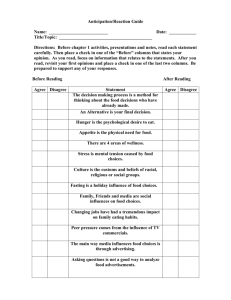
CSPI’s Petition to the FDA to Require Better
advertisement

CSPI’s Petition to the FDA to Require Better Sugar Labeling on Foods In addition to the Petition below, you can click on the following related items to find out more from CSPI: • Added Sugar Consumption in a 2,000 • Sugar Content of Popular Foods Calorie Diet • Sweet Tips for Consumers • CORN SYRUP • Added-Sugars Consumption • DEXTROSE (GLUCOSE, CORN SUGAR) • Consumption of Added Sugars Since 1983 • HIGH-FRUCTOSE CORN SYRUP • Where Added Sugar Comes From • SUGAR (SUCROSE) UNITED STATES DEPARTMENT OF HEALTH AND HUMAN SERVICES FOOD AND DRUG ADMINISTRATION Petition for Proposed Rulemaking to Establish a Daily Reference Value for “Added Sugars,” to Require Nutrition Labeling of “Added Sugars,” and to Make Corresponding Changes to Nutrient Content and Health Claim Regulations __________________________________ ) ) ) ) ) ) Docket No. _______ Submitted by the CENTER FOR SCIENCE IN THE PUBLIC INTEREST August 3, 1999 Michael F. Jacobson, Ph.D. Executive Director 1875 Connecticut Ave. NW #300 Washington, D.C. 20009-5728 202-332-9110 CONTENTS Page I. PRELIMINARY STATEMENT ..................................................................................................... 1 II. ACTION REQUESTED ................................................................................................................. 3 III. STATEMENT OF FACTUAL GROUNDS .................................................................................... 6 A. Introduction .................................................................................................................... 6 B. Health experts have made recommendations for appropriate intakes of added sugars ............ 7 C. New information invalidates the reasons given by the FDA in 1993 for not establishing a daily reference value and requiring nutrition labeling for added sugars .................................. 9 (1) Contrary to the FDA’s 1993 conclusion, there is a public health interest in reducing the consumption of added sugars ............................................................................................. 9 (a) Americans are consuming substantially more added sugars since the FDA’s reviews in 1986 and 1993 ....................................................................................... 9 (b) Added sugars have different nutritional consequences compared to naturally occurring sugars, because of the foods in which they occur ................................ 12 (i) Fruit ................................................................................................................ 13 (ii) Low-fat dairy products ................................................................................... 13 (c) Foods high in added sugars squeeze more healthful foods out of the diet ........... 14 (d) Added sugars increase blood-triglyceride levels and the risk of heart disease .... 18 (i) Sugars increase blood triglyceride levels in certain individuals..................... 18 (ii) Elevated blood triglycerides appears to be an independent risk factor for coronary heart disease .................................................................................... 21 (e) Added sugars contribute to obesity ....................................................................... 24 (f) Added sugars contribute to tooth decay ................................................................. 27 (2) Contrary to the FDA’s 1993 conclusion, there are ways to enforce regulations requiring disclosure of added sugars ................................................................................................ 28 (a) Analytical methods can distinguish added sugars from naturally occurring sugars in many foods ....................................................................................................... 29 (b) The enforcement of several existing FDA regulations requires distinguishing added sugars from naturally occurring sugars ...................................................... 31 (i) Sugars in ingredient lists ................................................................................ 31 (ii) Claims such as “no added sugar”.................................................................... 31 (iii) Percentages of fruit or vegetable juice .......................................................... 32 (iv) Standards of identity ...................................................................................... 32 (3) Contrary to the FDA’s 1993 conclusion, consumers would not be misled by information about added sugars............................................................................................................ 33 (4) The FDA’s conclusion in 1993 that naturally occurring sugars and added sugars have the same physiological impact ignores the adverse health impact of diets high in added sugars .................................................................................................................. 34 (5) The FDA’s conclusion in 1993 that there is no consensus on a daily reference value for added sugars ignored important information, which has been buttressed by new information .................................................................................................................. 35 D. Consumers need a disclosure of both the amount of added sugars and the “%DV” to help them gauge their added-sugars intake against recommended levels ...................................... 37 (1) Consumers need a disclosure of the amount of added sugars .......................................... 37 (2) Consumers need a disclosure of the “%DV” .................................................................... 38 E. Nutrient-content and health claims about added sugars should be held to the same standards as claims about fat, saturated fat, cholesterol, and sodium..................................................... 40 IV. STATEMENT OF LEGAL GROUNDS ....................................................................................... 41 A. In 1990 Congress decided that to assist consumers in maintaining healthy diets, the FDA should ensure that its nutrition-labeling regulations are consistent with new research and other information .................................................................................................................. 