Errors in Determining lnstar Numbers Through Head Capsule Measurements
of a Lepidopteran-a Laboratory Study and Critique1
FRED H. SCHMIDT, ROBERT K. CAMPBELL,
AND
STEPHEN]. TROTTER, JR. Pacific Northwest Forest and Range Experiment Station, USDA, Forest Service, Corvallis, Oregon 97331 ABSTRACT Larvae of Choristoneura viridis Freeman (Tortricidae)
were reared individually on an artificial medium in the
laboratory under controlled conditions. The larvae ex­
hibited developmental polymorphism, i.e., some larvae had
a total of 6 instars, others 7, and still others 8 instars.
Except for instar I, all larval head capsules were recov­
ered and widths measured for each larva in the study
population. A frequency distribution curve of capsule
widths suggested only 6 instars, when in fact over 57%
of individuals showed 7 or more instars. Head capsule
width ranges for successive instars, as suggested by the
multimodal frequency distribution curve, were not in
agreement with known values for the laboratory popula-
tion, nor were they in agreement when values for the
population were segregated by sex irrespective of larval
instar group. Peaks in the curve could be interpreted
only after the head capsule widths in the population were
segregated by instar group. In larval growth regressions
for the population as well as for head capsules broken
down by sex and/or instar group, standard error of esti­
mate values appeared to be more sensitive than R2 values
in reflecting relative precisions of equations. Generated
frequency distribution curves of head capsule widths sup­
port the argument that frequency distribution of head
capsule widths cannot be used to assign instar numbers
in lepidopterous species with developmental polymorphism.
Ecologists and applied entomologists are concerned
The model equation l nY = a + bX, where Y =
head capsule width and X = instar number, is an­
other method for determining the characteristic num­
ber of instars in species. Application of this method
to entomological work was 1st recognized by Dyar
( 1890) who found that "widths of the head of a
(le pidopte rous) larva in its successive stages follow
a regular geometrical progression." Dyar's original
purpose in proposing the generalization was to pro­
vide a method of discovering an overlooked instar
when trying to determine the number of molts or
instars of certain species (Dyar 1890, Richards
1949). The method is not always applicable (Gaines
and Campbell 1935, Fox et al. 1972).
Whether any method other than direct observa­
tion will adequately characterize instar number in
C horistoncura species (Lepidoptera: Tortricidae) is
questionable. In laboratory culture, instead of the
"typical" 6 instars that have been reported for the
western spruce budworm, C. occidental1's Freeman
(Bean and Batzer 1957, Lyon et al. 1972), and the
eastern spruce budworm, C. fumiferana (Clemens)
(McGugan 1954, Bean and Batzer 1957), larvae
with 5, 6, and 7 instars and 6, and 7 instars have
been found (Schmidt and Lauer 1977). Larvae
exhibiting 6. 7, or 8 instars were also found in
C. 'l1iridis Freeman. Head capsule width measure­
ments made on larvae of the 1st 2 species were
generally similar to those previously reported, even
though the actual number of instars may have been
other than the reported 62• The greatest deviation
from the expected, typical 6 instars occurred in C.
virid is where more than SO% of the insects had 7
or 8 instars. This study attempts to determine
whether or not methods other than the direct observa­
tion of developing larvae will clearly characterize the
number of instars in this species.
The derivation of the C. viridis stock, the artificial
with larval size, growth rate, age distribution within
a population, etc. Many of these factors are directly
related to the instars of a given species. Questions
of interest include, which instars are preyed upon by
given predators, which are parasitized by given
parasites, and which are most s usce ptib le to infection
by pathogens? How much of a given host is the
insect capable of consuming, and which instar (s)
consumes the most and thereby causes the most dam­
age? Are LDri0s for such agents as insecticides and
pathogens the same for different instars of a species
(Shepard 1951, Busvine 1957, Stairs 1965, Ahmad
and Forgash 1975, Magnoler 1975)? Can the instars
of a species be identified, so that seasonal development
of populations may be monitored to determine the
proper timing of insecticide applications in control
programs? Knowledge of the precise number of in­
stars of a species is of fundamental importance to
entomologists of varying interests.
