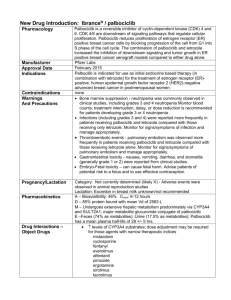
Effects of a non-steroidal aromatase inhibitor on ovarian function in cattle
advertisement

CSIRO PUBLISHING
Reproduction, Fertility and Development, 2012, 24, 631–640
http://dx.doi.org/10.1071/RD11239
Effects of a non-steroidal aromatase inhibitor on ovarian
function in cattle
M. Jimena YapuraA, Reuben J. Mapletoft B, Jaswant SinghA, Roger PiersonC,
Jonathan NaileD, John P. Giesy A,D, Hong ChangD, Eric Higley D,
Markus HeckerD and Gregg P. AdamsA,E
A
Department of Veterinary Biomedical Sciences, Western College of Veterinary Medicine,
University of Saskatchewan, Saskatoon, Saskatchewan S7N 5B4, Canada.
B
Department of Large Animal Clinical Sciences, Western College of Veterinary Medicine,
University of Saskatchewan, Saskatoon, Saskatchewan S7N 5B4, Canada.
C
Department of Obstetrics, Gynecology and Reproductive Sciences, College of Medicine,
University of Saskatchewan, Saskatoon, Saskatchewan S7N 0W8, Canada.
D
Toxicology Centre, University of Saskatchewan, Saskatoon, Saskatchewan S7N 0W8, Canada.
E
Corresponding author. Email: gregg.adams@usask.ca
Abstract. Effects of the non-steroidal aromatase inhibitor letrozole on ovarian function in cattle were determined. The
hypothesis that letrozole would arrest growth of the dominant follicle, resulting in emergence of a new follicular wave at a
predictable post-treatment interval, was tested. Heifers were assigned randomly to four groups 4 days after follicular
ablation (,2½ days after wave emergence) and given intravenous doses of 500 (n ¼ 9), 250 (n ¼ 10), or 125 mg kg1
(n ¼ 10) letrozole or phosphate-buffered saline (controls; n ¼ 10). Blood was collected and ovarian structures were
monitored daily by transrectal ultrasonography. Plasma concentrations of LH and FSH were measured by radioimmunoassay; plasma concentrations of letrozole were determined by high-performance liquid chromatography tandem mass
spectrometry. A single intravenous dose of letrozole did not induce regression of the dominant follicle present at the time
of treatment, nor did it directly affect FSH release. Conversely, treatment with letrozole increased endogenous
concentrations of LH and extended the lifespan of the dominant follicle, which delayed the next FSH surge and
subsequent follicular wave emergence. Letrozole continues to have potential as a non-steroidal treatment for controlling
ovarian function in cattle.
Additional keywords: bovine reproduction, oestradiol, ovarian synchronisation.
Received 20 September 2011, accepted 6 October 2011, published online 25 November 2011
Introduction
Of the strategies used to control ovarian function in cattle,
treatment with oestrogen in combination with progesterone has
been effective for synchronising follicle wave emergence and
ovulation (Bo et al. 1995a, 1995b). Steroid-induced wave synchronisation is brought about by regression of the dominant
follicle, followed by resurgence in circulating FSH and subsequent emergence of a new follicular wave consistently following treatment. The degree of synchrony achieved with protocols
involving oestradiol (E2) and progesterone has permitted
effective use of fixed-time AI in cattle (Bridges et al. 1999;
Martinez et al. 2000; Colazo et al. 2004) and more efficient use
of time and labour for multiple ovulation and embryo transfer, as
well as conventional breeding management (Bo et al. 1995b;
Mapletoft et al. 2003).
Journal compilation Ó CSIRO 2012
The use of natural or synthetic oestrogens in food-producing
animals has been the subject of considerable controversy (for a
review, see Umberger 1975). Increasing concern regarding the
toxicity of hormonal preparations used as growth promoters in
cattle and the potential carcinogenic effects of steroid hormone
residues in meat or milk has led to a prohibition of the use of E2
and other steroid hormones as growth promoters in animals
designated for human consumption in all member states of the
European Union as of 1 January 1989 (Andersson and Skakkebaek 1999; Fritsche and Steinhart 1999; Daxenberger et al.
2001; US Department of Agriculture, Foreign Agricultural
Service 2003). Further, the use of E2 and its ester derivatives
for purposes of reproductive management was prohibited in the
European Union on 14 October 2006 (Official Journal of
the European Union 2003). These actions in Europe led to the
www.publish.csiro.au/journals/rfd
632
Reproduction, Fertility and Development
subsequent prohibition of the use of E2 esters in lactating dairy
animals in New Zealand and Australia in 2007 (Lane et al.
2008). Although the use of E2 and zeranol (an oestrogen-like
compound) as growth promoters is still permitted in the US (US
Food and Drug Administration 2003) and Canada (Health
Canada Drugs and Health Products 2005), they cannot be used
for synchronisation of oestrus, except by prescription and
custom compounding. However, veterinary compounding of
pharmaceuticals for food-producing animals has recently come
under scrutiny in the US and is discouraged (US Food and Drug
Administration 2003; Gibbs 2004). This situation negatively
impacts implementation of reproductive technologies in cattle
production systems, limiting potential reproductive efficiency
and genetic improvement provided by the use of AI and embryo
transfer (Lane et al. 2008). Alternative methods for controlling
ovarian function in cattle that do not have harmful effects on
human or animal health are needed.
Aromatase inhibitors prevent the body from producing its
own oestrogens. Therefore, we hypothesised that aromatase
inhibitors may be an effective alternative to control ovarian
follicular development in cattle. Letrozole (4-[(4-cyanophenyl)(1,2,4-triazol-1-yl)methyl]benzonitrile), a non-steroidal aromatase inhibitor, inactivates the aromatase enzyme by reversibly
binding to the heme group of the P450 subunit of the aromatase
enzyme. Letrozole is used as an adjuvant treatment for hormoneresponsive breast cancer in post-menopausal women (Cohen
et al. 2002) and has been used as a fertility treatment for women
undergoing assisted reproduction because of its putative effect
on FSH secretion through removal of the negative feedback of
E2 (Requena et al. 2008). A 5-day regimen of letrozole (2.5 mg
day1 from Days 3 to 7; Day 0 ¼ beginning of menses) has been
used for ovarian stimulation in women (Mitwally and Casper
2002a) and larger or increasing doses of letrozole have been
used to induce ovarian superstimulation in women (Al-Fadhli
et al. 2006; Mitwally et al. 2008).
Contrary to the proposed hypothesis based on observations in
women (Requena et al. 2008), letrozole treatment of beef heifers
with single intravenous dose on Day 3 after ovulation or with a
3-day regimen from Day 1 to 3, Day 3 to 5 or Day 5 to 7 after
ovulation did not induce follicular atresia or hasten emergence
of a new follicular wave (Yapura et al. 2009). Letrozole
treatment did not induce increases in circulating FSH concentrations. Rather, letrozole treatment increased mean plasma LH
concentrations, which resulted in a prolonged period of dominance of the extant dominant follicle and delayed emergence of
the next follicular wave. Furthermore, a luteotrophic effect of
letrozole treatment was documented by larger corpus luteum
diameters in those heifers treated with letrozole in a 3-day
regimen.
