different forms, and
its applications, and the history of CE research at HP, leading to the new
HP CE instrument described in this issue.
" !"
,%#42/0(/2%3)3 )3 /.% /& 4(% -/34 0/7%2&5, )& 42!$)4)/.!,
!.!,94)#!, -%4(/$3 )#4/. !.$ ).$%2 !2$9 !.$ ,,)3 7%2%
452. /& 4(% #%.4529 0)/.%%23 ). 4(% !.!,93)3 /& ")/#/,,/)$3
02/4%).3 !.$ #!2"/(9$2!4%3 9 %,%#42/0(/2%4)# -%4(/$3
4(%9 7%2% !",% 4/ 7/2+ 7)4( 4(%3% 02%6)/53,9 ).42!#4!",%
-!4%2)!,3 2.% )3%,)53 53(%2%$ ). 4(% !'% /& ).3425-%.4!;
4)/. 7)4( ! $%-/.342!4)/. /& 4(% &)234 #%,, &/2 %,%#42/0(/2%4)#
!.!,93)3 ). 4(% 3 !.$ ). 4(% 3 %,%#42/0(/2%3)3 )3 /.%
/& 4(% -/34 6)3)",% )#/.3 /& 3#)%.#% 7)4( !.$ .%730!0%23
$!),9 $)30,!9).' %,%#42/0(/2%4)# 30/4 0!44%2.3
(% &53%$ 3),)#! #!0),,!29 ! ',!33 45"% !"/54 4(% 3):% /& !
(!)2 )3 ! 30).;/&& /& 4(% /04)#!, &)"%2 4 %8)343 -/2% "%#!53%
)4 #/5,$ "% -!$% "9 (%!4;$2!7).' ! ,!2'% ',!33 45"% ).4/ !
4).9 /.% 4(!. "%#!53% )4 7!3 3%%. !3 ! 0/7%2&5, !.!,94)#!,
4//, 4 )3 ./4 35202)3).' 4(!4 /.% /& )43 '2!.$%34 !00,)#!4)/.3
#!0),,!29 %,%#42/0(/2%3)3 7!3 ./4 &/2%3(!$/7%$
!2)/53 7/2+%23 ). 4(% %,%#42/0(/2%4)# &)%,$ 6%2!%243 *%2;
4%. )++%23 )24!.%. 7(),% !7!2% /& 4(% "%.%&)43 /& '/).'
4/ 3-!,, 3934%-3 $)$ ./4 %-0,/9 4(% &53%$ 3),)#! #!0),,!29
(% '2/74( /& 4(% -%4(/$ "%'!. 7(%. ! &%7 7/2+%23 ).;
#,5$).' 3#(/,!23 /2'%.3%. !.$ 5+!#3 /& 4(% .)6%23)49 /&
/24( !2/,).! ).$5342)!, 2%3%!2#(%23 #!.)'),, !.$ !5%2
/& !"/2!4/2)%3 !.$ /4(%23 ./4 .%#%33!2),9 3/0()34)#!4%$
). %,%#42/0(/2%3)3 "54 !7!2% /& 4(% 0/7%2 /& 4(% #!0),,!29 ).
'!3 #(2/-!4/'2!0(9 $%-/.342!4%$ "9 !9 !.$%.!5 !.$
2.)% %2%..%2 /& %7,%44;!#+!2$ ). #/.$5#4%$ 4(%
&)234 %80%2)-%.43 ). &53%$ 3),)#!
. !.!,94)#!, #(%-)3429 3%0!2!4)/. )3 ! &5.$!-%.4!, 02/#%33
#(%-)#!, 35"34!.#% )3 '%.%2!,,9 ).4)-!4%,9 -)8%$ 7)4(
-!.9 /4(%2 35"34!.#%3 !.$ )43 $%4%2-).!4)/. !.$ )$%.4)&)#!;
4)/. )3 -!$% #/.3)$%2!",9 %!3)%2 "9 )43 0(93)#!, 3%0!2!4)/.
&2/- 4(% -)8452% (53 !.!,94)#!, #(%-)343 (!6% 7/2+%$
(!2$ 4/ 5.$%234!.$ 4(% 3%0!2!4)/. 02/#%33 !.$ 4/ $%6%,/0
-!.9 -/$%3 /& 3%0!2!4)/.
