P T P7. ISE
advertisement

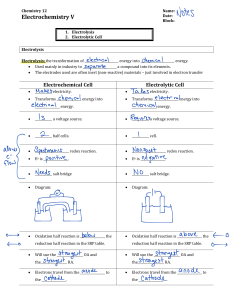
INSTRUMENT MAINTENANCE PRACTICAL TASK P7. ISE SENSITIVITY Outline Ion‐selective electrodes work by measuring the difference in concentration of the analyte ion across a conductive material sensitive to that species. Inside the electrode is a fixed concentration, outside is the sample, which obviously varies. The difference in concentration produces a voltage response. The voltage response is not linear with concentration changes; you need to plot log10[conc] to get a straight line calibration graph. The log10 factor means that the voltage changes by a constant amount for each 10 x change in concentration, e.g. from1 to 10 and from 10 to 100 gives the same voltage change. The amount of change, known as the sensitivity, depends on the ion and is equal to 59/n mV, where is the charge of the ion being measured. Therefore, for fluoride. it is ‐59 mV. Procedure 1. Pipette 25 mL of TISAB solution into two plastic beakers/cups. 2. Into one, pipette 25 mL of the supplied 5 mg/L F standard. 3. Into the other, pipette 25 mL of the supplied 50 mg/L F standard. 4. Dry the fluoride and reference electrodes and place in the 5 mg/L solution. 5. Ensure that the meter is reading correctly (mV). 6. Allow to stabilize before taking the reading or after 1 minute if the value does not stop changing. 7. Rinse and dry the electrodes and repeat with the 50 mg/L solution. 8. Rinse the electrodes and return them to a beaker with distilled water. Acceptable range: ‐54 to ‐64 mV. Reporting Requirements Use the standard Performance Check reporting sheet.