The overall objective of the present study was to develop an
effective, safe and steroid-free protocol for controlling ovarian
follicular wave dynamics in cattle. Specific objectives of the
study were to gain an understanding of the effects of an
aromatase inhibitor (letrozole) on bovine ovarian function and
to establish a minimum effective dose of letrozole in cattle. We
tested the hypothesis that letrozole will terminate the growth of
the extant dominant follicle (i.e. the dominant follicle present at
the time of treatment), resulting in a rise in circulating FSH
M. J. Yapura et al.
concentrations and followed by the emergence of a new wave of
follicular growth at a predictable interval after treatment.
Materials and methods
In vitro culture of bovine granulosa cells
Bovine granulosa cells were cultured in serum-free medium as
described previously (Zamberlam et al. 2011). Unless stated
otherwise, materials were obtained from Invitrogen Life Technologies (Burlington, ON, Canada). Briefly, bovine ovaries were
collected from adult cows at an abattoir and were transported to
the laboratory in phosphate-buffered saline (PBS) containing
penicillin (100 IU mL1) and streptomycin (100 mg mL1). Follicles between 2 and 5 mm in diameter were dissected and
granulosa cells were collected by rinsing the follicle wall with
Dulbecco’s modified Eagle’s medium Nutrient Mixture F-12
(DMEM/F12). The granulosa cells were washed twice by centrifugation at 980g for 20 min each time and suspended in
DMEM/F12 containing HEPES (15 mM), sodium bicarbonate
(10 mM), sodium selenite (4 ng mL1), bovine serum albumin
(BSA; 0.1%; Sigma-Aldrich, Oakville, ON, Canada), penicillin
(100 IU mL1), streptomycin (100 mg mL1), transferrin
(2.5 mg mL1), non-essential amino acid mix (1.1 mM), androstenedione (A4; 107 M at the start of culture and 106 M at each
medium change) and insulin (10 ng mL1). The number of cells
was counted with a haemocytometer and viable cells were
assessed by 0.4% Trypan blue dye exclusion. Cells were seeded
into 24-well tissue culture plates (Sarstedt, Montréal, QC,
Canada) at a density of 1 106 viable cells per well in 1 mL
medium. Cultures were maintained at 378C in 5% CO2 in air for 6
days, with 700 mL medium being replaced every 2 days. On Day
2 of culture, cells were treated with 1 ng mL1 FSH to stimulate
aromatase expression and E2 secretion. On Day 4, cells were
treated with medium including FSH alone, with FSH plus
letrozole (15 or 50 ng mL1) and without FSH for 48 h. Medium
samples were collected on Day 6 and stored at 208C until
steroid assay. All series of cultures were performed on at least
three different pools of cells collected on different occasions.
Cattle
Hereford-cross beef heifers (Bos taurus), 14–20 months of age
and weighing between 295 and 450 kg, were chosen from a herd
of 50 heifers maintained in outdoor corrals at the University of
Saskatchewan Goodale Research Farm (528N, 1068W). Heifers
were fed alfalfa and/or grass hay and grain to gain approximately 1.3 kg per head per day and had water available
ad libitum during the experimental period from July to October.
Heifers were initially examined by transrectal ultrasonography
(7.5 MHz linear-array transducer; SSD-900; Aloka, Tokyo,
Japan). The presence of a corpus luteum (CL) was used to
confirm that the heifers were postpubertal and cycling (Pierson
and Ginther 1987).
Treatments and examinations
Heifers in which a CL was detected during the initial examination were given 500 mg, i.m., cloprostenol (PGF; Estrumate;
Schering-Plough Animal Health, Pointe-Claire, QC, Canada)
to induce regression of the CL and to synchronise ovulation
Aromatase inhibitor and ovarian function in cattle
(Hafs Louis et al. 1974). Ovulation was confirmed by daily
transrectal ultrasonography and visualisation of follicle collapse. To synchronise wave emergence, transvaginal ultrasoundguided follicular aspiration of follicles $5 mm was performed
5–8 days after ovulation (Bergfelt et al. 1994). Heifers were
examined daily by transrectal ultrasonography to detect follicular wave emergence, which was expected 1–1.5 days after
follicular ablation (Bergfelt et al. 1994). Four days after follicular ablation (,2.5 days after follicular wave emergence and
around the time of follicular selection; Adams et al. 1993;
Ginther et al. 2001), heifers were assigned randomly to one of
the following treatment groups and given a single intravenous
dose of: (1) 500 mg kg1 letrozole (high dose group; n ¼ 9);
(2) 250 mg kg1 letrozole (medium dose group; n ¼ 10);
(3) 125 mg kg1 letrozole (low dose group; n ¼ 10); or (4) 20 mL
PBS (control group; n ¼ 10). The letrozole used in the present
study was manufactured by Xian Huayang Biological Science
and Technology (Xian, China). For practical purposes, the dose
of letrozole was calculated based on an average weight of 400 kg
for all heifers. The average oral dose used in women (2.5–5 mg
day1 for 5 days) was used to estimate a medium dose for cattle
(Mitwally and Casper 2002a; Requena et al. 2008). The high and
low doses were set as 200% and 50% of the medium dose,
respectively. The day of letrozole treatment was defined as
Day 0. For intravenous injection, letrozole was prepared in 95%
ethanol at a final concentration of 5 mg mL1, which resulted in
an injection volume of 10–40 mL. The letrozole preparation was
administered slowly via an intravenous catheter to monitor for
any adverse effects of the high volume of ethanol in the animals.
The experiment was performed in four replicates (n ¼ 2–3 per
group per replicate). Each heifer was used only once.
Ovarian ultrasonography
Observations from ultrasound examinations were recorded on
a sketch sheet in which each ovary and its structures (CL and
follicles $4 mm in diameter; Knopf et al. 1989) were represented in size and location. Ovulation was defined as the disappearance of any follicle $8 mm between two consecutive daily
examinations and was confirmed by the subsequent development
of a CL (Pierson and Ginther 1987). Follicular wave emergence
was defined retrospectively as the day the dominant follicle was
first identified at a diameter of 4 or 5 mm (Adams et al. 1993;
Ginther et al. 1997). The dominant follicle of a wave was defined
as the one that was destined to become the largest, whereas the
first subordinate follicle was defined as the second largest follicle
of that wave (Peter et al. 2009). If the dominant follicle was not
identified until it reached 6 or 7 mm, the previous day was considered the day of follicular wave emergence (Kastelic et al.
1990). Onset of follicular and/or luteal regression was defined as
the first day of an apparent constant decrease in follicular and
luteal diameters, respectively (Adams et al. 1993).
Collection of blood samples
Blood was collected by jugular or coccygeal venipuncture into
10-mL heparinised vacuum tubes (Becton Dickinson Vacutainer Systems, Franklin Lakes, NJ, USA). Blood was collected at
0, 0.25, 0.5, 1, 1.5, 2, 3, 4, 6, 8, 12, 24, 36 and 48 h after treatment
Reproduction, Fertility and Development
633
(Sioufi et al. 1997a) using an indwelling jugular catheter, as
described previously (Bergfelt et al. 1997), and daily thereafter
to the first post-treatment ovulation. Blood was centrifuged at
1500g for 20 min; the plasma was separated and stored in plastic
tubes at 208C.