(% 3%0!2!4)/. 02).#)0,% &/2 ! 0!24)#5,!2 3%0!2!4)/. -%4(/$
)3 4(% 0(93)#!, /2 #(%-)#!, 02/0%249 4(!4 6!2)%3 ). -!'.)45$%
!-/.' 4(% 35"34!.#%3 ). ! 3!-0,% . #(2/-!4/'2!0(9 &/2
%8!-0,% 4(% 3%0!2!4)/. 02).#)0,% )3 /&4%. #(%-)#!, !&&).)49
&/2 #(2/-!4/'2!0()# -!4%2)!,3 &%!452% /& )3 4(!4 )43 3%0;
!2!4)/. 02).#)0,% )3 /24(/'/.!, 4/ 4(!4 /& ,)15)$ #(2/-!4/';
2!0(9 /2 ). /4(%2 7/2$3 )4 (!3 ! #/-0,%4%,9 $)&&%2%.4
"!3)3
5.% %7,%44;!#+!2$ /52.!,
%0!2!4)/. )-0,)%3 0(93)#!, -/6%-%.4 /2 42!.30/24 . &2%%
3/,54)/. #!0),,!29 %,%#42/0(/2%3)3 4(% 3)-0,%34 &/2/& 42!.30/24 /& ! 0!24)#5,!2 #(%-)#!, 30%#)%3 )3 4(% 2%35,;
4!.4 /& 3%6%2!, $2)6).' &/2#%3 (% 30%#)%3 -/6%3 ). 2%30/.3%
4/ !. %,%#42)# &)%,$ 7()#( )3 )-0/24!.4 )& )4 )3 #(!2'%$ 4
-/6%3 ). 2%30/.3% 4/ 4(% &,/7 ). 4(% #(!..%, 7()#( #!. "%
#!53%$ "9 4(% %,%#42)# 0/4%.4)!, $)&&%2%.#% !#2/33 4(% &,5)$;
3),)#! ).4%2&!#% %,%#42//3-/4)# &,/7 /2 "9 -%#(!.)#!,
05-0).' /& 4(% &,5)$ ).!,,9 )43 -)'2!4)/. )3 !&&%#4%$ "9 4(%
&2)#4)/.!, $2!' )4 %80%2)%.#%3 7()#( $%0%.$3 /. )43 3):% !.$
3(!0% !.$ 4(% 6)3#/3)49 /& 4(% &,5)$
)3 2%!,,9 ! '2/50 /& 3%6%2!, 02/#%$52%3 /2 #!0),,!29
'%, %,%#42/0(/2%3)3 )3 !. %.(!.#%-%.4 /& !. /,$%2 4%#(.)15%
/2 )3/%,%#42)# &/#53).' (!3 !,3/ "%%. $/.% ). /4(%2 &/2;
-!43 !.$ )3 34),, ). 4(% 02/#%33 /& !$!04!4)/. 4/ 4(% #!0),,!29
/2 )3/4!#(/0(/2%3)3 (!3 ./4 &/5.$ 7)$% 53% "54 (!3
#/.3)$%2!",% 0/4%.4)!, &/2 02%0!2).' 052% #(%-)#!,3 /2 -)#%,,!2 %,%#42/+).%4)# #(2/-!4/'2!0(9 !,3/ #!,,%$ "9
/4(%2 !#2/.9-3 )3 ! #/-0,%4%,9 .%7 -%4(/$ 4(!4 #/-").%3
%,%#42/0(/2%3)3 !.$ 0!24)4)/. #(2/-!4/'2!0(9 (% 3)-0,%34
!.$ -/34 #(!2!#4%2)34)# -/$% ). 4%2-3 /& 7()#( !,, 4(%
/4(%23 #!. "% $%3#2)"%$ )3 4 )3 4(% &/2- -/34
02!#4)#%$ 4/$!9
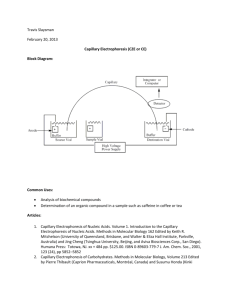
. ! #!0),,!29 #(!..%, 490)#!,,9 ! &%7 -)#2/-%4%23 4/
47/ (5.$2%$ -)#2/-%4%23 ). $)!-%4%2 )3 &),,%$ 7)4( ! #/.;
$5#4).' ,)15)$ -/34 /&4%. ! 7!4%2 3/,54)/. #/.4!).).' !. !#)$
/2 ! "!3% !.$ )43 3!,4 4%2-%$ ! "5&&%2 7()#( (!3 ! 0 4(!4 )3
).3%.3)4)6% 4/ 4(% !$$)4)/. /& 3-!,, !-/5.43 /& !#)$3 /2
High-Voltage
Power
Supply
Electroosmotic Flow
Buffer Solution
Anode
Cathode
Detector
Fused Silica Capillary
2%% 3/,54)/. #!0),,!29 %,%#42/0(/2%3)3
Si
O
+
Si
OH
Si
OH
Si
O–
Si
OH
$! )+/0 /,!01(. "!01.! +" %/ 0$0 0$! )+(!1(!/ %* * .! /1&!0 0+ 2!.5 (%00(! %/,!./%+* 3$%(! 0.2!(%*#
(+*# 0$! +(1)* $! "(+3 %* 0$! +(1)* +!/ *+0 ,,.!%7
(5 /0%. 1, 0$! * !1/! %0 %/ "(07,.+"%(! "(+3 %""17
/%+* %/ %*!/,(! * +!/ /,.! 0$! * 10 !1/!
