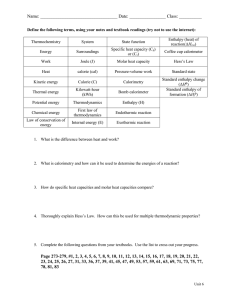
CHEMISTRY II Worksheet for Chapter 4 ENERGY
advertisement

CHEMISTRY II Worksheet for Chapter 4 ENERGY 1. Describe the energy transformations which occur when an electric hotplate is used to boil water 2. What are exothermic and endothermic reactions? give examples of each 3. Define enthalpy (H) and change in enthalpy (∆H) 4. What is Hess's Law? 5. Use Hess’s Law to find ∆H for the following reaction CO(g) + ½ O2(g) CO2(g) Given that C(s) + O2(g) CO2(g) 2C(s) + O2(g) 2CO(g) ∆H= -393kJ ∆H= -222kJ 6. Define the heat of formation of a compound 7. Calculate the heat of reactions of the following, using the heats of formation (∆Hf) in table 4.3 on page 37 of your class notes a) 2AlCl3 + 3H2O Al2O3 + 6HCl b) HCl + NaOH NaCl + H2O 8. Define bond energy 9. Calculate the ∆H of the following reaction – using the bond energies given in table 4.4 on page 38 of your class notes N2 + 3H2 2NH3 (Bonding in N2 is N≡N)