E4 A O
advertisement

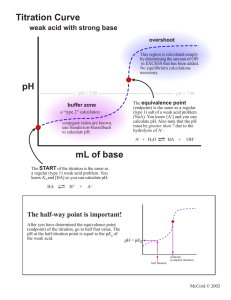
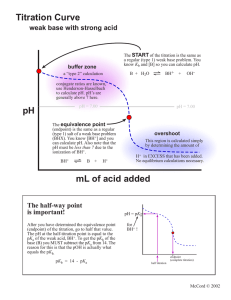
ANALYSIS OF ACIDITY IN ORANGE JUICE E4 You are required to submit ONE report from the group 4, 5 and 6. A report includes results, calculations and questions. PURPOSE To determine the pH of the endpoint of the titration of citric acid – the major acid in orange juice – with NaOH. To analyse the acid content of an orange juice product. PROCEDURE I. Determination of the pH at endpoint for citric acid Sample: 15 mL of 0.05 M citric acid Titrant: standardised 0.1 M NaOH 1. 2. 3. 4. Set up the solutions and equipment for a potentiometric acid-base titration. Perform the titration, collecting enough data points to determine the pH at endpoint accurately. The expected endpoint is around 20 mL. Plot the data on the laboratory computer, using Excel. Determine the pH at endpoint. This will be used in the analysis in Part II. II. Determination of acidity in orange juice drink Sample: 25 mL of fruit juice drink Titrant: standardised 0.1 M NaOH 1. 2. 3. 4. Using the same equipment as before, titrate an aliquot of the sample to the endpoint pH determined in part I. You do not have to record volume/pH data as you go, only the volume required to reach the specified pH. Repeat with another aliquot of sample. Weigh accurately about 0.1 g of citric acid into a 150 mL beaker. Pipette an aliquot of sample into the beaker. Mix well (make sure you rinse the stirring rod into the solution). This sample is known as a recovery check. Titrate as before. CALCULATIONS • include the graph from Part I in you report – no calculations are required beyond this • for samples 1 & 2 in Part II, perform the following: 1. average the titration volumes for samples 1 and 2 2. calculate the moles of NaOH involved in the titration using the average volume 3. divide the moles of NaOH by 3 to obtain the moles of citric acid 4. calculate the mass of citric acid (FW 192) from the moles (** this is equal to m2 in the equation below)) 5. convert the mass of citric acid in the 25 mL aliquot to g/100 mL • for the recovery check, repeat steps 1-4 above to obtain the mass of citric acid (this is equal to m1 in the equation below) • calculate the % recovery using the following equation (m3 is the mass of added citric acid) % re cov ery = 100 x (m1 − m2 ) m3 QUESTIONS 1. What is the typical acidity level of oranges? How does your analysis compare to this? Suggest reasons for any differences. Why is the acidity level calculated assuming that citric acid is the only acid in oranges? 2. 3. The recovery check is a way of seeing whether the analysis of an unknown sample is accurate. The acceptable range of % recovery values is 901-10% How does your result compare? Why can’t an indicator be used for this analysis? 4. 5. If you were analysing citric acid solutions (ie colourless and clear), would you use a potentiometric titration? Explain your answer. What indicator would be suitable for the titration of citric acid with NaOH? E4. ANALYSIS OF ACIDITY IN ORANGE JUICE DATA SHEET I. Endpoint pH Record the titration data in your logbook. Endpoint pH II. Acidity determination Fruit juice ID Brand Barcode Expiry date Analysis data Molarity NaOH Sample 1 titration volume (mL) Sample 2 titration volume (mL) Mass of citric acid (g) Recovery check titration volume (mL) Tester’s signature ________________________________ Date _______________ Supervisor’s signature ______________________________ Date ______________