N3. DETERMINATION OF PURITY OF AN ORGANIC ACID PROCEDURE
advertisement

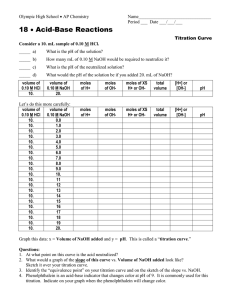
N3. DETERMINATION OF PURITY OF AN ORGANIC ACID Purpose: To determine the purity of a sample of an organic acid PROCEDURE 1. Obtain an organic acid sample, and note its identity (name, formula, reaction ratio with NaOH) 2. Weigh accurately about 0.2 – 0.25g (in triplicate) of the acid into a 250 mL conical flask 3. Add about 50 mL of purified water to each flask to dissolve the acid. (Gentle warming may be required for dissolution) 4. Add 1-2 drops of phenolphthalein and titrate to endpoint with standard NaOH CALCULATIONS As part of this exercise, you are required to design an Excel spreadsheet which performs the calculations. This must be done before leaving the laboratory. 1. 2. 3. 4. 5. Calculate the moles of NaOH used in the titration Using the reaction ratio, determine the moles of acid involved in the titration Calculate the mass of acid involved in the titration Calculate the % purity of the sample Average the individual results QUESTIONS 1. Explain why stronger organic acids are more likely to be soluble in water 2. Would it matter to the analysis results if the acid was insoluble in water? Explain your answer. 3. Explain why phenolphthalein was used as the indicator 4. Describe how you could determine the identity of an organic acid by titration, assuming that the sample was 100% pure.