Genetics
advertisement
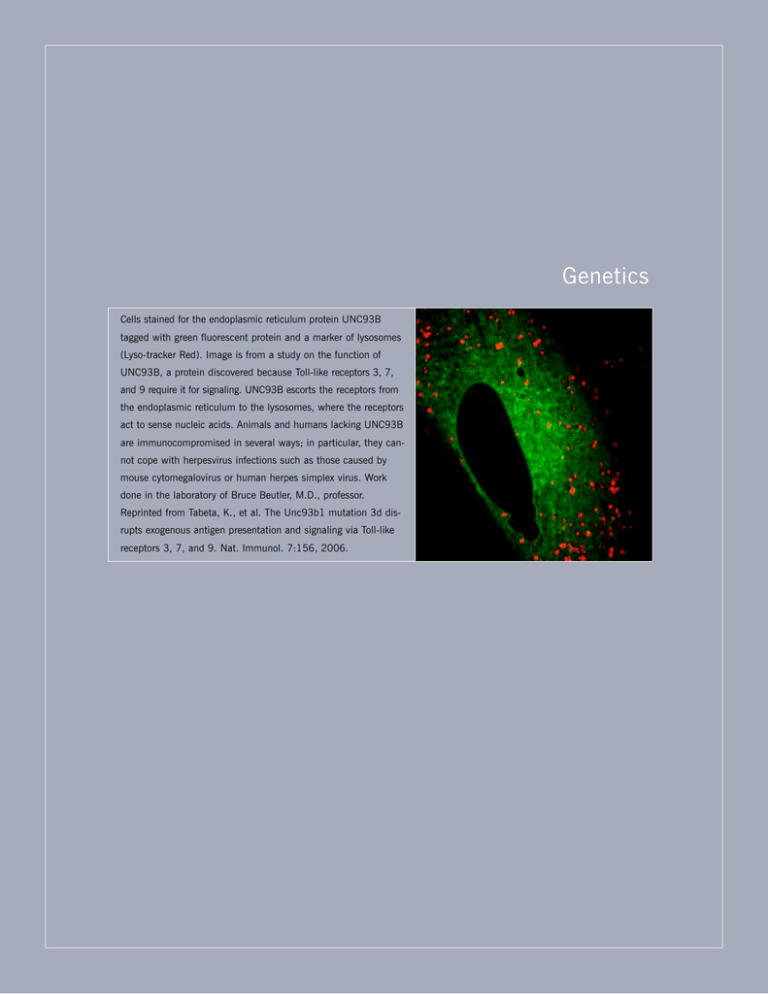
Genetics Cells stained for the endoplasmic reticulum protein UNC93B tagged with green fluorescent protein and a marker of lysosomes (Lyso-tracker Red). Image is from a study on the function of UNC93B, a protein discovered because Toll-like receptors 3, 7, and 9 require it for signaling. UNC93B escorts the receptors from the endoplasmic reticulum to the lysosomes, where the receptors act to sense nucleic acids. Animals and humans lacking UNC93B are immunocompromised in several ways; in particular, they cannot cope with herpesvirus infections such as those caused by mouse cytomegalovirus or human herpes simplex virus. Work done in the laboratory of Bruce Beutler, M.D., professor. Reprinted from Tabeta, K., et al. The Unc93b1 mutation 3d disrupts exogenous antigen presentation and signaling via Toll-like receptors 3, 7, and 9. Nat. Immunol. 7:156, 2006. Kevin Khovananth, Scientific Associate, Bruce Beutler, M.D., Professor and Chairman, and Yu Xia, Ph.D., Staff Scientist GENETICS 2008 THE SCRIPPS RESEARCH INSTITUTE 133 DEPAR TMENT OF GENETICS S TA F F R E S E A R C H A S S O C I AT E S Bruce Beutler, M.D. Professor and Chairman Carrie Arnold, Ph.D. Lei Sun, Ph.D. Michael Berger, Ph.D. Sungyong Won, Ph.D. Amanda Blasius, Ph.D. Nengming Xiao, Ph.D. S TA F F S C I E N T I S T S Xin Du, Ph.D. S C I E N T I F I C A S S O C I AT E Kevin Khovananth TECHNICAL WRITERS Katharina Brandl, Ph.D. Celine Eidenschenk, Ph.D. Eva Marie Y. Moresco, Ph.D. Philippe Krebs, Ph.D. Nora Smart, Ph.D. Yu Xia, Ph.D. Xiaohong Li, Ph.D. Chairman’s Overview iology is unique among the natural sciences for many reasons, not least because living things are constructed according to a blueprint that is largely open to inspection. This blueprint is the genome, and although many gaps remain in our understanding, we can at least read the genome to deduce the primary structure of most of the proteins that an organism can synthesize. This reading gives us a list of parts. Knowing the parts Bruce Beutler, M.D. is only a beginning, of course, but it is something. A more ambitious goal is to know how the parts fit together and how they function to make a living creature. The most complex clock ever built by humans is far simpler than the simplest eukaryotic cell. Even an inventory of all of the gears, levers, and springs would not tell a naive observer how the clock keeps time. If we imagine clocks with millions of component parts, presented to us disassembled and in no particular order, we begin to see the magnitude of the problem a biologist confronts. How do we ever determine what each part does? Luckily, biologists can do certain things clockmakers cannot. The first tool used in understanding the workings of a living organism is genetics. Genetics is not B merely the science of heredity; it is also the science of exceptions. Exceptions to the norm teach an enormous amount in biology. Here I refer to the process by which geneticists dissect complex phenomena, first splitting the phenomena into phenotypes and then finding out the most fundamental cause of each phenotype, the precise mutational change that was responsible. In practice, a scientist comes to the genetic approach with questions about a particular phenomenon. Rather than making guesses about how the phenomenon “works,” geneticists probe phenomenon is probed by introducing mutations into the host germ line at random by using the chemical mutagen N-ethyl-N-nitrosourea. When individuals with abnormal phenotypes are detected, the mutation that causes the abnormality can be tracked down by using positional cloning. This process can be repeated until we know approximately how many proteins are required for the phenomenon to occur as it normally does and until many or most of these proteins have been found. During the first year of existence of the Department of Genetics, researchers in the department have used genetics in mice to study an enormously complex phenomenon: how the mammalian host