0$! !4,!.%)!*0 %/ 2!.5 "/0 *+0 )1$ %""1/%+* +1./ * 0$!
"+.) +" 0$! 0!$*%-1! 0$! )! %1) %/ #!( %""1/%+* %/
./0%((5 /(+3! * ,.+ %#%+1/ .!/+(10%+* %/ $%!2(!
H2O
Si
(a)
Si
OH
Si
OH
+
H3O+
H2O
(b)
5 .0%+* +" .3 /%(% /1."! 0+ ,+(5/%(%% % !#0%2! $.#%*# +" /%(% 5 %+*%60%+* +" 0$! /1."!
/!/ $! 03+ !* / +" 0$! $**!( .! %))!./! %* 03+ .!/7
!.2+%./ 3$%$ .! $!( 0 %""!.!*0 !(!0.% ,+0!*0%(/ %* +0$!.
3+. / 2+(0#! %/ ,,(%! 0+ 0$! +(1)* * 0$! )+/0 1/1(
/%010%+* 0$%/ 3%(( .!/1(0 %* 0$! "(+3 +" 0$! (%-1% ".+) 0$!
*+ % .!/!.2+%. 0+ 0$! 0$+ % .!/!.2+%. $%/ %/ !(!0.++/7
)+0% "(+3 %# $! /1."! +" /%(% %* +*00 3%0$ * -1!+1/ )! %1)
3%0$ , *+ (+3!. 0$* +. /+ %/ (+ ! 3%0$ *!#0%2!
$.#! $%/ %/ !1/! /%(% $5 .0!/ * !+)!/ * % .!(!/%*# ,+/%0%2! $5 .+#!* %+*/ %*0+ 0$! )! %1) %# $! !4!// ,+/%0%2! $.#! %/ ,$5/%((5 (+(%6! 3%0$%* 2!.5 *..+3 6+*! (+/! 0+ 0$! /1."! %# %*! ,%((.%!/ !*(+/! 2!.5 /)(( $**!(/ * /%*! !(!7
0.+,$+.!0% 1..!*0/ .! ,.+,+.0%+*( 0+ 0$! .+//7/!0%+*(
.! +" 0$! $**!( %*2+(2!/ /)(( 1..!*0/ ".+) ".0%+* +" )%.+),!.! 0+ $1* .! )%.+),!.!/ +. /+
* 0$1/ /)(( )+1*0/ +" $!0 .!(0%2! 0+ +*2!*0%+*(
/(!/ +" !(!0.+,$+.!/%/ / .!/1(0 %0 %/ ,+//%(! 0+ ,,(5
)1$ (.#!. 2+(0#!/ * 4%( !(!0.% "%!( / %* 0$! $**!(
.! $%#$8$1* .! / +" 2+(0/ ,!. !*0%)!0!.
+)!3$!.! (+*# 0$! 01! / (+/! / ,+//%(! 0+ 0$! 0$7
+ ! !0!0+. %/ )+1*0! 0+ 2%/1(%6! 0$! * / / 0$!5
,// %*! /%(% %/ 0.*/,.!*0 0+ 1(0.2%+(!0 . %0%+* 0$!