The number of instars characteristic of a given
species may be determined in several ways including
direct observation of larvae reared through the entire
larval stage and through plots of frequency distri­
butions of head capsule widths taken from larvae
representative of the entire larval stage of the spe­
cies. It has been argued that the latter procedure
will provide a multimodal curve with each peak be­
ing representative of head capsules found within one
instar. The total number of peaks represents the
total number of instars exhibited for the species in
the collection of larvae examined. Such multimodal
curves, and the resultant classification of the num­
ber of instars characteristic of a species, have been
in the literature since 1928 (Peterson and Haeussler
1928, Taylor 1931), and are still in current use
(Kishi 1971, Fox et al. 1972, Parker and Moyer
1972, Hoxie and Wellso 1974, Vanderwerker and
Kulman 1974, Wilson 1974).
1 Received for publication Sept.
24, 1976.
750
)
2 Larval head capsule widths, of last instar larvae, irrespective
of the total number of instars exhibited by the species, are ca. the
same within the species (Schmidt and Lauer 1977).
September 1977]
SCHMIDT ET
AL.:
ERRORS IN lNSTAR NUMBERS
budworm medium, and the rearing methods have
been described in Schmidt and Lauer (1977) .
The widths of sloughed head capsules from the
2nd instar to pupation were measured to the nearest
0.016 mm using a calibrated, ocular micrometer (Mc­
Gugan 1954) . The instar group to which each larva
belonged was noted at pupation. Only head capsule
data from insects that later emerged as adults normal
in appearance were included in the results. The
adults were sexed. A total of 2218 head capsules was
measured from a total of 397 larvae. These included
head capsules (and larvae) from the following
groups: 500 ( 100) , 366 (61) , and 105 ( 15) from 6-,
7-, and 8-instar males, or 971 total head capsules (and
176 larvae) . In addition, 500 ( 100) , 600 (100) , and
147 (21) head capsules from 6-, 7-, and 8-instar fe­
males were measured for a total of 1247 head capsules
(and 22 1 larvae) .
To provide a representative frequency distribution
curve of larval head capsule widths, a population of
C. viridis was constructed by the random selection
(random number table) of 200 of the total 397 lar­
vae. The component groups of this population had
proportions equivalent to those observed in previous
experiments; i.e., 0.5:0.5 male to female frequency
and an instar frequency of 0.53, 0.45, and 0.02 for
6-, 7-, and 8-instar males, and 0.32, 0.63, and 0.05
for 6-, 7-, and 8-instar females. The curve included
widths from all head capsules recovered from all 200
larvae in the constructed population beginning with
instar II. A total of 1 122 head capsules was included.
These data were also employed in the calculation of
the mean head capsule width and standard deviation
for each instar and in each subsequent breakdown of
the population by sex and instar group. The same
data were used to compute larval growth regressions
for the population and for the breakdowns. The model
1nY = a + bX, where Y = head capsule width and
X = instar number, was used. In these regressions,
the standard errors (i.e., v'MSE, where MSE =mean
square error) are biased slightly downwards and R2s
upwards because ·all head capsules for each larva in
the population, and in each breakdown, were used
in the derivation of the resulting regressions.
The data suggested that frequency distribution
curves of larval head capsule widths might vary with
the structure of the population being examined. To
verify this, curves for populations with different
structures were simulated. Sample data were gen­
erated by a regression equation that well predicts
head capsule width (unpublished):
1nY = 0.830 + 0.804 X1 + 0.069 X2 - 0.062
X1 X2 + 0.007 X2X3, where Y represents head cap­
sule width, xl head capsule number, x2 instar group,
and X3 sex.
In a program developed by D. G. Niess, Systems
Analyst, Oregon State University, Corvallis, popula­
tion structures were varied by changing the propor­
tions of larvae in instar groups, maintaining a 50:50
sex ratio. Head capsule numbers were obtained with
a random number generator, and 11noise" was intro­
751
duced into the generated data by incorporating error
based on the standard error of estimate of the above
equation ( = v'MSE = 0.0739) . Finally, generated
data were plotted in the characteristic frequency
curve for comparison with distributions based on
other population structures.
