Determining the crystal structure of ADP-glucose pyrophosphorylase
advertisement
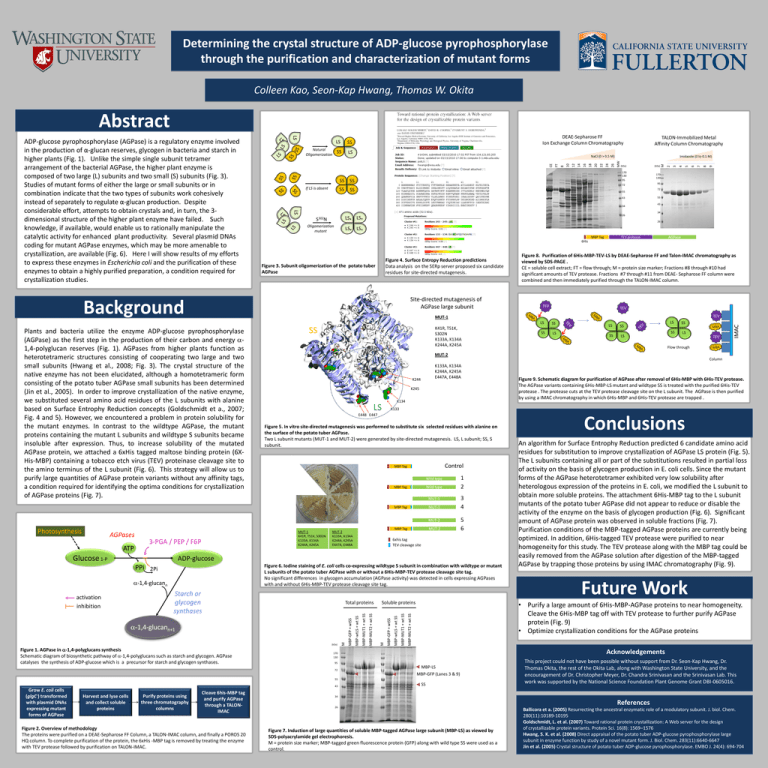
Determining the crystal structure of ADP-glucose pyrophosphorylase through the purification and characterization of mutant forms Colleen Kao, Seon-Kap Hwang, Thomas W. Okita Abstract If LS is absent SS 3-PGA / PEP / F6P S302N LS* LS* Oligomerization mutant LS* LS* 9 55 43 TEV protease AGPase 6His Figure 8. Purification of 6His-MBP-TEV-LS by DEAE-Sepharose FF and Talon-IMAC chromatography as viewed by SDS-PAGE . CE = soluble cell extract; FT = flow through; M = protein size marker; Fractions #8 through #10 had significant amounts of TEV protease. Fractions #7 through #11 from DEAE- Sepharose FF column were combined and then immediately purified through the TALON-IMAC column. Figure 4. Surface Entropy Reduction predictions Data analysis on the SERp server proposed six candidate residues for site-directed mutagenesis. TEV TEV TEV SS K41R, T51K, S302N K133A, K134A K244A, K245A LS SS SS LS LS SS SS LS LS SS SS LS TEV Flow through MUT-2 Column K133A, K134A K244A, K245A E447A, E448A K244 K245 K134 LS Figure 9. Schematic diagram for purification of AGPase after removal of 6His-MBP with 6His-TEV protease. The AGPase variants containing 6His-MBP-LS mutant and wildtype SS is treated with the purified 6His-TEV protease . The protease cuts at the TEV protease cleavage site on the L subunit. The AGPase is then purified by using a IMAC chromatography in which 6His-MBP and 6His-TEV protease are trapped . K133 E448 E447 Figure 5. In vitro site-directed mutagenesis was performed to substitute six selected residues with alanine on the surface of the potato tuber AGPase. Two L subunit mutants (MUT-1 and MUT-2) were generated by site-directed mutagenesis. LS, L subunit; SS, S subunit. Control MBP Tag G Wild type Wild type MBP Tag MUT-1 MUT-1 MUT-2 MBP Tag MUT-2 K133A, K134A K244A, K245A E447A, E448A 1 2 3 4 5 6 6xhis tag TEV cleavage site 95 MBP-LS MBP-GFP (Lanes 3 & 9) 55 43 An algorithm for Surface Entrophy Reduction predicted 6 candidate amino acid residues for substitution to improve crystallization of AGPase LS protein (Fig. 5). The L subunits containing all or part of the substitutions resulted in partial loss of activity on the basis of glycogen production in E. coli cells. Since the mutant forms of the AGPase heterotetramer exhibited very low solubility after heterologous expression of the proteins in E. coli, we modified the L subunit to obtain more soluble proteins. The attachment 6His-MBP tag to the L subunit mutants of the potato tuber AGPase did not appear to reduce or disable the activity of the enzyme on the basis of glycogen production (Fig. 6). Significant amount of AGPase protein was observed in soluble fractions (Fig. 7). Purification conditions of the MBP-tagged AGPase proteins are currently being optimized. In addition, 6His-tagged TEV protease were purified to near homogeneity for this study. The TEV protease along with the MBP tag could be easily removed from the AGPase solution after digestion of the MBP-tagged AGPase by trapping those proteins by using IMAC chromatography (Fig. 9). • Future Work Acknowledgements 170 130 72 Conclusions • Purify a large amount of 6His-MBP-AGPase proteins to near homogeneity. Cleave the 6His-MBP tag off with TEV protease to further purify AGPase protein (Fig. 9) • Optimize crystallization conditions for the AGPase proteins MBP-MUT2 + wt SS MBP-MUT1 + wt SS MBP-wtLS + wt SS MBP-GFP + wtSS Soluble proteins M MBP-MUT2 + wt SS MBP-MUT1 + wt SS MBP-wtLS + wt SS M MBP-GFP + wtSS Total proteins IMAC Figure 3. Subunit oligomerization of the potato tuber AGPase (kDa) Figure 2. Overview of methodology The proteins were purified on a DEAE-Sepharose FF Column, a TALON-IMAC column, and finally a POROS 20 HQ column. To complete purification of the protein, the 6xHis -MBP tag is removed by treating the enzyme with TEV protease followed by purification on TALON-IMAC. 8 72 26 MBP Tag Starch or glycogen synthases Cleave 6his-MBP tag and purify AGPase through a TALONIMAC 7 95 34 Figure 6. Iodine staining of E. coli cells co-expressing wildtype S subunit in combination with wildtype or mutant L subunits of the potato tuber AGPase with or without a 6His-MBP-TEV protease cleavage site tag. No significant differences in glycogen accumulation (AGPase activity) was detected in cells expressing AGPases with and without 6His-MBP-TEV protease cleavage site tag. Figure 1. AGPase in -1,4-polyglucans synthesis Schematic diagram of biosynthetic pathway of -1,4-polyglucans such as starch and glycogen. AGPase catalyses the synthesis of ADP-glucose which is a precursor for starch and glycogen synthases. 