41 B. In 1993 the FDA decided that using a “%DV” disclosure best complied with its Congressional mandate to present nutrition information in a way that facilitates the public’s understanding .................................................................................................................. 42 C. Congress directed the FDA to prohibit nutrient-content and health claims on food labels unless they are made in accordance with regulations issued by the Secretary ....................... 43 V. CONCLUSION .................................................................................................................. 44 VI. ENVIRONMENTAL IMPACT .................................................................................................... 44 VII. ECONOMIC IMPACT................................................................................................................ 44 VIII. CERTIFICATION .................................................................................................................. 44 Exhibits 1. Letter to FDA in support of petition 2. Proposed label 3. Added sugar contained in 28 different foods 4. Comparison of trends in consumption of milk and soda 5. Some current labels August 3, 1999 Dockets Management Branch United States Food and Drug Administration Department of Health and Human Services Room 1-23 12420 Parklawn Drive Rockville, MD 20857 CITIZEN PETITION I. PRELIMINARY STATEMENT In 1982, the Food and Drug Administration (“FDA”) proposed to affirm that various added sugars — corn sugar, corn syrup, invert sugar, and sucrose — posed no risk to public health at the levels that were then being consumed.1 At that time the FDA said: “The agency will undertake a new safety evaluation if total dietary consumption increases significantly.”2 [emphasis added] The per capita consumption of added sugars3 has risen by 28 percent since 1983. The Center for the Science in the Public Interest (“CSPI”)4 and other organizations, researchers, and nutritionists5 believe that the time has come for the FDA to honor that commitment by initiating a rulemaking to establish a Daily Reference Value (“DRV”) for added sugars, to require nutrition labeling of added sugars, and to make corresponding changes to regulations regarding nutrient-content and health claims. Reducing the consumption of added sugars is an essential public health measure. Diets high in added sugars — from such foods as soft drinks, fruit drinks, candy, cakes, and cookies — squeeze healthier foods out of the diet, thereby displacing foods that provide nutrients that reduce the risk of osteoporosis, cancer, heart disease, stroke, and other health problems. In some people, diets rich in added sugars contribute to obesity, the prevalence of which has risen dramatically in the last two decades in both youths and adults. Obesity, in turn, increases the risk of diabetes, heart disease, high blood pressure, and other health problems. In people who are “insulin resistant,” high intakes of added sugars increase levels of blood triglycerides, which may increase the risk of heart disease. In addition, frequent consumption of foods rich in added sugars promote tooth decay. Using current labels, it is impossible for consumers to determine how much sugar has been 1 added to foods such as yogurt, ice cream, puddings, frozen fruit bars, sorbet, canned or frozen fruit, fruit snacks, juice drinks (beverages, cocktails, etc.), jams, jellies, breakfast cereals, cereal bars, blueberry (or other fruit) muffins, and raisin (or other fruit) cookies. In addition, current labels fail to inform consumers how much of a reasonable day’s intake of added sugars a serving of any food — from ice cream to soda pop — provides. Action by the FDA is necessary to help consumers monitor — and, if appropriate, reduce — their added-sugars consumption. Though the U.S. Department of Agriculture (“USDA”) provided quantitative dietary recommendations for added sugars in The Food Guide Pyramid (“Pyramid”), without labeling of added sugars it is difficult for consumers to follow such recommendations. In 1999, the USDA recognized that Americans were consuming far more added sugars than can fit into a healthy diet and observed that the current nutrition label is not as helpful as it could be. USDA stated that “Added sugar consumption exceeds dietary targets” and that: The ability of consumers to moderate their consumption of added sugars and sweeteners is complicated by the fact that many added sweeteners are likely to be “hidden” in prepared foods....the [food] label does not distinguish total from added sugars, which may sometimes make it difficult for consumers to determine how much added sugar they are actually consuming.6 Action by the FDA is also necessary to comply with the bipartisan judgment of Congress when it passed the law mandating nutrition labeling on packaged foods. Section 2(a) of the Nutrition Labeling and Education Act of 1990 (“NLEA”)7 directs the Secretary of Health and Human Services to require labeling information about any specific nutrient if the Secretary determines that such information “will assist consumers in maintaining healthy dietary practices.” The FDA should recognize that good dietary practices can promote general health and should not insist that to be listed on labels nutrients must be directly linked to specific illnesses such as cancer and heart disease. While we recognize that there are costs involved when food labels are changed, we believe the action requested here8 represents a critical public health measure to give consumers the tools they need to reduce their intake of added sugars and that the compliance costs are reasonable in light of the public health benefits. In any case, the costs of adding an additional line to the food label would 2 generally be modest (some producers of cereals already voluntarily include a line disclosing the amount of “other carbohydrates,” and some companies list many more nutrients than are required to be listed).9 Moreover, the costs of complying with the regulations requested in this petition would, of course, be reduced greatly if the FDA required the changes we request be implemented at the same time as other changes. II. ACTION REQUESTED Specifically, CSPI requests that the FDA establish a Daily Reference Value (“DRV”) for “added sugars” of 40 grams and require a mandatory disclosure of