RESULTS AND DISCUSSION
Frequency Distribution Curve of Head Capsule
Widths and Determination of Number of Instars.­
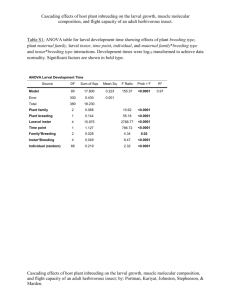
Fig. 1 shows the frequency distribution curve of head
capsule widths for all head capsules in the larval
population. The curve has 5 apparent peaks, with
means at ca. 0.3 (peak a) , 0.433 (peak b) , and
0.633 (peak c) , 1.167 (peak d) , and 1.7 mm (peak e) ,
presumably for instars II through VI. This suggests
that there are but 6 instars in the population. How­
ever, this cannot be the case because a substantial
number of head capsules were included from larvae
that had more than 6 instars. Therefore, 11secondary"
peaks in the curve, such as those at ca. 0.933 (peak
d1) and 1.4 mm (peak d2) may have functional
significance.
Since the origin of every head capsule in the
frequency distribution was known, it was possible to
determine a mean and variation about the mean (i.e.,
S.D.) for each instar of the population and to cal­
culate a larval growth regression using the means
of the successive instars. It was also possible to
segregate the capsules into sex and instar classes
and to make similar determinations of means and
variation about the means for each instar of each
subgroup. These calculated mean head capsule widths
are shown in Table 1 and also graphically at the top
of Fig. 1.
There is little apparent difference in head capsule
width between head capsules VII and VIII when
the mean head capsule widths of all larvae, irrespec­
tive of sex and instar group (i.e., the population),
are examined (top line in Fig. 1). Moreover, instars
VII and VIII might ordinarily be considered in­
distinguishable as evidenced by their close mean
widths and overlapping standard deviations; and, to
a large extent, these instars overlap those of instar
VI. When larvae for the respective instar numbers
are grouped by sex, irrespective of instar group, this
problem is still unresolved. There is little difference
in head capsule widths between head capsules VII
and VIII, and standard deviations overlap for head
capsules VI through VIII in both sexes. In females
of this sample, the mean width for instar VIII is
less than that for instar VII.
Head capsule width of successive instars are only
distinguishable without overlap when animals in the
population are broken down by instar group, irrespec­
tive of sex. Such a breakdown substantially reduced
the variability in head capsule widths from instar V
onward. If the population is broken down further
by both sex and instar, an even greater reduction in
this variability is evident in all subgroups except
male 8-instar animals, a subgroup containing but 2
animals.
752 POPULATION
SEX
180
-
160 ........ w
u
z
w
a: (..)
...,....
.,.
140
120
[Vol. 70, no. 5
ANNALS OF THE ENTOMOLOGICAL SoCIETY OF AMERICA
+
r l.
_.,._..J..'_ -/-
/'
1
../.. b
""/
--+"" -
..,. ,. -"';;::""'�-
;;
I
-L
--
/
--
-
--
-
>-+-<
-
4 2.5
6
54.0
7
3.5
SEX-INSTAR
d'
;oo;r
6
7
Q
-
--
53.0
45.0
6
2.0
lC501r
32.0
8
5.0
8
--
---
50.0 Q
-- -
,____.i_
--
--+--<
50.0 8
-+--
-
--
INSTAR GROUP
- - -- --- -
100% a'
7
63.0
!Qf;
""""'
100
(..)
0
lJ..
0
>­
u
z
w
::::>
0
w
a:: 60
lJ..
c
40
20
0.2 0.4
0.6
OB
1.0
1.2
1.4
HEAD CAPSULE WIDTH IN
1.6
mm 1.8
2.0 FIG. I.-Frequency distribution curve of head capsule widths for a larval "population" of C. viridis based on ob­
served widths of all head capsules, except instar I, recovered from individuals in the population. Head capsule width
means. ( ± SD) of each instar are given for the population and for each subgroup of the same population. Broken
lines connect common instar, or head capsule, numbers within a breakdown, and are presented only for the conveni­
ence of the reader.