Quantification of hormones
Plasma concentrations of LH were determined in duplicate
using a double-antibody radioimmunoassay (RIA; NIDDKbLH4; Evans et al. 1994; Rawlings et al. 1984). The minimum
and maximum values along the standard curve were 0.06 and
8 ng mL1, respectively. The intra- and interassay CV were
10.2% and 8.8%, respectively, for low reference samples (mean
0.9 ng mL1) and 9.4% and 9.1%, respectively, for high reference samples (mean 2.7 ng mL1).
Plasma concentrations of FSH were determined in duplicate
using a double-antibody RIA using NIDDK-anti-oFSH-1 primary antibody and expressed as US Department of Agriculture
bovine FSH-Il units (Evans et al. 1994; Rawlings et al. 1984).
The minimum and maximum values along the standard curve
were 0.12 and 16 ng mL1, respectively. Intra- and interassay
CV were 11.2% and 10.0%, respectively, for low reference
samples (mean 1.7 ng mL1) and 12.0% and 12.4%, respectively,
for high reference samples (mean 4.4 ng mL1).
Concentrations of E2 in the conditioned culture medium were
determined in duplicate by RIA without solvent extraction, as
described previously (Bélanger et al. 1990). Intra- and interassay
CV were 6% and 9%, respectively. The sensitivity of the assay
was 10 pg per tube, equivalent to 0.3 ng mg1 protein. Oestradiol
concentrations in the culture medium were corrected for cell
number by expressing the concentration per unit mass of cell
protein. Cells were lysed with 100 mL of 1 M NaOH for 2 h and
neutralised with 100 mL of 1 M HCl. Total cell protein was
measured by the Bradford protein assay (Bio-Rad, Mississauga,
ON, Canada).
Plasma concentrations of oestradiol were determined in
duplicate by ELISA (Cayman Chemical, Ann Arbor, MI, USA).
Plasma E2 competed with acetylcholinesterase-labelled E2 for
the binding site on polyclonal rabbit anti-steroid antibody. The
antiserum to E2 has been reported (Cayman Chemical) to crossreact with oestradiol-3-glucoronide (14%), oestrone (12%), and
oestriol (0.3%). For all other steroid hormones, cross-reactivity
has been reported to be ,0.1%. The minimum and maximum
values along the standard curve were 6.6 and 4000 pg well1,
respectively. The intra- and interassay CV were 11.7% and
12.7%, respectively, for reference samples analysed in duplicate.
A concentration procedure using diethyl ether extraction was
performed before the assay in all samples to increase E2 to
measurable concentrations (Valentini et al. 2002). A 3H-labelled
steroid was added to each plasma sample before extraction as an
internal recovery standard. After the extraction procedure, a
fraction of the final extract was quantified in a liquid scintillation
counter to test for recoveries (Hecker et al. 2005).
Plasma letrozole concentrations
Plasma concentrations of letrozole were determined using highperformance liquid chromatography tandem mass spectrometry
634
Reproduction, Fertility and Development
(LC/MS/MS). Letrozole was extracted from 250 mL plasma with
250 mL of 0.1 M ammonium acetate followed by the addition of
5 mL methyl t-butyl ether (MTBE) and vortexed for 15 s. The
organic layer was removed and transferred to a fresh 15-mL
plastic tube and dried by gentle nitrogen gas flow. The dried
extract was reconstituted in 1 mL of 100% ethanol, sonicated for
5 min and transferred to a labelled vial for further analysis.
Separation was accomplished by HPLC (Agilent 1200; Agilent,
Santa Clara, CA, USA) fitted with an analytical column
(50 2.1 mm, 3 mm particle size; Betasil C18; Thermo Scientific, Waltham, MA, USA) operated at 358C. Gradient conditions were used at a flow rate of 250 mL min1, starting at 85% A
(0.1% acetic acid) and 15% B (0.1% acetic acid in acetonitrile).
Initial conditions were held for 2 min and then ramped to 100%
B at 6 min, held until 9 min, decreased to 0% B at 11 min,
returned to initial conditions at 13 min and held constant until
15 min. Mass spectra were collected using a tandem mass
spectrometer (SCIEX 3000; Applied Bioscience, Foster City,
CA, USA) fitted with an electrospray ionisation source, operated in the negative ionisation mode. Chromatograms were
recorded using multiple reaction monitoring (MRM) mode,
where at least two transitions per analyte were monitored. The
following instrument parameters were used: desolvation temperature 4508C, desolvation (curtain) gas 6.0 arbitrary units
(AU), nebulizer gas flow 4 AU, ion spray voltage 4500 V, collision gas 12 AU, collision energy 46 AU, declustering potential
30 AU and a dwell time of 100 msec. Quantification using these
transitions was performed using Analyst 1.4.1 software provided by SCIEX (Applied Bioscience). The minimum and
maximum values along the standard curve were 0.25 and
500 mg mL1, respectively. The limit of quantification used in
this method was 250 ng mL1 and the mean recovery was 70%.
The concentration of letrozole in plasma as a function of time
(C–t) data for each heifer were analysed by non-compartmental
techniques using a computer modelling program (WinNonLin
Standard Edition Version 2.1; Pharsight, Mountain View, CA,
USA). Peak letrozole concentration in plasma (Cmax) and time
to peak letrozole concentration (tmax) were determined using
observed values. The apparent terminal rate constant (l) was
determined by linear regression of the last six to eight points on
the terminal phase of the logarithmic plasma concentration vs
time curve. The area under the C–t curve until the final plasma
sample (AUClast) was determined using the linear trapezoidal
rule. The total area under the curve extrapolated to infinity
(AUC0–N) was calculated by adding the Clast obs/l þ AUClast.
The terminal half-life (T½l) was calculated as ln2/l. The mean
residence time (MRT) was calculated as the area under the
moment curve extrapolated to infinity (AUMC0–N)/AUC0–N.
Systemic clearance (ClS) was determined using the dose divided
by AUC0–N. The apparent volume of distribution (Vl/f ) was
calculated by divided clearance by l.
Statistical analyses
Statistical analyses were performed using SAS Learning Edition
9.1 (SAS Institute, Cary, NC, USA). Time series hormone data,
plasma letrozole concentrations and follicular diameter profiles
were analysed by repeated measures, using the PROC MIXED
M. J. Yapura et al.
procedure. The main effects were treatment (high, medium and
low dose and control), time and their interactions. When no
differences were detected among doses of letrozole, data were
combined and reanalysed as a single letrozole treatment group.
Single point measurements (intervals from ablation to wave
emergence, treatment to wave emergence, treatment to ovulation, treatment to onset of follicular regression, and treatment to
onset of CL regression) were analysed by one-way ANOVA. For
in vitro data, doses of letrozole were used as the main effects and
culture replicate was included in the model as a random effect
using the PROC MIXED procedure. Differences between means
were tested with Tukey’s test. Paired t-tests were used to compare E2 concentrations before and after treatment within a
treatment group and a two-sample t-test was used to compare E2
concentrations at a single data point between letrozole and
control groups. An F-test was used to analyse whether the variability in the interval from treatment to wave emergence differed between letrozole-treated and control heifers. Because of
individual variability in circulating concentrations of LH and
FSH among heifers, and because our objective was to determine
the effect of treatment within individuals, LH and FSH data were
transformed to a percentage of the mean concentration of the
first three samples (i.e. 0, 15 and 30 min after treatment) for each
individual heifer. Residuals from percentage data were normally
distributed. All values are expressed as the mean s.e.m.
Animal procedures were performed in accordance with the
Canadian Council on Animal Care and were approved by
University of Saskatchewan Protocol Review Committee.
Results
In vitro results
Aromatase activity was compared between cells cultured with
FSH alone, FSH combined with two doses of letrozole and
medium without FSH. There were significant effects of treatment (P ¼ 0.002) on E2 production. Treatment with 1 ng mL1
FSH stimulated aromatase activity. In the presence of 1 ng mL1
FSH combined with either 15 or 50 ng mL1 letrozole, aromatase activity was inhibited and E2 levels were comparable with
those observed in cells cultured in absence of FSH (Fig. 1).
Plasma letrozole concentrations
Plasma concentrations of letrozole followed a dose-dependent
pattern (Fig. 2). Throughout the 8-day period, mean letrozole
concentrations in the high-, medium- and low-dose groups were
0.63 0.04, 0.44 0.04 and 0.27 0.04 mg mL1, respectively
(P , 0.0001). There were also dose-dependent differences in
Cmax and AUClast (P ¼ 0.007 and P , 0.0001, respectively). No
significant differences were detected among dose groups terms
of T½, Vz/f, ClS or MRT (Table 1).
Ovarian function
The interval from follicular ablation to emergence of the new
follicular wave did not differ among treatment groups (1.7, 1.6,
1.7 and 1.6 days for the high-, medium- and low-dose letrozole
and control groups, respectively). Consequently, treatment was
applied 2.4 0.1 days after follicular wave emergence, when
Aromatase inhibitor and ovarian function in cattle
E2 secretion (pg µg⫺1 of protein)
600.0
Reproduction, Fertility and Development
the growing dominant follicle was 7.1 0.3 mm. The interval
from ablation to treatment, and the diameter of the dominant
follicle at the time of treatment, did not differ among groups.
Although the dominant follicle diameter profiles after letrozole treatment followed a dose-dependent pattern, differences
among the letrozole dose groups were not significant (P ¼ 0.11).
Therefore, data from all letrozole dose groups were combined
for comparison with the control group.
The number of heifers in which the extant dominant follicle
ovulated did not differ among groups (5/9, 5/10, 7/10 and 4/10
heifers in the high-, medium- and low-dose and control groups,
respectively). Although dominant follicle profiles did not differ
between treated and control heifers in which the extant dominant
follicle ovulated (Fig. 3a), the interval from treatment to ovulation of the extant dominant follicle tended to be longer in
letrozole-treated heifers (P ¼ 0.07; Table 2). In heifers that did
not ovulate, the extant dominant follicle diameter profile in
letrozole-treated heifers was larger (P , 0.01) than in control
heifers (Fig. 3b). The intervals from treatment to onset of
dominant follicle regression and to emergence of a new follicular wave were also longer in heifers treated with letrozole
than in controls (P , 0.05; Table 2). The variability (degree
of synchrony) in intervals from treatment to wave emergence
or dominant follicle regression was not significantly different
between letrozole-treated and control groups (F ¼ 2.7; P . 0.05).
FSH 1 ng mL⫺1
FSH 1 ng mL⫺1 ⫹ Letrozole 15 ng mL⫺1
FSH 1 ng mL⫺1 ⫹ Letrozole 50 ng mL⫺1
FSH 0 ng mL⫺1
a
500.0
400.0
300.0
P-value ⫽ 0.002
200.0
b
100.0
b
b
0.0
Plasma letrozole concentration (µg mL⫺1)
Fig. 1. Mean ( s.e.m.) oestradiol concentrations (mean s.e.m.) in
conditioned media from in vitro cultured bovine granulosa cells after
treatment with 1 ng mL1 FSH, 1 ng mL1 FSH plus 15 ng mL1 letrozole,
1 ng mL1 FSH plus 50 ng mL1 letrozole or without FSH. Bars with
different superscript letters differ significantly (P , 0.002).
3
High
Medium
Low
2.5
2
Effect
Treatment
Time
Treat*time
1.5
P-value
⬍0.001
⬍0.001
⬍0.001
1
0.5
0
0 0.5 1
2
3 4 5 6 7 8
Hours from treatment
9
635
10 11 12
Fig. 2. Mean ( s.e.m.) plasma letrozole concentrations (determined by
high-pressure liquid chromatography tandem mass spectrometry) in heifers
after administration of a single intravenous dose of 500, 250 or 125 mg kg1
letrozole (n ¼ 4 per group).
Circulating hormone concentrations
During the 4 days after treatment, plasma FSH concentrations
did not differ among the high-, medium- and low-dose letrozole
groups. Therefore, data were combined for comparison with
saline-treated controls. Plasma FSH concentrations increased
in both letrozole and control groups (time effect P , 0.03), but
proportionately less in letrozole-treated animals (treatment
effect P , 0.03; Fig. 4).
Plasma LH concentrations did not differ among letrozoletreated groups during the 12-h period after treatment, so data
were combined for comparison with PBS-treated controls.
During the first 12 h following treatment, plasma LH concentrations in heifers treated with letrozole were higher than in salinetreated controls (P ¼ 0.05; Fig. 5). Similarly, plasma LH
concentrations during the 4-day period after treatment did not
differ among letrozole-treated groups. After combining the data,
Table 1. Pharmacokinetics of letrozole after administration of a single intravenous dose of 500, 250 or 125 lg kg21 letrozole to postpubertal beef
heifers, determined by non-compartmental analysis
Data are the mean s.e.m. Within rows, values with no common superscript differ significantly (P , 0.05). Cmax, maximum concentration AUClast, area under
the C–t curve until the final plasma sample; Vz/f, volume of distribution; CLS, systemic clearance; MRT, mean residence time
Letrozole (mg kg1)
Parameter
500 (n ¼ 9)
1
Cmax (mg mL )
Half-life (h)
AUClast (h mg mL1)
Vz/f (mL kg1)
ClS (mL h1 kg1)
MRT (h)
2.5 0.4
28.5 1.1a
28.1 2.2c
745.7 47.4a
18.5 1.7a
35.8 1.4a
c
Combined
250 (n ¼ 9)
125 (n ¼ 9)
1.7 0.2
26.6 1.2a
17.3 2.0b
592.9 66.1a
15.5 1.8a
33.7 3.0a
1.2 0.3a
26.9 1.0a
8.8 0.7a
566.2 44.0a
14.6 1.1a
31.7 2.0a
b
1.8 0.3
27.3 0.4
18.2 4.0
634.9 39.5
16.2 0.8
33.7 0.8
636
Reproduction, Fertility and Development
(a) 14
M. J. Yapura et al.
Letrozole
Table 2. Effects of letrozole on interval to follicle wave emergence,
ovulation, onset of follicular regression and onset of corpus luteum
regression in cattle
Data from high -, medium- and low-dose treatment groups were combined
and compared with saline-treated controls (regardless of whether ovulation
occurred after treatment). Values are expressed as the mean s.e.m. Within
rows, values with different superscript letters differ significantly (P , 0.05).