added sugars in both grams per serving and % Daily Value, i.e., the percentage of that DRV. (See Exhibit 2 for a mock-up of the proposed label.) CSPI also requests corresponding changes to the FDA’s labeling regulations prescribing nutrient-content and health claims. Those actions will require changes in the FDA’s nutrition-labeling regulations that include but are not limited to the following: 1. After 21 C.F.R. § 101.9(c)(6)(ii) — dealing with nutrition labeling of sugars in food — add a new subsection (iii) and renumber accordingly: “(iii) ‘Added sugars’: A statement of the number of grams of added sugars, as defined in 21 C.F.R. § 101.60(c)(2)(i)-(iii),10 in a serving, except that label declaration of added-sugars content is not required for products that contain less than 1 gram of added sugars in a serving if no claims are made about added sweeteners, added sugars, or added sugar alcohol content. Except as provided for in paragraph (f) of this section, if a statement of the added-sugars content is not required and, as a result, not declared, the statement ‘Not a significant source of added sugars’ shall be placed at the bottom of the table of nutrient values in the same type size. Added- sugars content shall be indented and expressed to the nearest gram, except that if a serving contains less than 1 gram, the statement ‘contains less than 1 gram’ or ‘less than 1 gram’ may be used as an alternative, and if the serving contains less than 0.5 gram, the content may be expressed as zero.” 2. In 21 C.F.R. § 101.9(c)(9) — dealing with DRVs — add the following to the table: under the food component column add “added sugars”; under the unit-of-measurement column add 3 “grams (g)”; and under the DRV column add “40”. 3. In 21 C.F.R. § 101.9(d)(9) — dealing with DRVs for diets of 2,000 and 2,500 calories — add the following to the table after the “dietary fiber” line: under the food-component column add “added sugars”; under the 2,000 column add “40 g”; and under the 2,500 column add “60”. 4. In 21 C.F.R. §§ 101.9(d)(12), (13) and 101.9(e)(5) — dealing with alternative sample labels — in the sample label in section 101.9(d)(12) change “Sugars” to “Total Sugars” and add a line after “sugars” stating “Added Sugars 3 g 8%”; in the sample label in section 101.9(d)(13) delete “Sugars” and add a line after “Dietary Fiber” stating “Added Sugars 8 g 153% 20% 6g 13 g 33%”; and in the sample label in section 101.9(e)(5) delete “Sugars” and add a line 15% 15%”.11 after “Dietary Fiber” stating “Added Sugars 6 g 5. In 21 C.F.R. § 101.9(f) — dealing with a simplified format — add “Added Sugars,” after the word “Sugars” throughout. 6. In 21 C.F.R. §§ 101.9(g)(5) and(6) — dealing with compliance — add “added sugars,” after “sugars”. 7. In 21 C.F.R. § 101.13(h) — dealing with disclosure of additional nutrient information for certain foods making a nutrient claim — in the first sentence of subsection (1) after “cholesterol,” add “8.0 grams of added sugars,”; in the first sentence of subsection (2) after “cholesterol,” add “16.0 grams of added sugars,”; and in the first sentence of subsection (3) after “cholesterol,” add “12.0 grams of added sugars,”.12 8. In 21 C.F.R. § 101.14(a)(4) -- prohibiting health claims for certain foods -- in the first sentence after “cholesterol,” add “added sugars,” and in the second sentence after “cholesterol,” add “8.0 grams of added sugars,”;13 in subsection (i) -- dealing with a meal product -- after “cholesterol,” add “16.0 g of added sugars,”; and in subsection (ii) -- dealing with a main dish product -- after “cholesterol,” add “12.0 g of added sugars,”. 9. In 21 C.F.R. § 101.60(c) -- dealing with sugar-content claims — add after subsection (4) a new subsection (5) and renumber accordingly: “(5) the terms ‘low added sugars,’ ‘few added sugars,’ ‘contains a small amount of added sugars,’ ‘low source of added sugars,’ or ‘low in added sugars’ may be used on the label or in 4 labeling of foods, except meal products as defined in subsection 101.13(1) and main dish products as defined in subsection 101.13(m), provided that: (i)(A) The food has a reference amount customarily consumed greater than 30 grams (g) or greater than 2 tablespoons and does not provide more than 2.0 grams of added sugars per reference amount customarily consumed;14 or (B) The food has a reference amount customarily consumed of 30 g or less or 2 tablespoons or less and does not provide more than 2.0 grams of added sugars per reference amount customarily consumed and per 50 g (for dehydrated food that must be reconstituted before typical consumption with water or a diluent containing an insignificant amount, as defined in subsection 101.9(f)(1), of all nutrients per reference amount customarily consumed, the per 50 g criterion refers to the ‘as prepared’ form). (ii) If a food meets these conditions without the benefit of special processing, alteration, formulation, or reformulation to vary the added-sugars content, it is labeled to clearly refer to all foods of its type and not merely to the particular brand to which the label attaches. (iii) The terms defined in paragraph (c)(5) of this section may be used on the label or in labeling of meal products as defined in § 101.13(l) or main dish products as defined in § 101.12(m) provided that: (A) the product contains 2.4 g of added sugars or less per 100 g;15 and (B) if the product meets this condition without the benefit of special processing, alteration, formulation, or reformulation to lower the added sugar content, it is labeled to clearly refer to all foods of its type and not merely to the particular brand to which it attaches.” 10. In 21 C.F.R. § 101.60 (c)(5) — dealing with when reduced-sugar or less-sugar claims can be made about the sugar content of a food — add “or added sugar” each time after “sugar” and add a new “and (iii) if the total amount of all sugars in the food does not meet the requirements for ‘reduced’ or ‘less’ in subsections (i) and (ii), then the claim shall contain the statement ‘not reduced in total sugars’.” 