As noted above, the frequency distribution ex­
hibited essentially 5 peaks, or 6 instars, and more
should have been evident because more than half of
the animals in the population had more than 6
instars. When the composition of individual peaks
in the distribution is examined, it is obvious ·that
at least some of the peaks in the curve cannot be
accounted for when instar means are computed
either for the population or for the subgroups broken
down by sex alone (the 3 top lines in Fig. 1). In­
consistencies in this regard occur with peaks d11 d,
and d2 on the curve. Judged by its position in the
curve, the d peak, the highest of the 3, probably
includes mostly instar V head capsules. When this
peak value is compared with means of instars actually
observed for the population and sexed subgroups, the
peak contains primarily head capsules from instar VI
larvae, which are mostly males. It is difficult to
account for the d peak because it occurs just within
one standard deviation from instar VII means of
the population and of the male subgroup. It was
not even within one standard deviation of the mean
of instar VI of the female subgroup. The d1 and d2
peaks on the curve are much closer to means actually
observed for the instars V and VI, respectively, for
the population and for each of the sexed subgroups.
September 1977]
SCHMIDT ET AL.: ERRORS IN lNSTAR NUMBERS
753
Table 1.-Mean head capsule widths ( HCW) for all C. viridis instars of the "population" and the respective break­
downs of the population in the study.
•
1. The "population" (N arvae in group= 200)
Instar
II
III
IV
v
VI
VII
VIII
HCW (mm)
±SD
0.297±0.011
0.413±0.027
0.620±0.065
0.966±0.156
1.425±0.262
1.756±0.165
1.748±0.110
2. Breakdown by sex irrespective of instar group (N arvae)
(N=100)
HCW (mm)
±SD
(N=100)
HCW (mm)
±SD
0.295±0.011
0.410±0.028
0.610±0.068
0.948±0.159
1.420±0.265
1.684±0.131
1.767±0.141
0.298±0.013
0.417±0.026
0.630±0.060
0.984±0.150
1.429±0.259
1.805±0.169
1.740±0.114
3. Breakdown by instar group irrespective of sex
6 (N=85)
Instar group (N,.,., )
HCW(mm)
Instar
±SD
7 (N=108)
HCW (mm)
±SD
8 (N=7)
HCW (mm)
±SD
0.295±0.009
0.403±0.026
0.587±0.054
0.861±0.088
1.238±0.109
1.782±0.133
0.286±0.030
0.383±0.040
0.531±0.050
0.760±0.044
1.031±0.066
1.352±0.049
1.748±0.110
Instar
II
IV
III
v
VI
VII
VIII
..
II
III
IV
v
VI
VII
0.300±0.009
0.428±0.018
0.669±0.041
1.116±0.083
1.694±0.126
VIII
4. Breakdown by sex and instar group
6
Instar· group
Sex (N,
•
.)
..,
Ins tar
II
III
IV
v
VI
VII
VIII
•
.
7
8
(N=53)
HCW (mm)
±SD
(N=32)
HCW (mm)
±SD
(N=45)
HCW(mm)
±SD
(N=63)
HCW(mm)
±SD
(N=2)
HCW (mm)
±SD
(N=5)
HCW (mm)
±SD
0.298±0.009
0.425±0.018
0.660±0.038
1.077±0.074
1.649±0.116
0.303±0.009
0.435±0.015
0.684±0.043
1.179±0.052
1.768±0.109
0.291 ±0.008
0.393±0.027
0.553±0.048
0.802±0.087
1.165±0.088
1.699±0.112
0.298±0.009
0.411±0.024
0.612±0.043
0.903±0.061
1.290±0.091
1.841±0.114
0.308±0.059
0.417±0.071
0.583±0.071
0.792±0.083
1.083±0.094
1.350±0.094
1.767±0.141
0.277±0.009
0.370±0.018
0.510±0.022
0.747±0.022
1.010±0.049
1.353±0.036
1.740±0.114
Data shown graphically at the top of Fig.
