Nitrogen Chemistry in Titan’s Upper Atmosphere
J. A.
1Division
1
Kammer ,
D. E.
2
Shemansky ,
X.
1
Zhang ,
Y. L.
1
Yung
of Geological and Planetary Sciences, California Institute of Technology, Pasadena, CA
2Planetary
and Space Science Division, Space Environment Technologies, Pasadena, CA
Introduction:
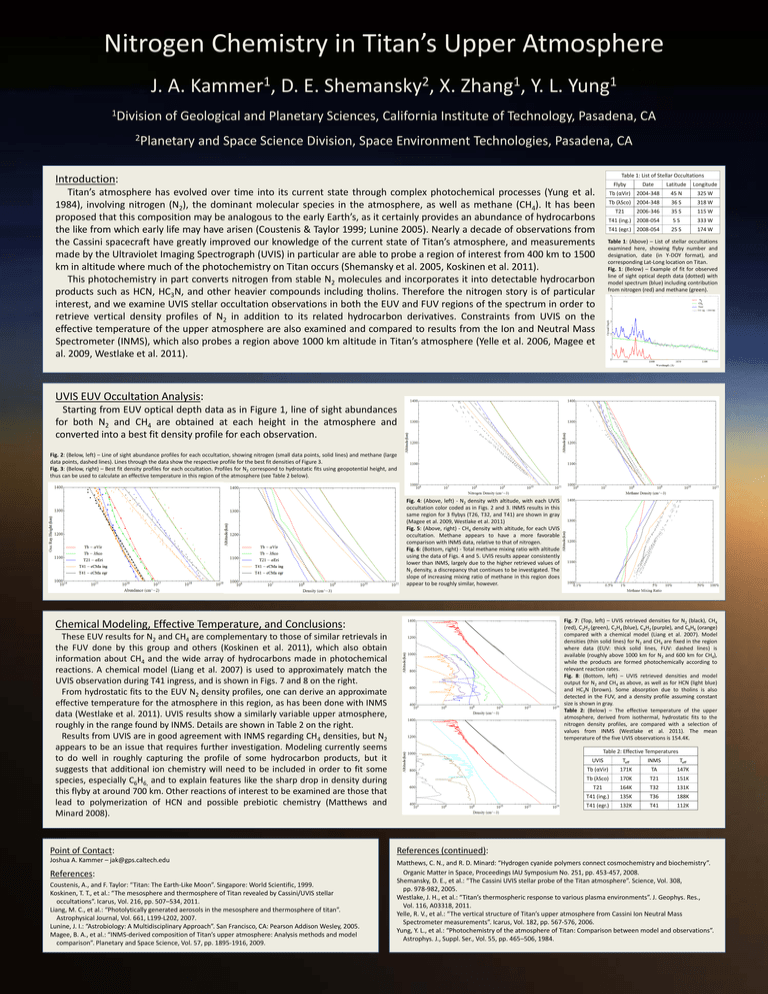
Table 1: List of Stellar Occultations
Titan’s atmosphere has evolved over time into its current state through complex photochemical processes (Yung et al.
1984), involving nitrogen (N2), the dominant molecular species in the atmosphere, as well as methane (CH4). It has been
proposed that this composition may be analogous to the early Earth’s, as it certainly provides an abundance of hydrocarbons
the like from which early life may have arisen (Coustenis & Taylor 1999; Lunine 2005). Nearly a decade of observations from
the Cassini spacecraft have greatly improved our knowledge of the current state of Titan’s atmosphere, and measurements
made by the Ultraviolet Imaging Spectrograph (UVIS) in particular are able to probe a region of interest from 400 km to 1500
km in altitude where much of the photochemistry on Titan occurs (Shemansky et al. 2005, Koskinen et al. 2011).
This photochemistry in part converts nitrogen from stable N2 molecules and incorporates it into detectable hydrocarbon
products such as HCN, HC3N, and other heavier compounds including tholins. Therefore the nitrogen story is of particular
interest, and we examine UVIS stellar occultation observations in both the EUV and FUV regions of the spectrum in order to
retrieve vertical density profiles of N2 in addition to its related hydrocarbon derivatives. Constraints from UVIS on the
effective temperature of the upper atmosphere are also examined and compared to results from the Ion and Neutral Mass
Spectrometer (INMS), which also probes a region above 1000 km altitude in Titan’s atmosphere (Yelle et al. 2006, Magee et
al. 2009, Westlake et al. 2011).
Flyby
Date
Latitude
Longitude
Tb (αVir)
2004-348
45 N
325 W
Tb (λSco) 2004-348
36 S
318 W
2006-346
35 S
115 W
T41 (ing.) 2008-054
5S
333 W
T41 (egr.) 2008-054
25 S
174 W
T21
Table 1: (Above) – List of stellar occultations
examined here, showing flyby number and
designation, date (in Y-DOY format), and
corresponding Lat-Long location on Titan.
Fig. 1: (Below) – Example of fit for observed
line of sight optical depth data (dotted) with
model spectrum (blue) including contribution
from nitrogen (red) and methane (green).
UVIS EUV Occultation Analysis:
Starting from EUV optical depth data as in Figure 1, line of sight abundances
for both N2 and CH4 are obtained at each height in the atmosphere and
converted into a best fit density profile for each observation.
Fig. 2: (Below, left) – Line of sight abundance profiles for each occultation, showing nitrogen (small data points, solid lines) and methane (large
data points, dashed lines). Lines through the data show the respective profile for the best fit densities of Figure 3.