CL, corpus luteum
Control
Follicle diameter (mm)
12
10
8
P-value
0.039
⬍0.001
0.288
Effect
Treatment
Time
Treat*time
6
4
2
-2
-1
0
1
2
3
4
5
6
7
8
9
Interval (days) from letrozole
treatment to:
Subsequent wave emergenceA
Ovulation of extant dominant
follicleB
Onset of regression of extant
dominant follicleB
Onset of CL regression
Letrozole (n)
Control (n)
7.5 0.3a (29)
9.0 0.4a (17)
5.9 0.5b (10)
8.0 0.9a (4)
8.7 0.5a (12)
5.2 0.7b (6)
6.1 0.4a (29)
5.1 0.6a (10)
Days from treatment
A
The difference in variability between groups was not significant.
The dominant follicle present at the time of treatment.
B
(b) 14
Letrozole
Control
250
10
8
Effect
Treatment
Time
Treat*time
6
4
2
-2
-1
0
1
2
3
P-value
⬍0.001
⬍0.001
0.064
4
5
6
7
8
9
Plasma FSH concentration (%)
Follicle Diameter (mm)
12
Letrozole
200
150
100
Effect
Treatment
Time
Treat*time
50
Days from treatment
Fig. 3. Mean ( s.e.m.) diameter of the (a) dominant follicle and (b) nonovulated dominant follicle in heifers treated with letrozole (high-, mediumand low-dose groups combined; n ¼ 29 and 12, respectively) 4 days after
follicular ablation (i.e. 2.5 days after wave emergence) compared with that in
saline-treated controls (n ¼ 10 and 6, respectively).
LH concentrations were higher in heifers treated with letrozole
than in saline-treated controls (P ¼ 0.01; Fig. 5).
Concentrations of E2 in plasma did not differ among the
letrozole-treated groups; hence, data were combined for comparison with saline-treated controls. Mean plasma concentrations of E2 over the 4-day period after treatment tended to be
higher in letrozole-treated heifers compared with control heifers
(P ¼ 0.06). This was due primarily to an increase in E2 concentrations between 4 and 12 h after treatment in letrozoletreated heifers (Fig. 6). Plasma E2 concentrations decreased by
nearly 50% from 0 to 24 h after treatment in letrozole-treated
heifers (from 15.2 3.01 to 8.0 1.5 pg mL1; P ¼ 0.03),
whereas no change occurred in control heifers (from
11.0 3.2 to 12.1 3.4 pg mL1; P ¼ 0.72).
Control
0
0
12
24
36
48
P-value
0.026
0.025
0.107
72
96
Hours from treatment
Fig. 4. Mean ( s.e.m.) plasma FSH concentrations (percentage change
after treatment) in heifers treated with letrozole (high-, medium- and lowdose groups combined; n ¼ 29,) 4 days after follicular ablation (i.e. 2.5 days
after wave emergence) compared with saline-treated controls (n ¼ 10).
Discussion
Letrozole has been used for several years to induce ovulation in
women (Mitwally and Casper 2001, 2002a, 2002b, 2004, 2005;
Casper 2003; Cortı́nez et al. 2005; Al-Fadhli et al. 2006; Bayar
et al. 2006; Jee et al. 2006; Mitwally et al. 2008); however, there
have been no reports on the use of letrozole to control ovarian
function in cattle. Based on clinical observations resulting from
infertility treatments in women, it was hypothesised that letrozole would induce a decrease in circulating E2 concentrations
followed by an increase in FSH concentrations, which would
cause the emergence of new follicular development (Mitwally
and Casper 2001, 2002a). However, results of the present study
Aromatase inhibitor and ovarian function in cattle
240
Plasma LH concentration (%)
220
200
180
Effect
Treatment
Time
Treat*time
Reproduction, Fertility and Development
P-value
0.054
0.703
0.329
Letrozole
637
Control
160
140
120
100
80
Effect
Treatment
Time
Treat*time
60
40
20
0 1 2 3 4
6
8
12
0 12 24 36 48
Hours from treatment
P-value
0.013
0.790
0.324
72
96
Fig. 5. Mean ( s.e.m.) plasma LH concentrations in heifers (percentage change after treatment) during the
first 12 h (left) and for 96 h (right) after a single intravenous dose of letrozole (high-, medium- and low-dose
groups combined; n ¼ 29) given 4 days after follicular ablation (i.e. 2.5 days after wave emergence) compared
with saline-treated controls (n ¼ 10).
Plasma estradiol (pg mL⫺1)
30
Letrozole
Control
25
P ⫽ 0.03
20
Effect
Treatment
Time
Treat*time
P-value
0.057
0.516
0.246
15
*
10
*
5
P ⫽ 0.72
0
01
4
12
24
48
96
Hours from treatment
Fig. 6. Mean ( s.e.m.) plasma oestradiol concentrations in heifers during
the first 96 h after a single intravenous dose of letrozole (high-, medium- and
low-dose groups combined; n ¼ 29) given 4 days after follicular ablation
(i.e. 2.5 days after wave emergence) compared with phosphate-buffered
saline (PBS)-treated controls (n ¼ 10). Within groups, differences in
oestradiol concentrations between 0 and 24 h after treatment were compared
by paired t-tests. *Values differed between groups (P , 0.03).
in cattle did not support this hypothesis. On the contrary, a single
treatment with letrozole on approximately Day 2.5 after wave
emergence in cattle, regardless of the dose, significantly
lengthened the period of dominance of the extant dominant
follicle, which resulted in a prolonged interval to emergence of a
new follicular wave. Letrozole treatment was associated with
elevated plasma LH concentrations, but it did not have the
expected effect on FSH concentrations. Further, the mean
diameter profile of the dominant follicle was significantly larger
in letrozole-treated heifers.
Extended growth and delayed regression of the extant dominant follicle can be attributed to elevated plasma LH concentrations (Adams et al. 1992; Stock and Fortune 1993), possibly
induced by letrozole treatment. Endogenous concentrations of
LH started to rise 2 h after letrozole was administered and
concentrations were elevated for at least 4 days after treatment.
Increasing concentrations of LH during this time may also have
elicited the rise in plasma E2 concentrations observed 12 h after
treatment.
Contrary to our expectations, plasma FSH concentrations
were lower in letrozole-treated heifers than in controls. Follicular products other than E2 also suppress FSH and may be
responsible for the observed effect (Miller et al. 1979, 1981;
Adams et al. 1992). Inhibin secreted by the dominant and
subordinate follicles, together with E2, has been associated with
the suppressive effects involved in follicular selection and
dominance (Bleach et al. 2001; Ginther et al. 2003). However,
treatment with letrozole was associated with overdominance
(prolonged growth and maintenance of the dominant follicle) in
the present study, which resulted in an extended period of FSH
suppression and delayed emergence of the next follicular wave.
It is of note that inhibition of E2 synthesis by an aromatase
inhibitor did not adversely affect the extant dominant follicle;
rather, it indirectly enhanced follicular dominance by permitting
elevated pituitary LH secretion.