1.
These were expected to be higher on the curve than
was found, and certainly higher than the d peak.
If the d peak has little relation to head capsule
widths for instar V, then 1.167 mm, the maximum
for the peak, cannot be assumed to be even a close
approximation of the mean head capsule width either
for that instar in the population or for that instar
in either sex of C. viridis. Therefore, the frequency
distribution curve of head capsule widths did not ac­
curately describe either the true number of instars
in this population, and presumably this species, or
the true mean head capsule widths of all instars
found in this population. Moreover, a breakdown by
sex did not appreciably improve this interpretation.
When head capsule widths are broken down by
instar group, irrespective of sex, a more reasonable
interpretation for the d1, d, and d2 peaks is possible.
The d peak is apparently a combination consisting
largely of the instar V head capsule of the 6-instar
group and the instar VI head capsule of the 7-instar
group. These groups represent ca. 96% of the larvae
in the population. The broad peak from ca. 0.833-
ANNALS OF THE ENTOMOLOGICAL SOCIETY OF AMERICA
754
0.933 mm (d1) consisted largely of the instar V head
capsule of the 7-instar group, a group representing
ca. 54% of the population. The peak at ca. 1.383 mm
(d2) consisted largely of the instar VII head capsule
of the 8-instar group, a group representing ca. 3%
of the population.
When head capsule widths are broken down by
both sex and instar group, interpretation becomes
easier. It is probable that the d peak consisted
largely of the instar VI head capsule of the male,
7-instar group and of tl].e instar V head capsule of
the female, 6-instar group, with a relatively minor
contribution of the instar VI head capsule of the
male, 8-instar group. These combined groups repre­
sent ca. 40% of the larvae in the population. The
d1 peak of the curve consisted mainly of instar V
head capsules of the female 7-instar group, repre­
senting ca. 32% of the population. The d2 peak
probably consisted mostly of the instar VII head
capsule of both males and females in the 8-instar
group, representing ca. 4% of the population. A
similar analysis of the probable composition of most
other peaks, both major and minor, of this popula­
tion, and presumably the species, can be made.
Gaines and Campbell (I935) used a modified ap­
proach to that described above in interpreting a com­
plex frequency distribution curve of head capsule
width of the black cutworm, Agrotis ipsilon ( =
ypsilon) (Hufnagel), from the data of Satterthwait
(I933). Satterthwait found that 6-, 7-, and 8-instar
"classes" occurred normally in that species. Gaines
and Campbell concluded that the frequency distri­
bution curve method of determining the number of
instars of a species "will give clear results only
[Vol. 70, no. 5
when the insects being measured are fairly homo­
geneous in rate of development and number of instars.
If the population being studied is a mixture of
individuals having n and n+ I instars and a cor­
responding difference in rate of development, it might
be difficult or impossible to interpret the frequency
distributions." This statement would accurately ap­
ply to C. viridis, as well as to other lepidopterous
species that exhibit postembryonic, developmental
polymorphism (Schmidt and Lauer I977).
Larval Growth Regressions from Head Capsule
Width Measurements of Successive Instars.-Table 2
shows larval growth regressions of head capsule
width measurements of successive instars for the
population and for the various subgroup breakdowns
shown in both Table I and Fig. 1.
In a comparison of R2 values for regressions of
the population and subsequent breakdowns of that
population, a relatively poor fit of the head capsule
width data to the regression was expected, and R2
values for the regressions of the various subgroup
breakdowns would reflect substantially better fits.
This was not the case. The R2 value was surprisingly
high, in view of the known heterogeneity in the
population. Little or no additional variability in the
data could be accounted for, as judged from R2
values, when the data was broken down into sub­
groups based on sex, irrespective of instar group.