11. In 21 C. F. R. § 101.65(d) — dealing with “healthy” food claims — make the following two changes: 5 (a) After subsection 101.65(d)(2)(iii) — dealing with general “healthy” food claims — add a new subsection and renumber accordingly: “(iv) Added sugars are not present at a level exceeding the disclosure level described in §101.13(h);” and (b) After subsection 101.65(d)(4)(iii) — dealing with “healthy” claims for main-dish and meal products — add a new subsection and renumber accordingly: “(iv) Added sugars are not present at a level exceeding 12 grams per labeled serving.” III. STATEMENT OF FACTUAL GROUNDS A. Introduction In early 1993 the FDA promulgated final regulations for nutrition labeling, as required by the NLEA.16 At that time, the FDA decided against establishing a DRV for added sugars and requiring nutrition labeling of added sugars. The FDA should now amend its food-labeling regulations to provide consumers with badly needed guidance on added sugars. The FDA should amend those regulations by embodying USDA’s quantitative recommendation in the form of a DRV for added sugars of 40 grams and requiring an “added sugars” declaration in the Nutrition Facts label. The FDA also should amend its regulations dealing with nutrient-content and health claims so as to treat added sugars in the same way as other nutrients that are associated with health problems are treated. Those amendments are essential because, as discussed below, the consumption of added sugars currently is far higher than recommended and is projected to rise even higher. New data from the USDA indicate that people who consume diets high in added sugars consume lower levels of a wide variety of nutrients. Those people also consume fewer servings of grains, fruits, vegetables, meats, and dairy products than people who consume less added sugars. By displacing protective nutrients and foods in the diet, added sugars may increase the risk of osteoporosis, cancer, high blood pressure, heart disease, and other health problems. Other research has indicated that consuming a diet high in added sugars can, in some “insulin-resistant” individuals, increase blood-triglyceride levels. Those higher levels, in turn, may increase the risk of coronary heart disease. And, of 6 course, other research has demonstrated that added sugars promote dental caries. Furthermore, the incidence of obesity has increased dramatically over the last two decades. During that time, calorie intakes also rose, due partly to an increase in the consumption of added sugars. Recent studies suggest that people do not compensate as efficiently for excess calories consumed as liquid as for those consumed as solids. That finding suggests that soft drinks, the single biggest source of added sugars, and fruit drinks, the third largest source of added sugars, have contributed to the rise in obesity. Additional research indicates that calorie-dense foods, which are typically high in sugar and/or fat, contribute to obesity. Those and other findings suggest that the recent increase in added-sugars consumption has contributed to the increased rates of obesity. The FDA should comply with the Congressional intent that the FDA’s labeling regulations be consistent with new research and other information. As discussed below, the new research and other information has invalidated each of the reasons given by the FDA in 1993 for not establishing a DRV and not requiring nutrition labeling for added sugars. B. Health experts have made recommendations for appropriate intakes of added sugars. In 1977, based on advice from its academic consultants and expert witnesses, the Senate Select Committee on Nutrition and Human Needs recommended that people limit their intake to ten percent of calories (see Section III.C(5) below).17 In the next 15 years, health agencies in numerous other nations developed similar guidelines. The average recommendation was to reduce consumption of added sugars to 10 percent of calories. (See II.C.(5) below.) In 1990, the World Health Organization (WHO), in Diet, Nutrition and the Prevention of Chronic Diseases, provided one of the first quantitative recommendations for consumption levels of added sugars.18 The WHO recommended that consumption of those sugars be limited to 10 percent of calories, or 50 grams per day for someone consuming 2,000 calories. The WHO was concerned about added sugars because of their ability to cause dental caries and because “free sugars” provide energy without associated nutrients and hence displace nutrient-containing foods. In 1992, the USDA offered the American public a more sophisticated recommendation for added-sugars intake in Food Guide Pyramid.19 Pyramid’s advice to consumers is based on nutrition 7 research at the USDA and HHS and is designed to give consumers information on choosing a diet that will promote better health and reduce the risks of certain diseases. Pyramid recommends that Americans consuming 1,600 calories a day should “try to limit” their consumption of added sugars to 6 teaspoons (about 24 g), people consuming 2,200 calories a day should limit their added sugars to 12 teaspoons (48 g), and people consuming 2,800 calories should limit their added sugars to 18 teaspoons (72 g).20 By interpolation, the recommendation for a 2,000-calorie diet is 10 teaspoons (about 40 g). Those recommendations recognize that someone who consumes fewer calories has less room in his or her diet for the empty calories provided by added sugars, and that someone who consumes large quantities of calories, such as a teenage boy, should have more room for the pure energy provided by added sugars. Thus, the suggested limits for a 1,600-calorie represents 6 percent of calories; the suggested limit for a 2,000-calorie represents 8 percent of calories (by interpolation); the suggested limit for a 2,200-calorie represents 9 percent of calories; and the suggested limit for a 2,800-calorie diet represents 10 percent of calories. The 1992 recommendation was reaffirmed in the 1996 edition of the Pyramid, which states on the first page that complying with that 40-gram recommendation would result in adherence to one of the seven dietary guidelines — “use sugars only in moderation”— that will help Americans “enjoy better health and reduce your chances of getting certain diseases.”21 While various Federal agencies and private health organizations had previously used generalities — “moderation” or “avoid too much sugar” -- of limited utility, the Pyramid was the first time that a Federal agency issued a quantitative recommendation. The recommended levels are both warranted and reasonable. To derive its quantitative recommendations, USDA calculated the number of calories in a diet of given calories that come from the recommended number of servings of each nutrient-bearing food group (i.e., bread, vegetable, fruit, milk, and meat groups). USDA assumed that the foods are in their lowest-fat form and contain no added sugars. Then USDA adjusted the diet to contain 30 percent of calories from fat. To determine the quantity of added sugars that could be added to the diet, USDA calculated the difference between the total-calorie level of the diet and the calories provided by the recommended servings from the nutrient-bearing food groups with the adjusted fat intake. 