6 5 4 3 2 MW 26 M 170 130 26 ADP-glucose Purify proteins using three chromatography columns 24 34 -1,4-glucann+1 Harvest and lyse cells and collect soluble proteins 22 43 MUT-1 K41R, T51K, S302N K133A, K134A K244A, K245A -1,4-glucann Grow E. coli cells (glgC-) transformed with plasmid DNAs expressing mutant forms of AGPase 20 55 (kDa) MUT-1 PPi 2Pi activation inhibition 18 SS MUT-2 Glucose 1-P 16 72 MBP Tag ATP (kDa) 95 Site-directed mutagenesis of AGPase large subunit Plants and bacteria utilize the enzyme ADP-glucose pyrophosphorylase (AGPase) as the first step in the production of their carbon and energy 1,4-polyglucan reserves (Fig. 1). AGPases from higher plants function as heterotetrameric structures consisting of cooperating two large and two small subunits (Hwang et al., 2008; Fig. 3). The crystal structure of the native enzyme has not been elucidated, although a homotetrameric form consisting of the potato tuber AGPase small subunits has been determined (Jin et al., 2005). In order to improve crystallization of the native enzyme, we substituted several amino acid residues of the L subunits with alanine based on Surface Entrophy Reduction concepts (Goldschmidt et a., 2007; Fig. 4 and 5). However, we encountered a problem in protein solubility for the mutant enzymes. In contrast to the wildtype AGPase, the mutant proteins containing the mutant L subunits and wildtype S subunits became insoluble after expression. Thus, to increase solubility of the mutated AGPase protein, we attached a 6xHis tagged maltose binding protein (6XHis-MBP) containing a tobacco etch virus (TEV) proteinase cleavage site to the amino terminus of the L subunit (Fig. 6). This strategy will allow us to purify large quantities of AGPase protein variants without any affinity tags, a condition required for identifying the optima conditions for crystallization of AGPase proteins (Fig. 7). AGPases 14 SS Background Background Photosynthesis Imidazole (0 to 0.1 M) 170 130 SS TALON-Immobilized Metal Affinity Column Chromatography NaCl (0 > 0.5 M) 12 LS 10 SS 8 SS FT Natural Oligomerization LS CE ADP-glucose pyrophosphorylase (AGPase) is a regulatory enzyme involved in the production of α-glucan reserves, glycogen in bacteria and starch in higher plants (Fig. 1). Unlike the simple single subunit tetramer arrangement of the bacterial AGPase, the higher plant enzyme is composed of two large (L) subunits and two small (S) subunits (Fig. 3). Studies of mutant forms of either the large or small subunits or in combination indicate that the two types of subunits work cohesively instead of separately to regulate α-glucan production. Despite considerable effort, attempts to obtain crystals and, in turn, the 3dimensional structure of the higher plant enzyme have failed. Such knowledge, if available, would enable us to rationally manipulate the catalytic activity for enhanced plant productivity. Several plasmid DNAs coding for mutant AGPase enzymes, which may be more amenable to crystallization, are available (Fig. 6). Here I will show results of my efforts to express these enzymes in Escherichia coli and the purification of these enzymes to obtain a highly purified preparation, a condition required for crystallization studies. DEAE-Sepharose FF Ion Exchange Column Chromatography SS This project could not have been possible without support from Dr. Seon-Kap Hwang, Dr. Thomas Okita, the rest of the Okita Lab, along with Washington State University, and the encouragement of Dr. Christopher Meyer, Dr. Chandra Srinivasan and the Srinivasan Lab. This work was supported by the National Science Foundation Plant Genome Grant DBI-0605016. 34 26 Figure 7. Induction of large quantities of soluble MBP-tagged AGPase large subunit (MBP-LS) as viewed by SDS-polyacrylamide gel electrophoresis. M = protein size marker; MBP-tagged green fluorescence protein (GFP) along with wild type SS were used as a control. References Ballicora et a. (2005) Resurrecting the ancestral enzymatic role of a modulatory subunit. J. biol. Chem. 280(11):10189-10195 Goldschmidt, L. et al. (2007) Toward rational protein crystallization: A Web server for the design of crystallizable protein variants. Protein Sci. 16(8): 1569–1576 Hwang, S. K. et al. (2008) Direct appraisal of the potato tuber ADP-glucose pyrophosphorylase large subunit in enzyme function by study of a novel mutant form. J. Biol. Chem. 283(11):6640-6647 Jin et al. (2005) Crystal structure of potato tuber ADP-glucose pyrophosphorylase. EMBO J. 24(4): 694-704