An improvement in R2 values was not evident until
the population was broken down by instar group,
irrespective of sex. When head capsule widths in
the population were broken down by both sex and
instar group, R2 values of regressions for the male
subgroups showed little or no change from the pre-
Table 2.-Larval growth regressions based on head capsule width measurements of all head capsules recovered
from 200 C. viridis larvae.
Breakdown
1. Population
Larval growth regression
(head capsule width (Y)
X instar number (X) )
In?
2. Sex
Male
Female
In?
In?
3. lnstar Group
6-instar
7-instar
8-instar
In?
In?
In?
4. Sex-Instar Group
Male
6-instar
7-instar
8-instar
Female
6-instar
7-instar
8-instar
*
**
In?
In?
In?
1n?
In?
In?
=
=
=
=
=
=
=
=
=
=
=
=
1.438+0.373. X
N in group
analyzed
'% (animals) of
group or subgroup
analyzed
R2*
S.E.
estimate**
0.953
O.I36
200
1122
IOO
1122
549
573
IOO
Head
Larvae capsules
1.426+0.373 X
1.4SI+0.372 X
0.948
0.957
0.14I
O.I32
200
IOO
IOO
1.271+0.44I X
1.425+0.364 X
1.538+0.308 X
0.988
0.983
0.985
0.070
0.082
0.077
200
85
108
7
1122
425
648
49
IOO
200
100
53
45
2
IOO
32
63
5
1122
549
265
270
14
573
I60
378
35
IOO
1.279+0.435 X
1.415+0.356 X
1.657+0.294 X
0.989
0.983
0.974
0.066
0.08I
0.103
1.256+0.452 X
1.433+0.369 X
1.49I+0.3I4 X
0.990
0.990
0.993
0.064
0.063
0.053
Coefficient of determination.
Standard error of estimate = V MSE .
50.0
50.0
42.5
54.0
3:5
50.0
50.0
53.0
45.0
2.0
32.0
63.0
5.0
September 1977]
SCHMIDT ET
AL.:
vious breakdown by instar group, irrespective of sex.
R:: values of female subgroup regressions, on the
other hand, did appear to be slightly higher than a
breakdown by instar group alone.
Standard error of estimate (SEE) proved to be
more sensitive than R2 values in reflecting the
increase in precision of regression equations associ­
ated with successive breakdowns of the population
into sex and instar group (Table 2) . Regressions
in subgroups broken down by sex, irrespective of
instar group, had SEE values of 0.141 and 0.132 for
males and females, respectively. These values were
little different from the SEE value of 0. 136 for the
population. In populations broken down by instar
group, irrespective of sex, the SEE values were
0.070, 0.082, and 0.077 for regressions of the 6-, 7-,
and 8-instar subgroups, or almost a 2-fold reduction
from the SEE value obtained for the population
regression. vVhen the population was broken down
by both sex and instar group, a still further reduc­
tion in SEE values usually occurred. This reduction
was substantial for the female-instar subgroups, re­
sulting in SEE values less than half those for the
population. Obviously in this sample, most of the
variation in the head capsule widths of C. viridis
has been accounted for by regression when the SEE
value is ca. 0.1 or less.
Computer Simulations of Frequency Distribution
Curves of Larval Head Capsule Widths.-The com­
puter simulations of larval head capsule widths were
plotted in a frequency distribution curve format.
Four populations of head capsules were generated.
Of the 4, 3 were generated with sex ratios of
0.5:0.5 each,
males
to
females.
All
the
755
ERRORS IN lNSTAR NUMBERS
simulated
head capsule widths of each simulated population
came from larvae of only one instar group. In one
population, all the represented larvae exhibited a
total of 6 instars (i.e.. 6-instar group) . In another
group, all the represented larvae exhibited a total of
7 instars. and in still another, all the represented
larvae exhibited a total of 8 instars. The resulting
frequency distribution curves are the top 3 in Fig. 2.
Since head capsule data for instar I larvae were
omitted in the derivation of the simulation model,
the number of peaks in the multimodal frequency
distribution curves should be one less than the num­
ber in the respective instar group. This proved to
be the case. with 5. 6, and 7 peaks being evident for
curves of the 6-, 7-, and 8-instar groups.