8 C. New information invalidates the reasons given by the FDA in 1993 for not establishing a daily reference value and requiring nutrition labeling for added sugars. In 1993, the FDA issued final regulations for nutrition labeling,22 but rejected CSPI’s request that a DRV for added sugars be established.23 As discussed below, none of the reasons given by the FDA for rejecting CSPI’s request remains valid in light of current information. (1) Contrary to the FDA’s 1993 conclusion, there is a public health interest in reducing the consumption of added sugars. In 1993 the FDA concluded that “Other than dental caries — the incidence of which has been declining considerably among the American population — no public health concern [relating to consumption of added sugars are] articulated by the comment [from a consumer group] or in the relevant reports.”24 As discussed below, consumption of added sugars has been increasing significantly in recent years. That increase may be squeezing health-promoting foods, such as fruits, vegetables, and lowfat dairy foods, out of the diet. The displacement of those foods — and the vitamins, minerals, fiber, and phytochemicals they contain — contributes to a variety of chronic diseases. If those added sugars lead to caloric intakes that exceed caloric expenditures, obesity, with its various sequelae, is a likely consequence. In recent years, evidence has accumulated that heavy consumption of added sugars can raise blood-triglyceride levels, which may increase the risk of heart disease. The consequences of heavy consumption of added sugars may be particularly detrimental to certain segments of the population, including insulin-resistant individuals; children and teenagers; people who consume few fruits, vegetables, and whole grains; and people prone to obesity and tooth decay. (a) Americans are consuming substantially more added sugars since the FDA’s reviews in 1986 and 1993. One of the reasons given by the FDA in January 1993 for not requiring the disclosure of added sugars was that — based on then-current levels of sugar consumption and a special review 9 conducted by the agency in the mid-1980s — “FDA concluded that other than the contribution to dental caries, there is no conclusive evidence that demonstrates that sugars intake from any source is associated with chronic disease conditions.”25 The FDA’s 1986 sugars report estimated that in 197778 Americans consumed 11 percent of calories from added sugars and predicted that per capita availability of sweeteners would decline slowly between 1984 and 1990.26 Similarly, in a detailed analysis of added-sugars intake based on its 1977-78 Nationwide Food Consumption Survey, USDA estimated that the average American was getting 12 percent of calories from added sugars, with teenagers and some younger children averaging as much as 13 percent to 15 percent of calories from added sugars.27 Data not available to the FDA in 1993 show that per capita consumption of added sugars has increased markedly since 1977-78 and the mid-1980s. While per capita consumption, as reflected in “disappearance” data, of caloric sweeteners rose by only 4 percent between 1970 (122.3 pounds) and 1986 (127.0) pounds, it increased by 23 percent between 1986 and 1998 (155.6 pounds).28 Furthermore, USDA’s 1997 Pyramid Servings Data indicates that in 1996 the average American consumed 16 percent of calories from added sugars, as compared to the 11 percent or 12 percent in 1977-78.29 Pyramid Servings Data indicates that in 1996 the average American consumed 1,969 calories per day and 20.1 teaspoons of sugar (twice what Pyramid recommends).30 The average teenager consumed 20 percent of calories from added sugar. Males 12 to 19 consumed an average of 2,739 calories and almost 34 teaspoons of sugar. Females 12 to 19 consumed 1,809 calories and almost 24 teaspoons of sugar. Indeed, in 1996 the average teenager got nine percent of his or her calories just from the sugars in soda pop.31 Teenagers, even though they have larger calorie intakes and commensurately larger sugar allowances, also consumed about twice as much added sugars as USDA advises. Two-day intake data from USDA’s Continuing Survey of Food Intakes of Individuals (“CSFII”) for 1994-9632 indicate that the percentage of calories from added sugars is extremely high in some segments of the population: * Among individuals aged 2 and over, the median intake of added sugars accounted for 14 percent of calories. (The mean intake was 16 percent of calories from added sugars.) However, 25 percent of the population consumed 21 percent or more of their calories from added sugars, and 5 percent of the population got 32 percent or more of their calories from added sugars. 10 * Among children aged 6 to 11, median intake of added sugars was 18 percent of calories. However, 25 percent of this age group consumed 24 percent or more of their calories from added sugars, and 5 percent got 32 percent or more of their calories from added sugars. * Among aged 12 to 19, median intake of added sugars was 19 percent of calories. However, 25 percent of teens consumed 25 percent or more of their calories from added sugars, and 5 percent consumed 37 percent or more of their calories from added sugars. Several considerations make it likely that the impact of added sugars on the diet and on health is greater than even those troubling figures indicate. First, Pyramid’s recommendations for added sugars presume that consumers have eaten the recommended quantities of fruits, vegetables, and other nutritious foods and obtained only 30 percent of their calories from fat.33 In fact, the vast majority of Americans does not consume such a diet and should consume even less added sugars than the USDA recommended.34 Second, it is widely recognized that dietary-recall surveys, such as CSFII on