Mean E2 concentrations in letrozole-treated heifers tended to
be higher than in controls for the first 4 days after treatment, but
this observation was attributed primarily to a significant and
sharp elevation at 12 h after letrozole treatment. The acute
elevation in E2 has not been reported in women, but a similar
increase in LH has been observed in rats and has been interpreted
to be the result of gonadotrophin release caused by letrozole
treatment (Sinha et al. 1998). Although plasma E2 concentrations decreased by nearly 50% by 24 h after letrozole treatment
in the present study, concentrations did not differ significantly
from those in controls.
The lack of an apparent suppressive effect of letrozole on
oestrogen concentrations in cattle in the present study may have
been the result of insufficient assay sensitivity and/or an
638
Reproduction, Fertility and Development
inadequate dose of letrozole. This is supported by the results of
the present study obtained in vitro, where the effectiveness of
letrozole to inhibit aromatase activity in bovine granulosa cells
has been confirmed. In women, basal and maximum circulating
E2 concentrations have been reported to be approximately
20 and 200 pg mL1, respectively (Baerwald et al. 2003). In
the heifers examined in the present study, basal plasma
E2 concentrations were below the detection limit of the assay
(3–4 pg mL1) and, on average, maximum concentrations did
not exceed 25 pg mL1during the first 8 days after ovulation. In
addition, the dose and duration of letrozole treatment used in our
experiment may not have been sufficient to inhibit E2 production in cattle compared with other species in which treatment
resulted in a reduction in circulating E2 concentrations of
97%–99% in post-menopausal women (Mitwally and Casper
2002a) and 88% in boars (At-Taras et al. 2006).
Although most of the studies in which letrozole has been used
to treat unexplained infertility in women are based on a 5-day
treatment regimen (total dose 12.5–20 mg letrozole), single dose
treatments of 20 mg administered orally on the third day of the
menstrual cycle have been reported to be equally effective in
suppressing circulating oestrogen concentrations. The half-life
of letrozole in humans has been reported to be approximately
2 days, which could result in effective suppression of E2
production for 4–6 days after a single administration (Mitwally
and Casper 2005). Although the pharmacokinetic parameters
reported in the present study are preliminary, it was estimated
that the half-life of letrozole in heifers was 27 h (cf. 48 h in
women (Sioufi et al. 1997a, 1997b). The MRT (average duration of persistence in the body) was estimated to be 34 h (cf. 59 h
in women; Sioufi et al. 1997a) and the volume of distribution
was estimated to be 635 mL kg1 (cf. 1870 mL kg1 in women;
Sioufi et al. 1997b). Together, these results are consistent with
the conclusion that cattle require a higher dose and a longer
period of exposure to achieve effective concentrations of letrozole in target tissues. This hypothesis is further supported by a
study in which albendazole (another imidazole derivative used
as an anthelminthic) significantly reduced follicular fluid E2
concentrations in ewes when given orally at 11.5 mg kg1
bodyweight (Mamali et al. 2008), which is 46-fold higher than
the medium dose used in the present study.
In summary, letrozole treatment in heifers was associated
with elevated circulating LH concentrations and an extended
period of dominance of the dominant follicle present at the time
of treatment, regardless of dose. Consequently, circulating
concentrations of FSH remained suppressed and emergence of
the next wave was delayed. These results were unexpected and
provide impetus for additional studies to elucidate the differences in pharmacokinetics of letrozole between the bovine and
human and to explore the potential of aromatase inhibitors as a
non-steroidal approach to the control of ovarian function in
cattle.
Acknowledgements
The authors thank Alan Chicoine for help in interpreting plasma letrozole
concentrations and Gustavo Zamberlam and Christopher Price from the
University of Montreal for their help in generating in vitro data. The authors
also thank Brad Blackmore and the staff at Goodale Research Farm for
M. J. Yapura et al.
assistance with handling and managing the cattle and our summer student,
Matthew Van Steelandt, for help with data collection. This research was
supported by Discovery Grants from the Natural Sciences and Engineering
Research Council of Canada (122236–08 and 326415–07) and a grant from
the Western Economic Diversification Canada (project no. 6578 and 6807).
The authors acknowledge the support of a Research Tools and Instruments
grant from the Natural Sciences and Engineering Research Council of
Canada (375327–09) and an infrastructure grant from the Canada Foundation for Innovation. JPG was supported by the Canada Research Chair
program and an at-large Chair in Marine Pollution at the Department of
Biology and Chemistry, State Key Laboratory, City University of Hong
Kong. RAP supported by the Canadian Institutes for Health Research.
References
Adams, G. P., Matteri, R. L., and Ginther, O. J. (1992). Effect of progesterone on ovarian follicles, emergence of follicular waves and circulating
follicle-stimulating hormone in heifers. J. Reprod. Fertil. 96, 627–640.
doi:10.1530/JRF.0.0960627
Adams, P., Kot, K., Smith, C., and Ginther, O. (1993). Selection of a
dominant follicle and suppression of follicular growth in heifers. Anim.
Reprod. Sci. 30, 259–271. doi:10.1016/0378-4320(93)90076-4
Al-Fadhli, R., Sylvestre, C., Buckett, W., Tan, S. L., and Tulandi, T. (2006).
A randomized trial of superovulation with two different doses of
letrozole. Fertil. Steril. 85, 161–164. doi:10.1016/J.FERTNSTERT.
2005.07.1283
Andersson, A., and Skakkebaek, N. (1999). Exposure to exogenous estrogens in food: possible impact on human development and health. Eur. J.
Endocrinol. 140, 477–485. doi:10.1530/EJE.0.1400477
At-Taras, E. E., Conley, A. J., Berger, T., and Roser, J. F. (2006). Reducing
estrogen synthesis does not affect gonadotropin secretion in the developing boar. Biol. Reprod. 74, 58–66. doi:10.1095/BIOLREPROD.105.
043760
Baerwald, A., Adams, G., and Pierson, R. (2003). Characterization
of ovarian follicular wave dynamics in women. Biol. Reprod. 69,
1023–1031. doi:10.1095/BIOLREPROD.103.017772
Bayar, U., TanrIverdi, H. A., Barut, A., Ayoglu, F., Özcan, O., and Kaya, E.
(2006). Letrozole vs. clomiphene citrate in patients with ovulatory
infertility. Fertil. Steril. 85, 1045–1048. doi:10.1016/J.FERTNSTERT.
2005.09.045
Bélanger, A., Couture, J., Caron, S., and Roy, R. (1990). Determination of
nonconjugated and conjugated steroid levels in plasma and prostate
after separation on C-18 columns. Ann. N. Y. Acad. Sci. 595, 251–259.
doi:10.1111/J.1749-6632.1990.TB34299.X
Bergfelt, D. R., Lightfoot, K. C., and Adams, G. P. (1994). Ovarian
synchronization following ultrasound-guided transvaginal follicle ablation in heifers. Theriogenology 42, 895–907. doi:10.1016/0093-691X
(94)90113-W
Bergfelt, D. R., Smith, C. A., Adams, G. P., and Ginther, O. J. (1997). Surges
of FSH during the follicular and early luteal phases of the estrous cycle in
heifers. Theriogenology 48, 757–768. doi:10.1016/S0093-691X(97)
00299-9
Bleach, E. C. L., Glencross, R. G., Feist, S. A., Groome, N. P., and Knight, P.
