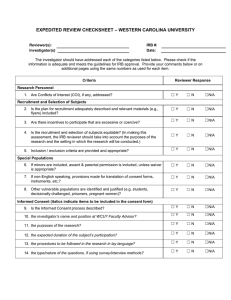
Human Subjects Research Change Form
advertisement

Human Subjects Research Change Form Any change to an approved protocol must be reviewed and approved by the IRB Any change to an approved protocol must be reviewed and approved by the IRB before the change is implemented. Such amendments could include changes to the study design, procedures, enrollment, methods of recruitment, personnel, funding source or the consent form/information sheet. This includes changes that appear to reduce risks to subjects. the change is implemented. Such amendments could include changes to the study design, procedures, enrollment, methods of recruitment, personnel, funding source or the consent form/information sheet. This includes changes that appear to reduce risks to subjects. Protocol #: Study Title: Principal Investigator (PI): Phone E-mail Mailing Address: Please describe each proposed amendment(s) and explain why it is being made. This includes changes to project personnel. Original Signature of Principal Investigator Date: