Document 12620393
advertisement
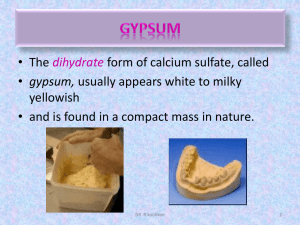
A number of gypsum products are used in dentistry as adjuncts to dental operation. 1. 2. 3. 4. 5. Type I: Impression plaster. Type II: Dental plaster. Type III: Dental stone (medium strength stone). Type IV: Improved stone (high strength stone) (die stone). Type V: high strength/high expansion stone. 12345- Impression plaster. Mounting the casts to the articulation. Form casts and dies. Used as a binder for silica. Used as a mold for processing dental polymers. 6- Used for bite registration (record centric jaw relation). Properties of ideal model material (gypsum products): Dimensional stability, no expansion or contraction during or after setting. High compressive strength to withstand the force applied on it. Hardness, soft material can be easily scratched. Reproduce the fine details. Produce smooth surface. Reasonable setting time. Compatible with the impression material. Can be disinfected without damaging the surface. Most gypsum products are obtained from natural gypsum rock. Because gypsum is the dihydrate form of calcium sulfate (CaSO4. 2H2O), on heating, it loses 1.5 g mol of its 2 g mol of H2O and is converted to calcium sulfate hemihydrate (CaSO4. 0.5H2O). When calcium sulfate hemihydrate is mixed with water, the reverse reaction takes place, and the calcium sulfate hemihydrate is converted back to calcium sulfate dihydrate. 1- Plasters are produced when the gypsum mineral is heated in an open kettle at a temperature of about 110° to 120°C (dry calcination). The hemihydrate produced is called β-calcium sulfate hemihydrate. Such a powder is known to have a somewhat irregular shape and is porous in nature. These plasters are used in formulating model and lab plasters. 2- Stones are produced when the gypsum is dehydrated under pressure and in the presence of water vapor at about 125°C (wet calcination), the product is called hydrocal. The powder particles of this product are more uniform in shape and denser than the particles of plaster. Calcium sulfate hemihydrate produced in this manner is designated as α-calcium sulfate hemihydrate. Hydrocal is used in making low- to moderate-strength dental stones. 3- High-strength stones are produced when the gypsum rock is boiling in a 30% calcium chloride solution, after which the chloride is washed away with hot water (100°C), the product is called densite, and the material is ground to the desired fineness. This variety is made by gypsum The calcium sulfate hemihydrate in the presence of 100°C water does not react to form calcium sulfate dihydrate because at this temperature their solubilities are the same. The powder obtained by this process is the densest of the types. 1- Potassium sulfate, and terra alba (set calcium sulfate dihydrate) are effective accelerators. 2- Sodium chloride in small amounts shortens the setting reaction but increases the setting expansion of the gypsum mass. 3- Sodium citrate is a dependable retarder. 4- A mixture of calcium oxide (0.1%) and gum arabic (1%) reduces the amount of water necessary to mix gypsum products, resulting in improved properties. The setting reaction is explained on the basis of difference in the solubilities of calcium sulfate dihydrate and hemihydrate. Hemihydrate is four times more soluble than dihydrate. When hemihydrate is mixed in water a suspension is formed which is fluid and workable. Hemihydrate dissolves until it forms a saturated solution. Some dihydrate is formed due to the reaction. Since solubility of dihydrate is much less than hemihydrate, the saturated hemihydrate is supersaturated with respect to the dihydrate. All supersaturated solutions are unstable. So the dihydrate crystals precipitate out. As the dihydrate precipitates out, the solution is no longer saturated with hemihydrate and so it continues to dissolve. The process continues until all hemihydrate converts to dihydrate. Other theories include . The mixing process, called spatulation, has a definite effect on the setting time and setting expansion of the material. Within practical limits an increase in the amount of spatulation (either speed of spatulation or time or both) shortens the setting time. Obviously when the powder is placed in water, the chemical reaction starts, and some calcium sulfate dihydrate is formed. During spatulation the newly formed calcium sulfate dihydrate breaks down to smaller crystals and starts new centers of nucleation, around which the calcium sulfate dihydrate can be precipitated. Because an increased amount of spatulation causes more nuclei centers to be formed, the conversion of calcium sulfate hemihydrate to dihydrate requires somewhat less time. The first effect of increasing temperature is a change in the relative solubilities of calcium sulfate hemihydrate and calcium sulfate dihydrate, which alters the rate of the reaction. As the temperature increases, the solubility ratios decrease, until 100°C is reached and the ratio becomes one. As the ratio of the solubilities becomes lower, the reaction is slowed, and the setting time is increased. The second effect is the change in ion mobility with temperature. In general, as the temperature increases, the mobility of the calcium and sulfate ions increases, which tends to increase the rate of the reaction and shorten the setting time. Practically, the effects of these two phenomena are superimposed, and the total effect is observed. Plaster can easily absorb water vapor from a humid atmosphere to form calcium sulfate dihydrate. The presence of small amounts of calcium sulfate dihydrate on the surface of the hemihydrate powder provides additional nuclei for crystallization. Increased contamination by moisture produces sufficient dihydrate on the hemihydrate powder to retard the solution of the hemihydrate. Experience has shown that the common overall effect of contamination of gypsum products with moisture from the air during storage is a lengthening of the setting time. Colloidal systems such as agar and alginate retard the setting of gypsum products. Accelerators such as potassium sulfate are added to improve the surface quality of the set CaSO4 .2H20 against agar or alginate. Liquids with low pH, such as saliva, retard the setting reaction. Liquids with high pH accelerate setting. The operator also can change the setting time of model plaster to a certain extent by changing the water/powder (W/P) ratio. The W/P ratio has a pronounced effect on the setting time. The more water in the mix of model; (plaster, dental stone, or high-strength dental stone); the longer the setting time. When set, gypsum products show relatively high values of compressive strength. The compressive strength is inversely related to the W/P ratio of the mix. The more water used to make the mix, the lower the compressive strength. Model plaster has the greatest quantity of excess water, whereas high-strength dental stone contains the least excess water. The set model plaster is more porous than set dental stone, causing the apparent density of model plaster to be lower. After most excess water is evaporated from the surface, the hardness will increase. Attempts have been made to increase the hardness of gypsum products by impregnating the set gypsum with epoxy or methyl methacrylate monomer that is allowed to polymerize. The tensile strength of model plaster and dental stone is important in structures in which bending tends to occur because of lateral force applications, such as the removal of casts from flexible impressions. Because of the brittle nature of gypsum materials, the teeth on the cast may fracture rather than bend. ANSI/ADA Specification No. 25 requires that types I and II reproduce a groove 75 μm in width, whereas types III, IV, and V reproduce a groove 50 μm in width. Air bubbles are often formed at the interface of the impression and gypsum cast because freshly mixed gypsum does not wet some rubber impression materials (e.g., some silicone types). The use of vibration during the pouring of a cast reduces the presence of air bubbles. Contamination of the impression with saliva or blood can also affect the detail reproduction. When set, all gypsum products show a measurable linear expansion. Under ordinary conditions, plasters have (0.2-0.3 %) setting expansion, low to moderate strength dental stone about (0.15-0.25 %), and highstrength dental stone only (0.08-0.10 %). Typically, (over 75 %) of the expansion observed at 24 hours occurs during the first hour of setting. Increasing the W/P ratio; reducing the setting expansion. If during the setting process, the gypsum materials are immersed in water, the setting expansion increases slightly. This is called hygroscopic expansion.