A 4th simulated population was structured by sex
and instar group according to classes shown in the
lowest curve of Fig. 2. Because this population had
the sex and instar group makeup of a natural popu­
lation, its frequency distribution curve is expected
to he similar to that found for the population shown
in Fig. 1. vVhile the curve (bottom Fig. 2) showed
a great deal of "noise." probably due to the error
term used with the model. similarities between the
2 curves are apparent. Peak maxima occurred at
ca. 0.3(a). 0.4 ( b). and 0.6 mm(c) and peak minima
at ca. 0.33 and 0.5 mm in both curves. The peak
located from ca. 1.5-1.95 mm (e) was evident in
100
80
60
100%
NCAPS
8 INSTAR
=
2002
40
20
w60
u
100% 7 I NSTAR
z
40
0::
:::>
NCAPS
=
1200
8 2o
0
l.L.
0
>-8
u
z
w
:::>6
0
w
0::
l.L.40
100% 6 INSTAR
NCAPS
=
1200
20
% IN INSTAR CLASS
40
SEX
6
7
8
d 53.0 45.0 2.0
9
32.0 63.0 5.0
1.5
HEAD CAPSULE WIDTH I N
2.0
mm
FIG. 2.-Computer simulation of frequency distribution
curves of head capsule widths using the model :
In?= 0.830 + 0.804 X1 + 0.069 X2
- o.062
X1X2 +
o.oo7 x2x3
Y represents head capsule width, X1 head capsule num­
ber, X2 instar group, and X3 sex. An expected peak for
instar I is omitted in each curve. The sex ratio in each
of the populations is 0.5: 0.5, males: females.
756
[Vol. 70, no. 5
ANNALS OF THE ENTOMOLOGICAL SOCIETY OF AMERICA
both curves. The simulated curve was much "noisier,"
and finding a precise location for the peak maximum,
or the mean head capsule width for the instar that
was presumed to be represented by it was, therefore,
precluded. The complex and difficult to interpret
region of the curve of the natural laboratory popula­
tion (i.e. between 0.75 and 1.5 mm, Fig. 1 ) , was
equally complex in the simulated curve. If the popu­
lation represented in the latter had been composed
exclusively or even predominately of 6-instar larvae,
a minimum would have been expected at ca. 0.8-0.9
mm. But this was not the case. The curves for the
homogeneous groups suggested that this peak is com­
posed of simulated head capsules V and VI of . the
7- and 8-instar groups. But since the 8-instar com­
plement to the population was only 3.5%, and there­
fore negligible, this peak consisted largely of instar
V head capsules of the 7-instar group. It is probable
that the peak between 1.0 and ca. 1.3 mm consisted
largely of simulated head capsules of instars V and
VI of the 6- and 7-instar groups. The findings by
computer simulations are in general agreement with
the conclusions made by examining the frequency
distribution curve of the actual population presented
in Fig. 1.
CONCLUSIONS
The use of frequency distribution curves to deter­
mine the characteristic number of instars of a species
or population may not always be reliable. As pointed
out by Gaines and Campbell ( 1935), such curves
"will give clear results only when the insects being
measured are fairly homogeneous in rate of develop­
ment and number of instars." In lepidopterous spe­
cies which show developmental polymorphism (e.g.,
C. 'l!iridis). a complex frequency distribution curve
can result. Conversely, it can be surmised that com­
plex frequency distributions for head capsule widths
in other species may indicate developmental poly­
morphism.
ACKNOWLEDGMENT
We thank R. B. Ryan and M. E. Martignoni,
Pacific Northwest Forest and Range Experiment
Station, and N. H. Anderson, Oregon State Univer­
sity for reading the manuscript, and P. Kanarek,
Oregon State University, for reviewing the statistical
presentation in the manuscript.
REFEREN CES CITED
1975. Toxicity of car­
baryl and diazinon to gypsy moth larvae: changes in
relation to larval growth. J. Econ. Entomol. 68:
803-6.