which Pyramid Servings Data is based, generally underestimate actual food intakes, particularly of fat and added sugars: • A Medical Research Council study in Cambridge, UK found: “Available evidence suggests that fat and sucrose are under reported, but not micronutrients such as vitamin C.”35 • A study conducted by the United Fresh Fruit and Vegetable Association that compared food diaries from 2,000 households with consumers’ self-reported food intake found that consumers overestimated fruit and vegetable consumption by up to one-third and underestimated consumption of fats and sweets by one-half.36 Third, USDA, using adjusted disappearance data, finds that the CSFII dietary recalls may underestimate sugar intake. USDA estimates that total disappearance of added sugars is 53 teaspoons per day.37 Because some of that sugar is wasted (by retailers, consumers, or food service) or lost due to other reasons (exported in processed foods, fermented in bread, etc.),38 the USDA reduces that level to an estimated 32 teaspoons per day.39 That intake is 60 percent higher than the 20 teaspoons a day of intake based on dietary recall (CSFII). Presumably, the actual amount of added sugars consumed by the average American is somewhere between 20 and 32 teaspoons per day. In any case, Americans are consuming far more than the FDA estimated in its earlier reviews. Added sugars may make up even a bigger part of the American diet a decade from now 11 unless preventive actions are taken. The USDA recently projected that if the consumption trend between 1992 and 1996 continues, per capita consumption of added sugars will increase almost 20 percent between 1996 and 2005.40 It is worth noting that consumption (as indicated by disappearance data) increased by 3.3 percent between 1996 and 1998. It is especially significant that added-sugars consumption has continued to rise sharply in recent years given the FDA’s comment in 1993 in which the agency recalled that in 198241 it had said that: it would monitor average daily consumption of these ingredients [sucrose, corn sugar, corn syrup, and invert sugar] and would reevaluate the safety of their use if total dietary consumption were to increase significantly. The agency concluded in those [1982] documents that there could be safety concerns if intake of these ingredients increased significantly over the current levels (approximately 50 gr).42 In fact, since the 1982 and 1986 reviews of sucrose and corn sugars, average consumption increased by more than 20 percent and is now about 80 grams per day, a far cry from that “approximately 50 gr.”43 (b) Added sugars have different nutritional consequences compared to naturally occurring sugars, because of the foods in which they occur. FDA’s current regulations — which require food labels to disclose only the amount of total sugar — fail to assist consumers in choosing a healthy diet because they treat all sugars as equal. While naturally occurring sugars are chemically identical to added sugars, treating them as equal for labeling purposes provides misleading dietary guidance. As USDA states: Although the human body cannot distinguish between naturally occurring and added sugars, dietary guidance focuses on added sugars because foods high in added sugars often supply calories but few nutrients. To the extent that consumers substitute the calories from less nutrient-dense sugary snacks like sweetened soft drinks and candy for nutrient-rich foods like fruits, vegetables, and whole grains, dietary intake of the fiber, vitamins, minerals, and other nutrients found in these foods may be reduced.44 Added sugars are found largely in soft drinks, sweet baked goods, fruit drinks, candies, and other empty-calorie or nutrient-poor foods that most Americans should eat in smaller quantities. According to CSFII 1994-96 data, the largest sources of added sugars are:45 Soft drinks 33% Cakes, cookies, pies, etc. 14% 12 Fruit drinks, ades, etc. 10% Dairy desserts Candy Breakfast cereals Tea Other 6% 5% 4% 3% 25% In contrast, naturally occurring sugars are found in fruits and dairy products. Fruits and low-fat dairy products are nutrient-dense foods that are associated with a lower risk of disease and that Americans should eat in greater quantities. The Dietary Guidelines Advisory Committee46 observed in 1995 that “sugars and starches occur naturally in many foods — including milk, fruit, some vegetables, breads, cereals and grains — that also supply other nutrients.”47 The Advisory Committee then noted that some foods that are high in added sugars “supply calories but few or no nutrients.”48 Growing evidence makes it clear that the public should consume greater quantities of fruit and low-fat dairy products, notwithstanding their content of sugars. For example: (i) Fruit. Numerous studies suggest that a diet rich in fruits (and vegetables) is associated with a lower risk of several cancers.49 Other studies have found lower rates of stroke in people who eat more fruits and vegetables.50 That finding is supported by the recent DASH study, which found that a low-fat diet rich in fruits (as well as vegetables, low-fat dairy products, etc.) lowered blood pressure in people with high-normal levels.51 Research is ongoing to determine the contribution of the phytochemicals, fiber, folic acid, potassium, or other components of fruit to the reduced risk of cancer, stroke, and other health problems. However, regardless of which nutrients provide which benefits, the Dietary Guidelines, the National Cancer Institute, and other health authorities agree that the public should eat more fruit. Food labels should provide information that enables consumers to distinguish added sugars from the naturally occurring sugars in such essential foods as fruits and vegetables. (ii) Low-fat dairy products. Milk and many other dairy products contain lactose. A large body of research indicates that 13 adequate calcium intakes reduce the risk of osteoporosis by increasing peak bone mass or by raising (or maintaining) bone density. Low-fat and fat-free milk are rich in calcium. Yet average calcium intakes fall far below recommended levels, especially among teenage girls and women, who face a high risk of osteoporosis in their later years. In addition, in a recent clinical trial, calcium supplements lowered the risk of adenomas of the colon in people who had already had at least one adenoma