Fig. 3: (Below, right) – Best fit density profiles for each occultation. Profiles for N2 correspond to hydrostatic fits using geopotential height, and
thus can be used to calculate an effective temperature in this region of the atmosphere (see Table 2 below).
Fig. 4: (Above, left) - N2 density with altitude, with each UVIS
occultation color coded as in Figs. 2 and 3. INMS results in this
same region for 3 flybys (T26, T32, and T41) are shown in gray
(Magee et al. 2009, Westlake et al. 2011)
Fig. 5: (Above, right) - CH4 density with altitude, for each UVIS
occultation. Methane appears to have a more favorable
comparison with INMS data, relative to that of nitrogen.
Fig. 6: (Bottom, right) - Total methane mixing ratio with altitude
using the data of Figs. 4 and 5. UVIS results appear consistently
lower than INMS, largely due to the higher retrieved values of
N2 density, a discrepancy that continues to be investigated. The
slope of increasing mixing ratio of methane in this region does
appear to be roughly similar, however.
Chemical Modeling, Effective Temperature, and Conclusions:
Fig. 7: (Top, left) – UVIS retrieved densities for N2 (black), CH4
(red), C2H2 (green), C2H4 (blue), C4H2 (purple), and C6H6 (orange)
compared with a chemical model (Liang et al. 2007). Model
densities (thin solid lines) for N2 and CH4 are fixed in the region
where data (EUV: thick solid lines, FUV: dashed lines) is
available (roughly above 1000 km for N2 and 600 km for CH4),
while the products are formed photochemically according to
relevant reaction rates.
Fig. 8: (Bottom, left) – UVIS retrieved densities and model
output for N2 and CH4 as above, as well as for HCN (light blue)
and HC3N (brown). Some absorption due to tholins is also
detected in the FUV, and a density profile assuming constant
size is shown in gray.
Table 2: (Below) – The effective temperature of the upper
atmosphere, derived from isothermal, hydrostatic fits to the
nitrogen density profiles, are compared with a selection of
values from INMS (Westlake et al. 2011). The mean
temperature of the five UVIS observations is 154.4K.
These EUV results for N2 and CH4 are complementary to those of similar retrievals in
the FUV done by this group and others (Koskinen et al. 2011), which also obtain
information about CH4 and the wide array of hydrocarbons made in photochemical
reactions. A chemical model (Liang et al. 2007) is used to approximately match the
UVIS observation during T41 ingress, and is shown in Figs. 7 and 8 on the right.
From hydrostatic fits to the EUV N2 density profiles, one can derive an approximate
effective temperature for the atmosphere in this region, as has been done with INMS
data (Westlake et al. 2011). UVIS results show a similarly variable upper atmosphere,
roughly in the range found by INMS. Details are shown in Table 2 on the right.
Results from UVIS are in good agreement with INMS regarding CH4 densities, but N2
appears to be an issue that requires further investigation. Modeling currently seems
to do well in roughly capturing the profile of some hydrocarbon products, but it
suggests that additional ion chemistry will need to be included in order to fit some
species, especially C6H6, and to explain features like the sharp drop in density during
this flyby at around 700 km. Other reactions of interest to be examined are those that
lead to polymerization of HCN and possible prebiotic chemistry (Matthews and
Minard 2008).
Point of Contact:
Joshua A. Kammer – jak@gps.caltech.edu
References:
Coustenis, A., and F. Taylor: “Titan: The Earth-Like Moon”. Singapore: World Scientific, 1999.
Koskinen, T. T., et al.: “The mesosphere and thermosphere of Titan revealed by Cassini/UVIS stellar
occultations”. Icarus, Vol. 216, pp. 507–534, 2011.
Liang, M. C., et al.: “Photolytically generated aerosols in the mesosphere and thermosphere of titan”.
Astrophysical Journal, Vol. 661, L199-L202, 2007.
Lunine, J. I.: “Astrobiology: A Multidisciplinary Approach”. San Francisco, CA: Pearson Addison Wesley, 2005.
Magee, B. A., et al.: “INMS-derived composition of Titan’s upper atmosphere: Analysis methods and model
comparison”. Planetary and Space Science, Vol. 57, pp. 1895-1916, 2009.
Table 2: Effective Temperatures
UVIS
Teff
INMS
Teff
Tb (αVir)
171K
TA
147K
Tb (λSco)
170K
T21
151K
T21
164K
T32
131K
T41 (ing.)
135K
T36
188K
T41 (egr.)
132K
T41
112K
References (continued):
Matthews, C. N., and R. D. Minard: “Hydrogen cyanide polymers connect cosmochemistry and biochemistry”.
Organic Matter in Space, Proceedings IAU Symposium No. 251, pp. 453-457, 2008.
Shemansky, D. E., et al.: “The Cassini UVIS stellar probe of the Titan atmosphere”. Science, Vol. 308,
pp. 978-982, 2005.
Westlake, J. H., et al.: “Titan’s thermospheric response to various plasma environments”. J. Geophys. Res.,
Vol. 116, A03318, 2011.
Yelle, R. V., et al.: “The vertical structure of Titan’s upper atmosphere from Cassini Ion Neutral Mass
Spectrometer measurements”. Icarus, Vol. 182, pp. 567-576, 2006.
Yung, Y. L., et al.: “Photochemistry of the atmosphere of Titan: Comparison between model and observations”.
Astrophys. J., Suppl. Ser., Vol. 55, pp. 465–506, 1984.