Bean, J. L., and H. 0. Batzer. 1957. Mean head widths
for spruce budworm larval instars in Minnesota and
associated data. Ibid. 50: 499.
Ahmad, S., and A. J. Forgash.
Busvine, J. R.
1957. A Critical Review of the Tech­
niques for Testing Insecticides, Commonwealth In­
stitute of Entomology, London. 208 pp.
Dyar, H. G. 1890. The number of molts of Lepidopter­
ous larvae. Psyche 5: 420-22.
Fox, R. C., N. H. Anderson, S. C. Garner, and A. I.
Walker. 1972. Larval head-capsules of the N an­
tucket pine tip moth. Ann. Entomol. Soc. Am. 65 :
513-4.
Gaines, }. C., and F. L. Campbell. 1935. Dyar's rule
as related to the number of instars of the corn ear
worm, H eliothis obsoleta (Fab.) , collected in the
field. Ibid. 28: 445-61.
Hoxie, R. P., and S. G. Wellso. 1974. Cereal leaf bee­
tle instars and sex, defined by larval head capsule
widths. Ibid. 67: 183-6.
Kishi, Y. 1971. Reconsideration of the method to meas­
ure the larval instars by use of the frequency distri­
bution
of
head-capsule
Entomol. 103: 1011-5.
widths
or
lengths.
Can.
Lyon, R. L, C. E. Richmond, J. L. Robertson, and B. A.
Lucas. 1972. Rearing diapause and diapause-free
western spruce budworm ( Choristoneura occiden­
talis) (Lepidoptera : Tortricidae) on an artificial
diet. Ibid. 104: 417-26.
1975. Bioassay of nucleopolyhedrosis vi­
rus against larval instars of Malacosom.a neustria. ].
Invertebr. Pathol. 25: 343-8.
McGugan, B. M. 1954. N eedle-mining habits and lar­
val instars of the spruce budworm. Can. Entomol.
86: 439-54.
Parker, D. L., and M. W. Moyer. 1972. Biology of a
leafroller, Archips negundamts, in Utah (Lepidop­
tera: Tortricidae) . Ann. Entomol. Soc. Am. 65:
1415-8.
Peterson, A., and G. }. Haeussler. 1928. Some obser­
vations on the number of larval instars of the orien­
tal peach moth, Laspeyresia molesta Busck. ]. Econ.
Entomol. 21: 843-52.
Richards, 0. W. 1949. The relation between measure­
ments of the successive instars of insects. Proc. R.
Entomol. Soc. London (A) . 24: 8-10.
Satterthwait, A. F. 1933. Larval instars and feeding
of the black cutworm, Agrotis ypsilon Rott. J. Agric.
Res. 46: 517-30.
Schmidt, F. H., and W. L. Lauer. 1977. Developmen­
tal polymorphism in Choristoneura spp. (Lepidoptera:
Tortricidae) . Ann. Entomol. Soc. Am. 70: 112-8.
Shepard, H. H. 1951. The Chemistry and Action of
Insecticides, McGraw-Hill, N ew York. 504 pp.
Stairs, G. R. 1965. Quantitative differences in suscepti­
bility to nuclear-polyhedrosis virus among larval in­
stars of the forest tent caterpillar, Malacosoma dis­
stria (Hubner) . ]. Invertebr. Pathol. 7: 427-9.
Taylor, R. L. 1931. On 'Dyar's Rule' and its applica­
tion to sawfly larvae. Ann. Entomol. Soc. Am. 24:
451-66.
Vanderwerker, G. K., and H. M. Kulman. 1974. Sta­
dium and sex determination of yellowheaded spruce
sawfly larvae, Pikou.ema alaskensis. Ibid. 67: 29-31.
Wilson, L. F. 1974. Life history and habits of a leaf
tier, Aroga argutiola (Lepidoptera: Gelechiidae) , on
sweet fern in Michigan. Can. Entomol. 106: 991-4.
Magnoler, A.