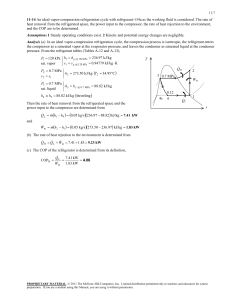
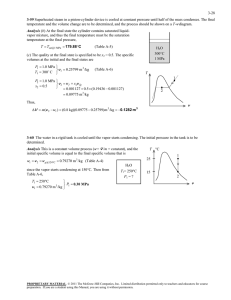
using three methods. , N , CO
advertisement
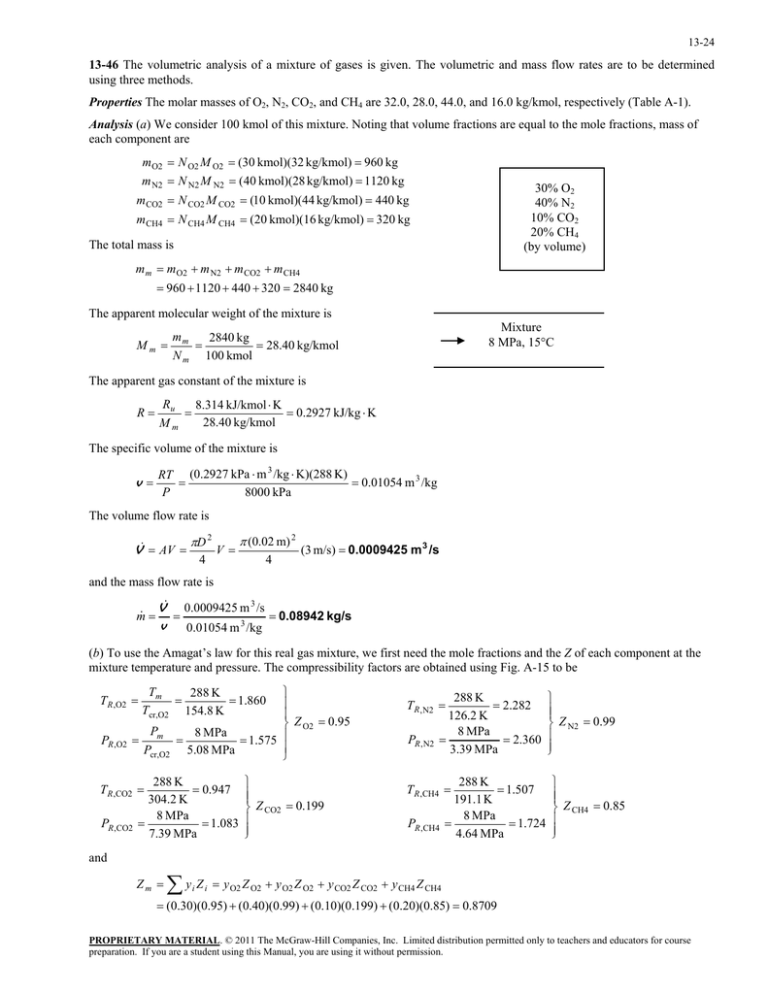
13-24 13-46 The volumetric analysis of a mixture of gases is given. The volumetric and mass flow rates are to be determined using three methods. Properties The molar masses of O2, N2, CO2, and CH4 are 32.0, 28.0, 44.0, and 16.0 kg/kmol, respectively (Table A-1). Analysis (a) We consider 100 kmol of this mixture. Noting that volume fractions are equal to the mole fractions, mass of each component are m O2 N O2 M O2 (30 kmol)(32 kg/kmol) 960 kg m N2 N N2 M N2 (40 kmol)(28 kg/kmol) 1120 kg 30% O2 40% N2 10% CO2 20% CH4 (by volume) m CO2 N CO2 M CO2 (10 kmol)(44 kg/kmol) 440 kg m CH4 N CH4 M CH4 (20 kmol)(16 kg/kmol) 320 kg The total mass is m m m O2 m N2 m CO2 m CH4 960 1120 440 320 2840 kg The apparent molecular weight of the mixture is Mm Mixture 8 MPa, 15C mm 2840 kg 28.40 kg/kmol N m 100 kmol The apparent gas constant of the mixture is R Ru 8.314 kJ/kmol K 0.2927 kJ/kg K 28.40 kg/kmol Mm The specific volume of the mixture is v RT (0.2927 kPa m 3 /kg K)(288 K) 0.01054 m 3 /kg P 8000 kPa The volume flow rate is V AV D 2 V 4 (0.02 m) 2 4 (3 m/s) 0.0009425 m 3 /s and the mass flow rate is m V 0.0009425 m 3 /s 0.08942 kg/s v 0.01054 m 3 /kg (b) To use the Amagat’s law for this real gas mixture, we first need the mole fractions and the Z of each component at the mixture temperature and pressure. The compressibility factors are obtained using Fig. A-15 to be Z O2 0.95 8 MPa 1.575 5.08 MPa T R ,O2 Tm 288 K 1.860 Tcr,O2 154.8 K T R , N2 PR ,O2 Pm Pcr,O2 PR , N2 288 K 0.947 304.2 K 8 MPa 1.083 7.39 MPa T R ,CO2 PR ,CO2 Z CO2 0.199 288 K 2.282 126.2 K 8 MPa 2.360 3.39 MPa 288 K 1.507 191.1 K 8 MPa 1.724 4.64 MPa T R ,CH4 PR ,CH4 Z N2 0.99 Z CH4 0.85 and Zm y Z i i y O2 Z O2 y O2 Z O2 y CO2 Z CO2 y CH4 Z CH4 (0.30)(0.95) (0.40)(0.99) (0.10)(0.199) (0.20)(0.85) 0.8709 PROPRIETARY MATERIAL. © 2011 The McGraw-Hill Companies, Inc. Limited distribution permitted only to teachers and educators for course preparation. If you are a student using this Manual, you are using it without permission. 13-25 Then, v Z m RT (0.8709)(0.2927 kPa m 3 /kg K)(288 K) 0.009178 m 3 /kg P 8000 kPa V 0.0009425 m 3 /s m V 0.0009425 m 3 /s 0.10269 kg/s v 0.009178 m 3 /kg (c) To use Kay's rule, we need to determine the pseudo-critical temperature and pseudo-critical pressure of the mixture using the critical point properties of mixture gases. Tcr , m yT i cr ,i y O2 Tcr ,O2 y N2 Tcr , N2 y CO2 Tcr ,CO2 y CH4 Tcr ,CH4 (0.30)(154.8 K) (0.40)(126.2 K) (0.10)(304.2 K) (0.20)(191.1 K) 165.6 K Pcr , m y P i cr ,i y O2 Pcr ,O2 y N2 Pcr , N2 y CO2 Pcr ,CO2 y CH4 Pcr ,CH4 (0.30)(5.08 MPa) (0.40)(3.39 MPa) (0.10)(7.39 MPa) (0.20)(4.64 MPa) 4.547 MPa and TR PR Tm ' Tcr, m Pm ' Pcr, m Z m 0.92 8 MPa 1.759 4.547 MPa 288 K 1.739 165.6 K (Fig. A-15) Then, v Z m RT (0.92)(0.2927 kPa m 3 /kg K)(288 K) 0.009694 m 3 /kg P 8000 kPa V 0.0009425 m 3 /s m V 0.0009425 m 3 /s 0.009723 kg/s v 0.09694 m 3 /kg PROPRIETARY MATERIAL. © 2011 The McGraw-Hill Companies, Inc. Limited distribution permitted only to teachers and educators for course preparation. If you are a student using this Manual, you are using it without permission.