G. (2001). Plasma inhibin A in heifers: relationship with follicle
dynamics, gonadotropins, and steroids during the estrous cycle and
after treatment with bovine follicular fluid. Biol. Reprod. 64, 743–752.
doi:10.1095/BIOLREPROD64.3.743
Bo, G. A., Adams, G. P., Caccia, M., Martinez, M., Pierson, R. A., and
Mapletoft, R. J. (1995a). Ovarian follicular wave emergence after
treatment with progestogen and estradiol in cattle. Anim. Reprod. Sci.
39, 193–204. doi:10.1016/0378-4320(95)01389-H
Bo, G. A., Adams, G. P., Pierson, R. A., and Mapletoft, R. J. (1995b).
Exogenous control of follicular wave emergence in cattle. Theriogenology 43, 31–40. doi:10.1016/0093-691X(94)00010-R
Aromatase inhibitor and ovarian function in cattle
Bridges, P. J., Lewis, P. E., Wagner, W. R., and Inskeep, E. K. (1999).
Follicular growth, estrus and pregnancy after fixed-time insemination in
beef cows treated with intravaginal progesterone inserts and estradiol
benzoate. Theriogenology 52, 573–583. doi:10.1016/S0093-691X(99)
00153-3
Casper, R. F. (2003). Letrozole: ovulation or superovulation? Fertil. Steril.
80, 1335–1337. doi:10.1016/J.FERTNSTERT.2003.05.004
Cohen, M. H., Johnson, J. R., Li, N., Chen, G., and Pazdur, R. (2002).
Approval summary: letrozole in the treatment of postmenopausal
women with advanced breast cancer. Clin. Cancer Res. 8, 665–669.
Colazo, M. G., Kastelic, J. P., Martı́nez, M. F., Whittaker, P. R., Wilde, R.,
Ambrose, J. D., Corbett, R., and Mapletoft, R. J. (2004). Fertility
following fixed-time AI in CIDR-treated beef heifers given GnRH or
estradiol cypionate and fed diets supplemented with flax seed or
sunflower seed. Theriogenology 61, 1115–1124. doi:10.1016/J.THER
IOGENOLOGY.2003.06.005
Cortı́nez, A., De Carvalho, I., Vantman, D., Gabler, F., Iñiguez, G., and
Vega, M. (2005). Hormonal profile and endometrial morphology in
letrozole-controlled ovarian hyperstimulation in ovulatory infertile
patients. Fertil. Steril. 83, 110–115. doi:10.1016/J.FERTNSTERT.
2004.05.099
Daxenberger, A., Ibarreta, D., and Meyer, H. H. D. (2001). Possible health
impact of animal oestrogens in food. Hum. Reprod. Update 7, 340–355.
doi:10.1093/HUMUPD/7.3.340
Evans, A. C. O., Adams, G. P., and Rawlings, N. C. (1994). Endocrine and
ovarian follicular changes leading up to the first ovulation in prepubertal
heifers. J. Reprod. Fertil. 100, 187–194. doi:10.1530/JRF.0.1000187
Fritsche, S., and Steinhart, H. (1999). Occurrence of hormonally active
compounds in food: a review. Eur. Food Res. Technol. 209, 153–179.
doi:10.1007/S002170050475
Gibbs, J. N. (2004). Is veterinary compounding illegal under federal law?
IJPC 8, 449–451.
Ginther, O. J., Kot, K., Kulick, L. J., and Wiltbank, M. C. (1997). Emergence
and deviation of follicles during the development of follicular waves
in cattle. Theriogenology 48, 75–87. doi:10.1016/S0093-691X(97)
00192-1
Ginther, O. J., Bergfelt, D. R., Beg, M. A., and Kot, K. (2001). Follicle
selection in cattle: relationships between growth rate, diameter ranking,
and capacity for dominance. Biol. Reprod. 65, 345–350. doi:10.1095/
BIOLREPROD65.2.345
Ginther, O. J., Beg, M. A., Donadeu, F. X., and Bergfelt, D. R. (2003).
Mechanism of follicle deviation in monovular farm species. Anim.
Reprod. Sci. 78, 239–257. doi:10.1016/S0378-4320(03)00093-9
Hafs, H. D., Louis, T. M., Noden, P. A., and Oxender, W. D. (1974). Control
of the estrous cycle with prostaglandin F2{alpha} in cattle and horses.
J. Anim. Sci. 38, 10–21.
Health Canada Drugs and Health Products (2005). ‘Veterinary Products.
Questions and Answer: Hormonal Growth Promoters.’ (Veterinary
Drugs Directorate, Health Products and Food Branch, Health Canada:
Ottawa, ON.) Available at http://www.hc-sc.gc.ca/dhp-mps/vet/faq/
growth_hormones_promoters_croissance_hormonaux_stimulateurs-eng.
php[Verified 25 January 2009].
Hecker, M., Park, J.-W., et al. (2005). Effects of atrazine on CYP19 gene
expression and aromatase activity in testes and on plasma sex steroid
concentrations of male African clawed frogs (Xenopus laevis). Toxicol.
Sci. 86, 273–280. doi:10.1093/TOXSCI/KFI203
Jee, B. C., Ku, S. Y., Suh, C. S., Kim, K. C., Lee, W. D., and Kim, S. H.
(2006). Use of letrozole versus clomiphene citrate combined with
gonadotropins in intrauterine insemination cycles: a pilot study. Fertil.
Steril. 85, 1774–1777. doi:10.1016/J.FERTNSTERT.2006.02.070
Kastelic, J. P., Knopf, L., and Ginther, O. J. (1990). Effect of day of
prostaglandin F2[alpha] treatment on selection and development
of the ovulatory follicle in heifers. Anim. Reprod. Sci. 23, 169–180.
doi:10.1016/0378-4320(90)90001-V
Reproduction, Fertility and Development
639
Knopf, L., Kastelic, J. P., Schallenberger, E., and Ginther, O. J. (1989).
Ovarian follicular dynamics in heifers: test of two-wave hypothesis by
ultrasonically monitoring individual follicles. Domest. Anim. Endocrinol. 6, 111–119. doi:10.1016/0739-7240(89)90040-4
Lane, E. A., Austin, E. J., and Crowe, M. A. (2008). Oestrous synchronisation in cattle: current options following the EU regulations restricting use
of oestrogenic compounds in food-producing animals. A review. Anim.
Reprod. Sci. 109, 1–16. doi:10.1016/J.ANIREPROSCI.2008.08.009
Mamali, P., Samartzi, F., Batzias, G. C., Theodosiadou, E., Vainas, E.,
Goulas, P., Belibasaki, S., and Saratsis, F. (2008). The effect of
albendazole administration on the concentration of ovarian steroids in
the follicular fluid and the maturation of oocytes in the ewe. Reprod.
Domest. Anim. 43, 192.
Mapletoft, R. J., Martinez, M. F., Colazo, M. G., and Kastelic, J. P. (2003).
The use of controlled internal drug release devices for the regulation of
bovine reproduction. J. Anim. Sci. 81, E28–E36.
Martinez, M. F., Adams, G. P., Kastelic, J. P., Bergfelt, D. R., and Mapletoft,
R. J. (2000). Induction of follicular wave emergence for estrus synchronization and artificial insemination in heifers. Theriogenology 54,
757–769. doi:10.1016/S0093-691X(00)00388-5
Miller, K. F., Critser, J. K., Rowe, R. F., and Ginther, O. J. (1979). Ovarian
effects of bovine follicular fluid treatment in sheep and cattle. Biol.
