CH908 UNIVERSITY OF WARWICK MASTER OF SCIENCE EXAMINATIONS:
advertisement
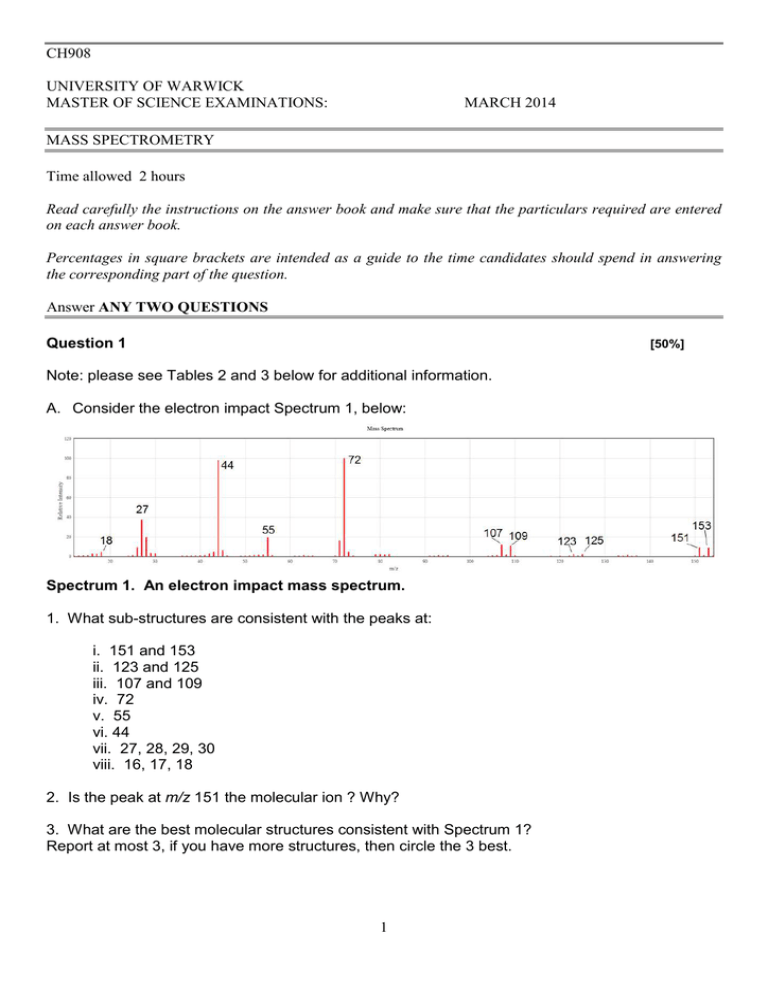
CH908 UNIVERSITY OF WARWICK MASTER OF SCIENCE EXAMINATIONS: MARCH 2014 MASS SPECTROMETRY Time allowed 2 hours Read carefully the instructions on the answer book and make sure that the particulars required are entered on each answer book. Percentages in square brackets are intended as a guide to the time candidates should spend in answering the corresponding part of the question. Answer ANY TWO QUESTIONS Question 1 [50%] Note: please see Tables 2 and 3 below for additional information. A. Consider the electron impact Spectrum 1, below: Spectrum 1. An electron impact mass spectrum. 1. What sub-structures are consistent with the peaks at: i. 151 and 153 ii. 123 and 125 iii. 107 and 109 iv. 72 v. 55 vi. 44 vii. 27, 28, 29, 30 viii. 16, 17, 18 2. Is the peak at m/z 151 the molecular ion ? Why? 3. What are the best molecular structures consistent with Spectrum 1? Report at most 3, if you have more structures, then circle the 3 best. 1 B. Consider the electron impact spectrum 2: Spectrum 2. An electron impact mass spectrum The molecule in Spectrum 2 generates a [M+H]+ ion at 128.1 from electrospray mass spectrometry. 1. What sub-structures are consistent with the peaks at: i. 127 ii. 100 iii. 87 iv. 84 v. 68 and 70 vi. 44 vii. 41 viii. 27, 29, 30, 31 ix. 14, 15, 16, 17, and 18 2. What are the best molecular structures consistent with Spectrum 2? 2 Question 2 [50%] (a) The spectrum below shows a high resolution electrospray ionisation tandem mass spectrum of a peptide called L3BBS using electron capture dissociation: Note: please see tables 2 and 3 below for additional information. (i) How does electron capture dissociation work? (ii) Which peak represents the precursor ion peak? (iii) This spectrum is for a peptide with the sequence, pQKLGNQWAVGHLM-NH2, where ‘pQ’ indicates a ‘cyclized’ pyroglutamic acid and ‘–NH2’ indicates an amidated c-terminus. Make a cleavage diagram and mark all of the labelled peaks on it. (iv) Are there any complementary fragment ions observed? If so, which? (v) This peptide has modified endgroups. The c-terminius is amidated, and the n-terminus is cyclised to a pyroglutamic acid. How would these modifications be detectable in the spectrum? (vi) Draw the full structure of the c3 ion. (vii) Draw the full structure of the z7 ion (sidechains can be defined as ‘RX’ where x is the single letter amino acid code). (b) (i) Outline the principles of Electrospray ionization. (ii) Why is it necessary to ionize a molecule to measure its mass using a mass spectrometer? (c) (i) How does a quadrupole mass spectrometer measure mass? (ii) Sketch a Mathieu stability diagram and note its key features. (iii) How is the low mass cut-off of a quadrupole mass analyzer running in the rf-only mode calculated? 3 Table 1 Element H C N O F Cl Br Atomic mass 1.007825 2.014102 12.000000 13.003355 14.003074 15.000109 15.994915 16.999132 17.999160 18.998403 34.968853 36.9659 78.918338 80.916291 Stable Isotopes 1 H 2 H 12 C 13 C 14 N 15 N 16 O 17 O 18 O 19 F 35 Cl 37 Cl 79 Br 81 Br 4 Isotope Abundance (%) 99.99 0.01 98.9 1.1 99.6 0.4 99.76 0.04 0.20 100.0 68.0 32.0 50.7 49.3 Table 2 5 6






