– WESTERN CAROLINA UNIVERSITY EXPEDITED REVIEW CHECKSHEET
advertisement
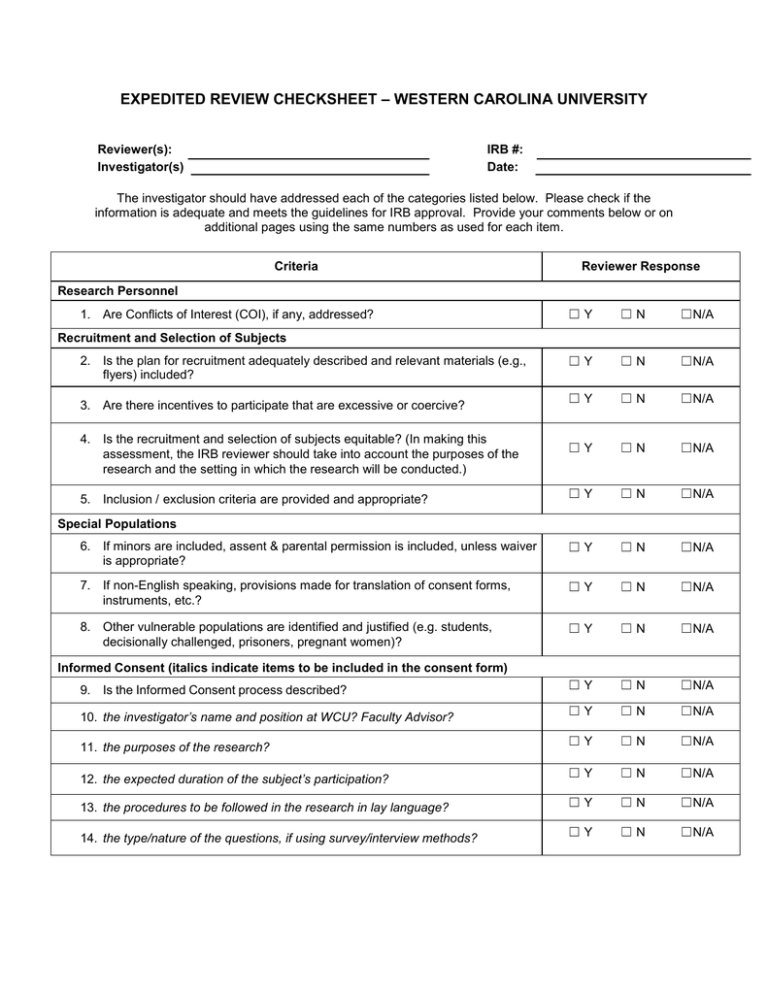
EXPEDITED REVIEW CHECKSHEET – WESTERN CAROLINA UNIVERSITY Reviewer(s): Investigator(s) IRB #: Date: The investigator should have addressed each of the categories listed below. Please check if the information is adequate and meets the guidelines for IRB approval. Provide your comments below or on additional pages using the same numbers as used for each item. Criteria Reviewer Response Research Personnel ☐Y ☐N ☐N/A ☐Y ☐N ☐N/A ☐Y ☐N ☐N/A ☐Y ☐N ☐N/A ☐Y ☐N ☐N/A 6. If minors are included, assent & parental permission is included, unless waiver is appropriate? ☐Y ☐N ☐N/A 7. If non-English speaking, provisions made for translation of consent forms, instruments, etc.? ☐Y ☐N ☐N/A 8. Other vulnerable populations are identified and justified (e.g. students, decisionally challenged, prisoners, pregnant women)? ☐Y ☐N ☐N/A 9. Is the Informed Consent process described? ☐Y ☐N ☐N/A 10. the investigator’s name and position at WCU? Faculty Advisor? ☐Y ☐N ☐N/A 11. the purposes of the research? ☐Y ☐N ☐N/A 12. the expected duration of the subject’s participation? ☐Y ☐N ☐N/A 13. the procedures to be followed in the research in lay language? ☐Y ☐N ☐N/A 14. the type/nature of the questions, if using survey/interview methods? ☐Y ☐N ☐N/A 1. Are Conflicts of Interest (COI), if any, addressed? Recruitment and Selection of Subjects 2. Is the plan for recruitment adequately described and relevant materials (e.g., flyers) included? 3. Are there incentives to participate that are excessive or coercive? 4. Is the recruitment and selection of subjects equitable? (In making this assessment, the IRB reviewer should take into account the purposes of the research and the setting in which the research will be conducted.) 5. Inclusion / exclusion criteria are provided and appropriate? Special Populations Informed Consent (italics indicate items to be included in the consent form) 15. statement that participation is voluntary, and that they may discontinue the study at any time for any reason without penalty, and that they may ask questions at any time? ☐Y ☐N ☐N/A 16. participants may skip any question they do not wish to answer, if a survey/interviewing method is being used? ☐Y ☐N ☐N/A 17. any reasonable, foreseeable risks or discomforts are stated? ☐Y ☐N ☐N/A 18. any benefits to the participant, which may reasonably be expected from the research are stated? ☐Y ☐N ☐N/A 19. whether participation and the data collected is anonymous or confidential? ☐Y ☐N ☐N/A 20. IRB contact information included? ☐Y ☐N ☐N/A 21. whether or not compensation will be awarded and the details thereof? ☐Y ☐N ☐N/A 22. Will signed consent be obtained? ☐Y ☐N ☐N/A 23. If no to #22, are the criteria in 45 CFR 46.117 met? ☐Y ☐N ☐N/A 24. Are data collection instruments included and procedures adequately described? ☐Y ☐N ☐N/A 25. Could identification of subjects be damaging to employability, insurability, or reputation? ☐Y ☐N ☐N/A ☐Y ☐N ☐N/A ☐Y ☐N ☐N/A ☐Y ☐N ☐N/A 29. There is no/minimal risk of breaching confidentiality? ☐Y ☐N ☐N/A 30. There is no/minimal risk of evoking emotional distress or other negative emotional response(s)? ☐Y ☐N ☐N/A 31. There is no/minimal foreseeable risk to subject’s reputation and/or social status? ☐Y ☐N ☐N/A 32. There is no/minimal foreseeable legal risk to subject? ☐Y ☐N ☐N/A 33. There is no/minimal foreseeable risk to subject’s employability or employment status? ☐Y ☐N ☐N/A 34. If deception will be used in the study, necessity is justified, and debriefing procedures are described? ☐Y ☐N ☐N/A 35. There are immediate benefits to the individual OR probable benefits to the target population? ☐Y ☐N ☐N/A Data Collection, Storage, and Confidentiality 26. If appropriate, does the research plan make adequate provisions for monitoring collected data to ensure safety of subjects, protection of privacy, and confidentiality of the data? 27. Are the investigator’s disclosures to subjects about anonymity/confidentiality adequate? 28. Is data storage clearly described (3 years after close of study)? Risk & Benefit Analysis 36. The research will likely contribute to the field of study/organization? Off-campus Sites 37. If project will involve work off campus, all sites are listed? 38. Authorization letters are provided from all off-campus sites? ☐Y ☐N ☐N/A ☐Y ☐N ☐N/A ☐Y ☐N ☐N/A Decision 39. Based on the above materials this proposal should be: ☐ Accepted as submitted ☐ Accept contingent on minor revisions ☐ Accept contingent on major revisions ☐ Not be accepted (full board required to disapprove)


![Lesson Study Project Informed Consent for Students 2011-12 [TEMPLATE]](http://s2.studylib.net/store/data/011897429_1-e9cd20ac12fa907a0c9dbbb5866bfc98-300x300.png)