defends itself from infection. There are many aspects to host resistance, which might be considered the reciprocal of host susceptibility. For any aspect of immune defense we wish to study, however broadly or narrowly defined, an appropriate genetic screen can be devised. We might ask, How many genes are required for a mouse to defend itself against mouse cytomegalovirus? The answer appears to be a minimum of 300 and likely is 1000 or so. To date, 48 mutations that disrupt defense have 1 3 4 GENETICS 2008 been created, and 26 of these have been identified at the molecular level. The others likely will be identified soon. How many genes can be mutated to give exaggerated resistance to this same virus? Perhaps a similar number; 10 mutations that do so are now under study, and possibly some of these will point to important new targets for antiviral therapy. Mutations that cause susceptibility to a virus can affect many different processes. Specific proteins are required to sense the virus, signal its presence to other cells, and activate those cells to eliminate the virus before it spreads out of control. Other proteins enable the host to tolerate the immune response itself, and without them, trivial infections would surely be lethal. Examples have been found in each category. The screen for viral susceptibility is therefore extremely broad. Other screens target immune phenomena that are much more tightly defined. How does the antibody response to an infectious agent get started? How do natural killer cells, antigen-presenting cells, or cytotoxic T cells become activated? And how do these cells differentiate in the first place to assume their proper roles in the immune system? Genetics can tell us, and we have begun to understand each process. During the past year, a mutation called spin revealed that a classical autoimmune disease, known in mice as the motheaten phenotype, is actually promoted by microbes and cannot occur in mice that are germ-free. Moreover, the disease depends on signaling by the cytokine IL-1 and on the proteins myeloid differentiation factor 88 and interleukin-1 receptor–associated kinase-4, which convey IL-1 signals into the cell. This research has revealed the important role of SHP-1, a protein phosphatase, as a “brake” on the immune sensing and response apparatus. Without this protein, microbes drive an inexorable process in which more and more IL-1 is produced, ultimately triggering a severe inflammatory and autoimmune disease. This study was performed by B. Croker, Department of Genetics, in collaboration with A. Theofilopoulos and B. Lawson, Department of Immunology and Microbial Science, and investigators at Jackson Laboratory, Bar Harbor, Maine, and the Karolinska Institutet, Stockholm, Sweden. Genetics creates phenotypes in all realms of biological function, and even when certain phenomena are not kept under surveillance, some declare themselves so loudly that they cannot be ignored. During the past year, we identified a mutation in mice called mask. This mutation causes loss of truncal (but not facial) hair and iron deficiency anemia. In collaboration with E. Beutler, Depart- THE SCRIPPS RESEARCH INSTITUTE ment of Molecular and Experimental Medicine, we deduced that mask disrupts a specific proteolytic enzyme and that mice require this protease to detect iron deficiency and respond appropriately to correct the deficiency. Now that a foothold has been gained, the biochemical basis of iron homeostasis may be understood as never before. Another bizarre phenotype is add, so named because affected mice are wildly hyperkinetic. They are also deaf and have a defect in vestibular function. The add mutation was also tracked down by X. Du, Department of Genetics. She found the phenotype is a fundamentally new inherited disease, stemming from a mutation in a gene that had not previously been annotated. This gene encodes a new form of the enzyme catecholamine-O-methytransferase (COMT2). In collaboration with U. Müller and M. Schwander, Department of Cell Biology, and G.F. Koob and H. Richardson, Committee on the Neurobiology of Addictive Disorders, we examined the mechanism of the phenotype and found that the new enzyme is required for proper function of hair cells in the inner ear. The enzyme may also be required for normal cerebral function. Interestingly, mutations of the classical COMT enzyme (now called COMT1) have been associated with schizophrenia in humans. Possibly, combining mutations of both COMT isoforms will create an authentic mouse model of this disease, because neither mutation alone does so. We also have found that certain cases of deafness in humans are due to mutations of the COMT2 gene and thus have identified a new genetic disease in humans. This discovery was made in collaboration with R. Smith, University of Iowa, Iowa City. Still other strange phenotypes in our collection include Possum (the mice appear to “play dead” when handled gently) and Woodrat (the mice have abnormalities of coat color, metabolism, and immune function). Finding such mutations becomes progressively easier with the technologic advances in DNA sequencing. But understanding the mutations mechanistically remains difficult. Obviously, a multidisciplinary approach is essential. For that reason, we are displaying all mutations on a special Web site (http://mutagenetix.scripps.edu), where scientists from Scripps Research and around the world can learn about the mutations, offer insight, and initiate collaborations. The Web site is maintained and constantly expanded by. E.M. Moresco and N. Smart. As stated at the beginning of this overview, many gaps remain in our understanding of a genome. Epigenetic control of gene expression is an example. Why is a liver cell so different from a brain cell when both have GENETICS 2008 THE SCRIPPS RESEARCH INSTITUTE 135 exactly the same genetic content? The genes are differently expressed and are programmed to maintain a certain level of expression through mysterious processes that operate during development. The rules for epigenetic silencing or activation of genes have only been defined in the most limited way and are partly embodied in the structure and behavior of chromosomes, visible bodies within the cell nucleus that are composed of both DNA and protein and contain most of the genes we have. The Department of Genetics continues to seek outstanding new faculty to answer these questions, and we will undergo a sizeable expansion during the coming year. It is my intention that the department will remain a tightly integrated group of scientists, each with strong collegial ties to other members of the department and to Scripps Research as a whole and committed to exploring biological questions at the most fundamental level. Probing Normal Function by Disrupting It: The Genetic Approach to Understanding Disease cancer, schizophrenia, nutritional deficiency disorders, immunodeficiency, and autoimmunity. Indeed, we have discovered fundamentally new diseases that afflict humans but were not previously recognized as heritable defects. To date, we have created and preserved more than 230 phenotypes, mapped 132 of them to chromosomes, and identified the responsible mutation in 119 instances (nearly 60 of these during the past year; see http://mutagenetix .scripps.edu for a detailed description). The following is a brief description of a few such phenotypes. The mutation mask, which has a visible phenotype marked by truncal (but not facial) hair loss and iron deficiency anemia, revealed how mammals sense iron deficiency and respond to it. The mask mutation indicated that a cell-surface protease, TMPRSS6, is necessary for the absorption of adequate amounts of iron from dietary sources. In the absence of TMPRSS6, which is expressed chiefly in the liver, a protein called hepcidin is produced in excess. Hepcidin is known as the master regulator of intestinal iron absorption. Through the identification of the mask phenotype and positional cloning of the mutation, patients with a comparable mutation responsible