)+/0 +*2!*%!*0 !0!0+. +*/%/0/ +" (), +* +*! /% ! +"
0$! +(1)* * ,$+0+ !0!0+. +* 0$! +0$!. /% ! $! * /
/+. 0$! (%#$0 / 0$!5 )+2! %*0+ 0$! ,0$ +" 0$! (), .5/
* 0$! /%#*( %/ %/,(5! / /!.%!/ +" ,!'/ /%)%(. 0+ 0$!
")%(%. ,!'/ +" $.+)0+#.,$5 (0$+1#$ 1*(%'! $.+)0+7
#.,$% ,!'/ 0$! -1*0%05 +" )0!.%( .!,.!/!*0! 5 ,.7
0%1(. ,!' !,!* / +* 3$!* %0 ,//!/ 0$! !0!0+. / 3!((
/ %0/ /%6! %#/ * /$+3 05,%( !(!0.+,$!.+#.)/ +7
0%*! 5 * .!/,!0%2!(5
$! )01.! ,.0%! +" !(!0.+,$+.!/%/ * 0$! *!3 0!$*+(7
+#5 +" "1/! /%(% ,%((.%!/ $2! !!* +)%*! 0+ ,!."+.)
,.!2%+1/(5 1*.!(%6! *(5/!/ "+. 0$! -1*0%00%+* +"
+(%#+*1(!+0% ! "%(1.! /!-1!*!/ * 10+)0(! /!7
-1!*%*# 0+ ,!."+.) +0$!./ 3%0$ 1*,.!! !*0! /,!! *
.!/+(10%+* "/0!. .!/0.%0%+* ".#)!*0 (!*#0$ ,+5)+.,$7
%/) *(5/%/ * ,!,0% ! ),,%*# 0$* (%-1% $.+7
)0+#.,$5 * 0+ ,.+2% ! (5 *!! ! +.0$+#+*(
/!,.0%+* ,.%*%,(! "+. (( (%-1% 7,$/! *(5/%/ 0$! +)7
%*0%+* +" $.#! * ".%0%+*( .# %/ *+0 &1/0 * %*7
.!)!*0( %),.+2!)!*0 +* !4%/0%*# )!0$+ / .0$!. %0
((+3/ !*0%.!(5 *!3 0$%*#/ 0+ ! +*! 0 %/ 0$%/ ,. %#)7
/$%"0%*# $.0!. 0$0 )'!/ 00.0%2! %* * %* 1/0.5
Capillary Inner Surface
Positive Ions
0!. )+(!1(!/ %* 0$! *..+3 6+*! +" ,+/%0%2! $.#! .!
/1&!0! 0+ 0$! ,1(( +" 0$! ,+/%0%2! $.#! / %0 )+2!/ 0+
0$! 0$+ ! * "0 0$! !*0%.! +(1)* +" "(1% %/ .##! *
+*0./0 0+ 3$0 3+1( $,,!* %" 0$%/ /)! +(1)* +" "(1%
3!.! )+2! 5 ,%/0+* 0$!.! %/ *+ ()%*. "(+3 *+ 3((
.# 0!* %*# 0+ ,.+ 1! ,.+(% "(+3 ,.+"%(! +%/!1((!
"(+3 $! "(+3 %/ ,.+,+.0%+*( 0+ 0$! !(!0.% "%!( * *
.!$ 2!(+%05 +" )%((%)!0!./ ,!. /!+* 0 0$! *+ ! 0$%* 6+*! +" )%401.! +" *(50!/ %* -1!+1/
/+(10%+* %/ %*0.+ 1! %*0+ 0$! ,%((.5 $! )+(!1(!/ %*
0$! 6+*! .! %))! %0!(5 /1&!0 0+ 0$! /0.+*# !(!0.% "%!(
* 0$! 1(' "(+3 +" 0$! !(!0.+(50! $!5 .! %),! ! 5
0$! )+(!1(!/ +" !(!0.+(50! 0$.+1#$ 3$%$ 0$!5 )1/0 )+2!
$! %""!.!*0 /,!%!/ !$ )+2! 3%0$ $.0!.%/0% 2!(+7
%05 * #!*!.( 0$!5 )+2! 0+3. 0$! 0$+ ! 10 %" /0.+*#(5
*!#0%2! * 01((5 ! !4,!((! %*0+ 0$! *+ % .!/!.2+%.