Reprod. 21, 537–544. doi:10.1095/BIOLREPROD21.3.537
Miller, K. F., Wesson, J. A., and Ginther, O. J. (1981). Interaction of estradiol
and a nonsteroidal follicular fluid substance in the regulation of gonadotropin secretion in the mare. Biol. Reprod. 24, 354–358. doi:10.1095/
BIOLREPROD24.2.354
Mitwally, M. F., and Casper, R. F. (2001). Use of aromatase inhibitor for
induction of ovulation in patients with an inadequate response to
clomiphene citrate. Fertil. Steril. 75, 305–309. doi:10.1016/S00150282(00)01705-2
Mitwally, M. F., and Casper, R. F. (2002a). Aromatase inhibition for ovarian
stimulation: future avenues for infertility management. Curr. Opin.
Obstet. Gynecol. 14, 255–263. doi:10.1097/00001703-20020600000003
Mitwally, M. F., and Casper, R. F. (2002b). Aromatase inhibition improves
ovarian response to follicle-stimulating hormone in poor responders.
Fertil. Steril. 77, 776–780. doi:10.1016/S0015-0282(01)03280-0
Mitwally, M. F. M., and Casper, R. F. (2004). Aromatase inhibition reduces
the dose of gonadotropin required for controlled ovarian hyperstimulation. J. Soc. Gynecol. Invest. 11, 406–415. doi:10.1016/J.JSGI.
2004.03.006
Mitwally, M. F. M., and Casper, R. F. (2005). Single-dose administration
of an aromatase inhibitor for ovarian stimulation. Fertil. Steril. 83,
229–231. doi:10.1016/J.FERTNSTERT.2004.07.952
Mitwally, M. F., Said, T., Galal, A., Chan, S., Cohen, M., Casper, R. F., and
Magarelli, P. C. (2008). Letrozole step-up protocol: a successful superovulation protocol. Fertil. Steril. 89, S23–S24. doi:10.1016/J.FERTN
STERT.2008.02.071
Official Journal of the European Union. (2003). ‘L 262, 14/10/2003.
Directive 2003/74/EC of the European Parliament and of the Council
on 22 September 2003 amending Council Directive 96/22/EC concerning the prohibition on the use in stockfarming of certain substances
having a hormonal or thyristatic action and of beta-agonist.’ (EUR-Lex:
Brussels.) Available at http://www.fve.org/veterinary/pdf/food/directive_
2003_74.pdf.
Peter, A. T., Levine, H., Drost, M., and Bergfelt, D. R. (2009). Compilation
of classical and contemporary terminology used to describe morphological aspects of ovarian dynamics in cattle. Theriogenology 71,
1343–1357. doi:10.1016/J.THERIOGENOLOGY.2008.12.026
Pierson, R. A., and Ginther, O. J. (1987). Reliability of diagnostic ultrasonography for identification and measurement of follicles and detecting
the corpus luteum in heifers. Theriogenology 28, 929–936. doi:10.1016/
0093-691X(87)90043-4
640
Reproduction, Fertility and Development
M. J. Yapura et al.
Rawlings, N. C., Jeffcoate, I. A., and Rieger, D. L. (1984). The influence of
estradiol-17[beta] and progesterone on peripheral serum concentrations
of luteinizing hormone and follicle stimulating hormone in the ovariectomized ewe. Theriogenology 22, 473–488. doi:10.1016/0093-691X(84)
90047-5
Requena, A., Herrero, J., Landeras, J., Navarro, E., Neyro, J. L., Salvador,
C., Tur, R., Callejo, J., Checa, M. A., Farre, M., Espinos, J. J., Fabregues,
F., and Grana-Barcia, M. (2008). Use of letrozole in assisted reproduction: A systematic review and meta-analysis. Hum. Reprod. Update 14,
571–582. doi:10.1093/HUMUPD/DMN033
Sinha, S., Kaseta, J., Santner, S. J., Demers, L. M., Bremmer, W. J., and
Santen, R. J. (1998). Effect of CGS 20267 on ovarian aromatase and
gonadotropin levels in the rat. Breast Cancer Res. Treat. 48, 45–51.
doi:10.1023/A:1005937900788
Sioufi, A., Gauducheau, N., Pineau, V., Marfil, F., Jaouen, A., Cardot, J. M.,
Godbillon, J., Czendlik, C., Howald, H., Pfister, C. H., and Vreeland, F.
(1997a). Absolute bioavailability of letrozole in healthy postmenopausal
women. Biopharm. Drug Dispos. 18, 779–789. doi:10.1002/(SICI)1099081X(199712)18:9,779::AID-BDD64.3.0.CO;2-5
Sioufi, A., Sandrenan, N., Godbillon, J., Trunet, P., Czendlik, C., Howald, H.,
Pfister, C., and Ezzet, F. (1997b). Comparative bioavailability of letrozole
under fed and fasting conditions in 12 healthy subjects after a 2.5 mg single
oral administration. Biopharm. Drug Dispos. 18, 489–497. doi:10.1002/
(SICI)1099-081X(199708)18:6,489::AID-BDD36.3.0.CO;2-P
Stock, A. E., and Fortune, J. E. (1993). Ovarian follicular dominance in
cattle: relationship between prolonged growth of the ovulatory follicle
and endocrine parameters. Endocrinology 132, 1108–1114. doi:10.1210/
EN.132.3.1108
Umberger, E. J. (1975). Products marketed to promote growth in foodproducing animals: steroid and hormone products. Toxicology 3, 3–21.
doi:10.1016/0300-483X(75)90003-7
US Department of Agriculture. Foreign Agricultural Service (2003). ‘Historic Overview and Chronology of EU’s Hormone Ban. GAIN Report
E23206.’ (USDA: Washington, DC.) Available at http://www.fas.usda.
gov/scriptsw/attacherep/gain_display_report.asp?Rep_ID=145986773
[Verified 3 November 2011].
US Food and Drug Administration (2003). ‘Compliance Policy Guides
Manual, Sec. 608.400. Compounding of Drugs for Use in Animals.’
(Department of Health and Human Services: Silver Spring, MD.)
Available at http://www.fda.gov/ora/compliance_ref/cpg/cpgvet/cpg608400compounding.pdf [Verified 25 August 2011].
Valentini, F., Compagnoneb, D., Gentilic, A., and Palleschia, G. (2002). An
electrochemical ELISA procedure for the screening of 17-estradiol in
urban waste waters. Analyst 127, 1333–1337. doi:10.1039/B204826B
Yapura, M. J., Mapletoft, R. J., Singh, J., Pierson, R. A., and Adams, G. P.
(2009). Effects of a single dose of a nonsteroidal aromatase inhibitor on
ovarian function in cattle. Reprod. Fertil. Dev. 22, 271. doi:10.1071/
RDV22N1AB227
Zamberlam, G., Portela, V., de Oliveira, J. F. C., Gonçalves, P. B. D., and
Price, C. A. (2011). Regulation of inducible nitric oxide synthase
expression in bovine ovarian granulosa cells. Mol. Cell. Endocrinol.
335, 189–194. doi:10.1016/J.MCE.2011.01.013
www.publish.csiro.au/journals/rfd