for refractory iron deficiency anemia have been identified. Moreover, mask has enlightened us about an important physiologic process. The mutant gene sphinx provides a model of liver tumors as well as a disturbance of function of natural killer cells and CD8 cells. The mutation spin offers a model of autoimmunity promoted by microbes and signaling by myeloid differentiation factor 88, specifically signaling via the receptor for IL-1. And the mutation add has a phenotype in which hyperkinesis, deafness, and vestibular disease have led to the identification of a completely new gene that encodes a protein required for maintenance of the hair cells of the inner ear. Among the mutations created so far, 48 compromise the ability of the host to cope with mouse cytomegalovirus, a common pathogen of mice with a human equivalent that causes severe disease in immunodeficient B. Beutler, C. Arnold, M. Barnes, M. Berger, A. Blasius, K. Brandl, K. Crozat, C. Domingo, X. Du, C. Eidenschenk, N. Gnauck, E. Hanley K. Hoebe, M. Kastner, K. Khovananth, P. Krebs, B. Layton, E. Moresco, N. Nelson, B. Ortiz, X. Li, S. Sovath, O. Siggs, N. Smart, K. Whitley, Y. Xia, N. Xiao enetics is the science of exceptions. It begins with a “phenotype,” 1 of 2 or more alternative states of a phenomenon of interest. To understand why most animals are lean, we might find an exceptional animal that is obese; to understand why most animals resist a particular infection, we might find an animal that cannot. With current technology, the genetic cause of such an exception can be determined in short order. Geneticists can thus elucidate the fundamental molecular requirements for a given phenomenon. Without ever making hypotheses, they can sometimes draw profound conclusions about the functions of specific genes and the proteins the genes encode. Phenotypes are the raw material for genetic inquiry, and much effort goes into making them. We have developed a prolific mutagenesis effort and have used forward genetics primarily to study immunologic phenomena: how humans resist infection by specific microbes, and why inflammatory or autoimmune diseases sometimes develop. Beyond the immune system, we have also identified mutations that cause neurobehavioral, metabolic, developmental, hematologic, neoplastic, ocular, auditory, and pigmentation disorders. In so doing, we have broken new ground in understanding a wide variety of human diseases, including deafness, blindness, arthritis, obesity, G 1 3 6 GENETICS patients and newborn infants. Of the 48 mutations, 26 have been identified. Among them was a mutation (3d) that foretold the existence of an equivalent human herpesvirus-susceptibility phenotype, which we have continued to investigate and understand mechanistically. Another mutation (warmflash) has pointed to a potential means of treating human herpesvirus infections. We have also collected a total of 28 mutations that impair awareness of infection mediated by the Toll-like receptors, key sensors of microbes in mammals. A total of 18 of these mutations have been identified at the molecular level, and all have contributed to our understanding of how the immune system becomes activated during infection (Fig. 1). 2008 THE SCRIPPS RESEARCH INSTITUTE methods for sequencing become more advanced, most likely strange phenotypes will be found even more quickly, within days rather than months or years. In anticipation of this situation, we are striving to devise methods to find aberrant phenotypes faster than ever before and to make the resulting mutational models available to the scientific community. In years to come, we think that 50 to 100 phenovariants will typically be “solved” annually. Through interactions with other members of the Scripps Research community, in particular, members of the expanding Department of Genetics, we hope to study the proteins that are found and to understand their functions in mechanistic detail. PUBLICATIONS Beutler, B. Neo-ligands for innate immune receptors and the etiology of sterile inflammatory disease. Immunol. Rev. 220:113, 2007. Beutler, B. The Toll-like receptors. In: Genetic Susceptibility to Infection. Kaslow, R.L., McNicholl, J., Hill, A.V.S. (Eds.). Oxford University Press, New York, 2008, p. 165. Beutler, B., Du, X., Xia, Y. Precis on forward genetics in mice. Nat. Immunol. 