removed.52 While the trial used calcium supplements, not calcium-rich foods, the results suggest that calcium-rich, low-fat dairy products may reduce the risk of colon cancer. Furthermore, low-fat dairy products were a component of the DASH diet, which lowered blood pressure in a clinical study. It is impossible to attribute the reduction in blood pressure to low-fat dairy products alone or to the specific nutrients in low-fat dairy products. However, it is clear that food labels should provide information that enables consumers to distinguish added sugars from the naturally occurring sugars (for instance, lactose) in such valuable foods as low-fat dairy products. While fruit and low-fat dairy products appear to lower the risk of major illnesses that threaten Americans’ health, foods that are high in added sugars offer no known benefits other than providing calories. Instead, they increase the risk of health problems or displace foods and nutrients that appear to reduce the risk of disease. The added sugars they provide should be distinguished on food labels from naturally occurring sugars. Without that information, it is difficult for consumers to know how much of the total sugar in numerous processed foods is added and how much comes from fruit or dairy products. Those foods include fruit snacks; fruit yogurt; cereal bars; ice cream; frozen yogurt; canned or frozen fruit; puddings; juice drinks; jams; jellies; sorbet; frozen fruit bars; and breakfast cereals, cookies or muffins that contain fruit. (c) Foods high in added sugars squeeze more healthful foods out of the diet. In its 1986 sugars report, the FDA concluded: There is no firm evidence that sugars as currently consumed interfere with the bioavailability of vitamins, minerals, or trace nutrients, nor is there scientific evidence supporting the notion that dietary imbalances are preferentially caused by increased sugars consumption.53 [emphasis added] 14 More specifically, the report stated: ...there is not persuasive evidence that sugars as they are commonly used and consumed: (1) have unique properties or uses relative to the production of “empty calorie” diets; (2) [nor is there persuasive evidence that added sugars] have been identified as a significant cause of nutrient deficiencies with [sic] the U.S....54 [emphasis added] Contrary to the FDA’s 1986 thinking, we do not believe that it should be essential to demonstrate that added sugars are uniquely responsible for nutrient-poor diets in order to require labeling that would help consumers to lower their intake of added sugars. Indeed, the Select Committee on GRAS Substances reported to the FDA in 1976: “. . . It is likely that some individuals may eat enough [sucrose] to exclude adequate amounts of other foods that furnish required nutrients.”55 Furthermore, there is compelling new evidence that a high intake of added sugars does compromise the nutrient content and healthfulness of the diet. Since the FDA’s report was issued, the added-sugars content of the average American’s diet has jumped from an estimated 11-12 percent of calories in 1986 to 16 percent in 1996. That increase is particularly disturbing because few Americans are consuming the recommended minimum of five servings of fruits and vegetables a day, more low-fat dairy products, and more fiber-rich whole grains and beans. New data from the USDA indicate that people who consume diets high in added sugars consume lower levels of protein; fiber; vitamins A, E, C, B-6, B-12, riboflavin, niacin, and folate; calcium; iron; zinc; and magnesium.56,57 They also consume fewer servings of grains, fruits, vegetables, meats, and dairy products than people who consume less added sugar.58 In addition, a recent study indicates that the small percentage of Americans who consume the number of servings from each food group recommended by USDA’s Food Guide Pyramid consumes less sugars than others.59 As Meir Stampfer of the Harvard School of Public Health said at the March 9, 1999, meeting of the Dietary Guidelines Advisory Committee, added sugars’ “main adverse effect is that it’s displacing foods that do provide nutrients.”60 At the same meeting, committee member Shiriki Kumanyiki added, “[I]t’s very clear that it’s a displacement issue . . . it’s replacing things that are needed.”61 The impact of added sugars on the nutrient quality of Americans’ diets and the public’s 15 health is substantial. Many of the 14 nutrients that are negatively associated with added-sugars consumption have a key role in promoting health and preventing disease. For example: * Calcium. Adequate intakes of calcium can help reduce the risk of osteoporosis62 and possibly high blood pressure63 and colon cancer.64 * Fiber. High intakes of fiber are associated with a lower risk of heart disease65 and diabetes.66 * Vitamin E. Women who consume more vitamin E from foods have a lower risk of heart disease.67 * Folate. Diets rich in folate may help prevent heart disease, colon cancer, and birth defects.68,69,70,71 * Vitamin B-6. Women who consume more vitamin B-6 (from foods or supplements) have a lower risk of heart disease.72 Soda pop, the single biggest source of added sugars for the average American, illustrates why the FDA must consider not just the impact on health of added sugars, per se, but also the impact of foods high in added sugars. Higher intakes of soft drinks are associated with lower intakes of more healthful beverages. For example, among children aged 2 to 17, those who consume the most soft drinks consume lower levels of milk and fruit juice.73 A study of 105 children aged 24 to 36 months found a similar inverse relationship between consumption of soft drinks versus milk and fruit juice.74 Researchers at USDA reported an inverse relationship between milk and soft-drink consumption in the late 1970s, when soda consumption was significantly lower than it is now.75 Using data from USDA surveys, twenty years ago teenagers consumed twice as much milk as soft drinks; in 1994-96 they consumed twice as much soft drinks as milk (see Exhibit 4). The potential impact on osteoporosis rates several decades from now is obvious. Preliminary research indicates that drinking soft drinks instead of milk contributes to broken bones in children and adults. One study found that children 3 to 15 years old who had suffered broken bones had lower bone density, which can result from low calcium intake.76 Another study found a significantly higher rate of bone fractures among former college athletes who consumed more soft drinks.77 The authors concluded: These results, if confirmed, may have important public health implications because of the 300% increase in carbonated beverage consumption combined with a decline in milk consumption in the U.S. over the last three decades. 