/ 0$!5 )+2! 0$!5 /!,.0! %*0+ * / !*.%$! %* 0$!
2.%+1/ /,!%!/ .!(0%2! 0+ 0$! +.%#%*( )%401.! * Neutrals
Silanoate Ions
in Surface
Narrow Zone of Excess Positive
Charge near Negative Surface
* !(!0.++/)+0% "(+3 +1(! (5!. +" ,+/%0%2! * *!#7
0%2! $.#!/ "+.)/ *!. 0$! /1."! +" 0$! ,%((.5
1*! !3(!007'. +1.*(
the manipulation and recognition of critically important DNA
molecules, such as those that cause inherited traits (genetic
diseases) or those that can identify individuals. The synthesis
of these molecules is carried out one letter at a time and is
prone to mistakes at each step. After a series of steps, a popĆ
ulation of failure sequences exists along with the desired
materials. These mistakes are most easily detected and quanĆ
titated by capillary gel electrophoresis (CGE). Neither the
traditional slab gel techniques of electrophoresis nor LC has
the resolving power to distinguish the authentic material
from these attendant failures.
UV Absorbance at 205 nm (AU)
3 5
4
8
1
6
0
5
7
10
Migration Time (min)
9
15
20
Fig. 4. An FSCE separation of an amine mixture. Peaks: 1.
pyridine. 2. benzylamine. 3. diphenylamine. 4. adenine. 5.
aminophenylboronic acid hemisulfate. 6. 4Ćaminoantipyrine.
7. xanthene. 8. 2ĆaminoĆ5Ćnitrophenol. 9. hypoxanthine.
Buffer: 0.1M phosphate, pH 2.7. Separation conditions: anode
20 kV, cathode 10 kV. Column: 40 cm. InjectorĆdetector: 15
cm.
that, while not growing as explosively as the computer inĆ
dustry, has been changing rapidly, with products becoming
obsolete in a matter of months.
Most of the separations performed in the mainstream chemiĆ
cal industry, as well as the food and drug industries, can be
carried out and are being tried by CE. In some instances,
notably the largeĆmolecule areas of bioscience, good appliĆ
cations have already been found and are on their way to
validation. In many others, sometimes surprising ones inĆ
volving molecules with no charge differences, there is great
interest. The high resolving power of CE sometimes outĆ
weighs an inappropriate separation principle, for example in
the case of chiral drug molecules. These molecules are unĆ
charged and have the same frictional drag and therefore
cannot be separated with ordinary media. Their separation in
CE comes from transport through a medium that is itself
chiral, and the low dispersion of electroosmotic transport
preserves it for examination.
CE and the Bioanalytical Market
In the biotechnical segment of the analytical chemical market
HP has had only a few products. One of the most important
is the liquid chromatography (LC) system, which is used to
characterize chemical products and validate their identities
(traditional quality control). LC is very powerful in the field
of general chemicals, along with gas chromatography (GC),
but the new bioengineered pharmaceuticals, the nucleic
acids, and peptides and proteins challenge even its power.
CE is a welcome partner method that shows promise in solvĆ
ing some of these challenges.
Oligonucleotide Failure Sequences
The oligonucleotide, which is a sequence of molecular letters
of DNA code, is used in the research and development effort
in the new biotechnical industry. These segments, a few to
perhaps a hundred letters in length, are synthesized to be
used as templates, or more properly stencils, for operations
that create large amounts of the informational molecule
(polymerase chain reactions or PCR) or as implements for
8
June 1995 HewlettĆPackard Journal
Peptide Mapping
In FSCE, the most important mode in terms of the number of
analyses carried out, an important bioanalytical target is the
protein digest. Most of the bioengineered pharmaceuticals
being produced or contemplated are proteinaceous. The
identity of a protein product is determined by using specific
proteins to break it down catalytically into a mixture of
smaller proteins (peptides) which identifies its origin just as
a geometrical pattern of closely spaced curved ridges identiĆ
fies the fingertip on which it occurs. The peptide mixture can
be analyzed with LC to produce the molecular analog of the
fingerprint, called a peptide map. The mixture can also be
analyzed with CE to produce an equally characteristic map
more quickly. Both maps are useful, and at this stage of
manufacturing practice, their complementarity is beginning
to be widely appreciated.