8:659, 2007. Beutler, B., Eidenschenk, C., Crozat, K., Imler, J.L., Takeuchi, O., Hoffmann, J.A., Akira, S. Genetic analysis of resistance to viral infection. Nat. Rev. Immunol. 7:753, 2007. Beutler, B., Moresco, E.M.Y. Akirins vs infection. Nat. Immunol. 9:7, 2008. Croker, B., Crozat, K., Berger, M., Xia, Y., Sovath, S., Schaffer, L., Eleftherianos, I., Imler, J.L., Beutler, B. ATP-sensitive potassium channels mediate survival during infection in mammals and insects. Nat. Genet. 39:1453, 2007. Du, X., She, E., Gelbart, T., Truksa, J., Lee, P., Xia, Y., Khovananth, K., Mudd, S., Mann, N., Moresco, E.M., Beutler, E., Beutler, B. The serine protease TMPRSS6 is required to sense iron deficiency. Science 320:1088, 2008. F i g . 1 . Mutagenesis can be used to analyze biochemical path- ways by which infection is sensed. Each red X depicts a mutation. The names of phenotypes are shown in yellow; protein names (where identified) are shown in pink. These mutations were identified by screening macrophages from mutant mice that did not produce TNF in response to stimulation with a Toll-like receptor. Freudenberg, M.A., Tchaptchet, S., Keck, S., Fejer, G., Huber, M., Schütze, N., Beutler, B., Galanos, C. Lipopolysaccharide sensing an important factor in the innate immune response to gram-negative bacterial infections: benefits and hazards of LPS hypersensitivity. Immunobiology 213:193, 2008. One of the most recent success stories in immunology is the Elektra mutation. Identified because mice homozygous for the mutation died when infected with a normally harmless inoculum of mouse cytomegalovirus, Elektra seems to affect a protein that responds to viral infection within various immune cells, sending a signal to activate the T-cell response. The Elektra protein is also required for the development of a class of immune cells called natural killer T cells, which have resistance functions that are still poorly understood. Interestingly, Elektra points to a member of a family of proteins, and most likely the other members of the family have related functions. The pace of identifying mutations has gathered speed with the development of special tools for the automated exploration of critical regions by DNA sequencing. As Han, J.H., Akira, S., Calame, K., Beutler, B., Selsing, E., Imanishi-Kari, T. Class switch recombination and somatic hypermutation in early mouse B cells are mediated by B cell and Toll-like receptors. Immunity 27:64, 2007. Georgel, P., Du, X., Hoebe, K., Beutler, B. ENU mutagenesis in mice. Methods Mol. Biol. 415:1, 2008. Kiemer, A.K., Senaratne, R.H., Hoppstädter, J., Diesel, B., Riley, L.W., Tabeta, K., Bauer, S., Beutler, B., Zuraw, B.L. Attenuated activation of macrophage TLR9 by DNA from virulent mycobacteria. J. Innate Immun., in press. Kim, S.O., Ha, S.D., Lee, S., Stanton, S., Beutler, B., Han, J. Mutagenesis by retroviral insertion in chemical mutagen-generated quasi-haploid mammalian cells. Biotechniques 42:493, 2007. Koehn, J., Huesken, D., Jaritz, M., Rot, A., Zurini, M., Dwertmann, A., Beutler, B., Korthäuer, U. Assessing the function of human UNC-93B in Toll-like receptor signaling and major histocompatibility complex II response. Hum. Immunol. 68:871, 2007. Otsuka, M., Jing, Q., Georgel, P., New, L., Chen, J., Mols, J., Kang, Y.J., Jiang, Z., Du, X., Cook, R., Das, S.C., Pattnaik, A.K., Beutler, B., Han, J. Hypersusceptibility to vesicular stomatitis virus infection in Dicer1-deficient mice is due to impaired miR24 and miR93 expression. Immunity 27:123, 2007. Xia, C.H., Liu, H., Cheung, D., Wang, M., Cheng, C., Du, X., Chang, B., Beutler, B., Gong, X. A model for familial exudative vitreoretinopathy caused by LPR5 mutations. Hum. Mol. Genet. 17:1605, 2008.






