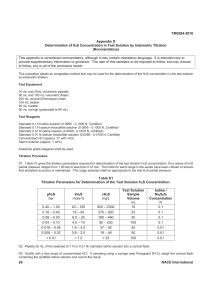
N6. ANALYSIS OF HYPOCHLORITE BLEACH PREWORK:
advertisement

N6. ANALYSIS OF HYPOCHLORITE BLEACH PREWORK: Using Vogel identify a method other than the iodometric method for the analysis of the available chlorine in bleach • Write the balanced equations for the reaction of hypochlorite and iodide; and iodine and thiosulfate • PROCEDURE 1. For liquid bleaches containing sodium hypochlorite, transfer a 10 mL aliquot to a 100 mL volumetric flask. 2. Add 25 mL purified water to the flasks. 3. Add 12.5 g of potassium iodide and make the flask up to the mark. 4. Pipette 20 mL of the diluted bleach solution into a conical flask, in triplicate 5. Add approximately 8 mL 2.5M sulfuric acid and 3 drops of 3% ammonium molybdate solution 6. Titrate the liberated iodine using standardised 0.1 M sodium thiosulfate solution until the brown colour of fee iodine is almost discharged (a pale straw colour) 7. Add an iodometric indicator (fresh starch or Vitex) and complete the titration to the disappearance of the deep blue colour. CALCULATIONS As part of this exercise you are required to write an Excel spreadsheet which performs the calculations. This must be done before leaving the laboratory. 1. Use the equations identified in the prework 2. Calculate the average moles of thiosulfate used in the titration 3. Calculate the moles of iodine and then hypochlorite involved in the titration. 4. Calculate the molarity of hypochlorite in the diluted solution and the original sample 5. Calculate the chlorine content as %w/v of liquid bleach QUESTIONS 1. Why is the molybdate added? 2. What role does potassium iodide play in this titration? 3. Why can’t sodium thiosulfate, a reducing agent, be used to directly titrate easily reduced species such as hypochlorite? 4. Sulfur dioxide can be determined by reaction with a known excess of standardised iodine solution, followed by back titration with thiosulfate. A 5.0347g sample of dried fruit containing SO2 as a preservative, was digested to liberate the SO2, which was distilled into 25.0 mL of 0.0102 M iodine solution. A distillation was complete, the remaining iodine was back titrated with 11.2 mL of 0.0218 M thiosulfate. Calculate the %w/w of sulfur dioxide in the dried fruit. REFERENCE Vogel’s Textbook of Quantitative Inorganic Analysis