The Future of CE: Integrated LiquidĆPhase Analysis
CE will continue to find applications. Its growth rate as a
liquidĆphase analytical method will be higher than its more
mature cousin, LC. Most of LC's applications are firmly enĆ
trenched and sufficient for immediate needs. Improvements
in these will be incremental enhancements involving a variĆ
ety of techniques including CE.
CE in the future will likely be a part of a complex strategy.
Workers are now combining the unique separating power
and efficiency of CE with the versatility of LC and the mass
spectrometer's ability to identify unknowns. New forms of
separation continue to spin off the CE experiment (most reĆ
cently CEC, or capillary electrochromatography) and will also
play a part in the integrated future of liquidĆphase analysis.
Absorbance at 260 nm (AU)
2
0.000
Migration Time (min)
Fig. 5. A CGE separation of oligoadenylic acids. The oligomĆ
ers range from 40 to 60 units in length.
17.500
Douglass McManigill of HP Laboratories became interested in
CE as a result of the publication of Professor Jim Jorgenson's
first paper describing the use of fused silica capillaries for
electrophoresis.2 McManigill, in what is now the chemical
systems department of the Analytical/Medical Laboratory of
HP Laboratories, was working in the area of supercritical
fluid chromatography, but was intensely interested in the
physics of transport processes. Along with Henk Lauer of the
HP Waldbronn Analytical Division, he built a CE apparatus
and began an investigation of the CE separation process.
At roughly the same time, Paul Bente and Joel Myerson of
HP Genenchem were working on an electrophoresis instruĆ
ment to do gel separations of proteins and peptides. While
they had limited success instrumentally, the pressurized gel
synthesis they invented was the only reproducible technique
for making the gels for several years.
In time, Lauer left the company, and over several years,
McManigill led the project, working with many others, inĆ
cluding Sally Swedberg, who later worked in Waldbronn on
CE, mechanical engineer Stu Lerner, who is no longer with
HP, Jim Young, mechanical engineer and designer whose
contributions included a method of coating columns with a
resistive layer, Mark Bateman, computer scientist and electriĆ
cal engineer, who created the first software for the instruĆ
ment, mechanical engineer John Christianson, who worked
on the first prototype and is now at the HP Vancouver DiviĆ
sion, the author, a chemist from the medical group at HP LabĆ
oratories, Don Rose from Jorgenson's group at the University
of North Carolina, Jürgen Lux from MaxĆPlanck Institut für
Kohleforschung in Mülheim, Germany, Cathy Keely, an
engineer who made hundreds of CE runs to explore the
unexpected behavior of columns with a conductive coatĆ
ing, Tom van de Goor, a former student of Everaerts in EindĆ
hoven, the Netherlands, who also joined the study of the
control of electroosmotic flow, and Wes Cole, an electrical
engineer who modeled electric fields at the ends of capilĆ
laries.
Another group at HP Laboratories, led by Gary Gordon,
worked on several issues surrounding a CE instrument.
Physicist Dick Lacey helped design a detector. Gary develĆ
oped the bubble cell," which offers a greater optical path
length. An automated system for the fabrication of bubble
cell capillaries was developed by Rich Tella, Henrique
Martins, Bill Gong, and Frank Lucia of the manufacturing
systems and technology department.
Christmas season 1989 saw the transfer of HP Laboratories
technology into the capable hands of Alfred Maute, Fred
Strohmeier, Martin Bäuerle, Fritz Bek, Franz Bertsch, BernĆ
hard Dehmer, Monika Dittmann, Ulrike Jegle, Patrick KaltenĆ
bach, Alwin Ritzmann, Werner Schneider, Klaus Witt, and
HansĆPeter Zimmerman of the Waldbronn Analytical DiviĆ
sion. The result is the instrument described in this issue.
The help of Doug McManigill is acknowledged in the conĆ
struction of this history.
1. R. Dandenau and E.H. Zerenner, An Investigation of Glasses in
Capillary Gas Chromatography," , Vol. 2, 1979, p.
351.
2. J.W. Jorgenson and K.D. Lukacs, Zone electrophoresis in opentubular glass capillaries," , Vol. 53, 1981, p.
1298.
June 1995 HewlettĆPackard Journal