16 In addition to the impact of soft drinks on milk consumption, new data from USDA indicate that foods high in added sugars also replace fruit, vegetables, low-fat dairy products, high-fiber whole grains, and other healthful foods.78 Diets rich in those foods are associated withlower risk of cancer,79 heart disease,80,81 stroke,82, 83 diabetes,84 and osteoporosis.85 However, it is more difficult to consume adequate amounts of foods that reduce the risk of those illnesses if one consumes a diet high in added sugars. According to a study by USDA and NCI nutrition experts, the fewer nutrition objectives that children age 2 to 11 met, the greater their consumption of added sugars.86 Children who met all five guidelines (for grains, vegetables, fruit, dairy, and meat) consumed 11.6 percent of their calories in the form of added sugars. Those meeting two or three guidelines consumed about 14 percent of calories from added sugars. Those who met just one guideline consumed about 17 percent of their calories from added sugars. And children who failed to meet any of the guidelines consumed 20.2 percent of their calories from added sugars. The landmark report Food, Nutrition and the Prevention of Cancer: a global perspective published by the World Cancer Research Fund expressed concern about the impact of added sugars on nutrient intake and cancer risk. The report stated: In particular, individuals with high sucrose or sugar intakes (proportional to energy intake) tend to have lower intakes of a number of foods or dietary constituents which have probable or possible protective roles in colorectal cancer. These include vegetables, fruits, cereals, fibre, folate, carotenoids and other antioxidants. Associations observed between sucrose intake and colorectal cancer could therefore, at least partly, be accounted for by low intake of such protective dietary constituents. . . . On balance, the panel judged the evidence to show a possible causal relationship between refined sugars and colorectal cancer.87 That same report’s “best-guess” estimate is that increasing consumption of fruits and vegetables (excluding potatoes and legumes) by 1.5 servings per day would reduce overall cancer risk by about 20 percent.88 The most conservative estimate was a 7 percent decrease in risk. We would expect, if people reduced overall added-sugars intake, many consumers would replace at least a portion of those calories with fruits and vegetables. That salutary change would be likelier to occur if the FDA accompanied added-sugars labeling with an educational campaign (recall that the “E” of NLEA stands for Education). 17 (d) Added sugars increase blood-triglyceride levels and the risk of heart disease. In 1986 the FDA concluded that “Current levels of sugars consumption have not been demonstrated to be an adverse risk factor in terms of blood lipid and lipoprotein profiles for normal individuals.”89 In 1993, when rejecting the declaration on labels of added sugars, the FDA relied largely on that 1986 report and did not acknowledge recent evidence on blood lipids. In fact, evidence then available, as well as new scientific evidence, indicates that levels of sugar consumption that are now current may raise blood triglycerides in insulin-resistant individuals, who comprise a substantial proportion of the population. Higher triglycerides, in turn, appear to increase the risk of heart disease in insulin-resistant individuals. (i) Sugars increase blood triglyceride levels in certain individuals. The FDA’s dismissal in 1986 of studies by USDA on sugars and blood lipids rested in part on the uncertainty surrounding what the USDA researchers called carbohydrate sensitivity. “Carbohydrate sensitivity has been suggested to be an early manifestation of diabetes; however, an association with diabetes has not been shown,” states the FDA 1986 sugars report.90 Research has since identified “Syndrome X” or the “Metabolic Syndrome,” a constellation of risk factors, including insulin resistance and high triglyceride levels, that are associated with a higher risk of heart disease.91 The FDA’s 1986 sugar report acknowledged that USDA’s research showed that “carbohydrate-sensitive males...exhibited adverse blood lipid risk profiles as sucrose was increased in their diet.”92 However, the report then essentially dismissed that entire series of sugar studies by stating, “when these individuals are fed in a gorging meal pattern (75 to 90% of total daily calories in a single meal), they can demonstrate impaired glucose tolerance as dietary sucrose is increased in amounts above those currently consumed in the U.S.”93 It is not clear whether the FDA’s criticism about gorging is valid. In any case, FDA’s dismissal was inappropriate because other USDA studies did not use a gorging meal pattern. For example, in one study that found higher triglyceride levels after carbohydrate-sensitive men consumed diets containing 7.5 percent or 15 percent fructose, the researchers fed 15 percent of calories at breakfast, 30 percent at lunch, and 55 percent at dinner.94 A second study found higher triglycer18 ide levels in carbohydrate-sensitive men after they and normal men were fed diets containing 20 percent fructose rather than 20 percent starch. In that study, the subjects ate 22 percent of their calories at breakfast, 29 percent at lunch, and 49 percent at dinner.95 Moreover, a 1984 study at the Stanford University School of Medicine found similar results without feeding a large number of calories at one meal.96 The FDA’s 1986 report also dismissed the evidence linking added sugars to high triglyceride levels by noting that “the reports are inconsistent. In some studies, high-sucrose or -fructose intake did not lead to any changes in serum cholesterol, triglyceride or lipoprotein patterns, while in others all these parameters were affected by sugars consumption.”97 However, those inconsistencies do not warrant a conclusion that large amounts of added sugars are safe. As one reviewer stated: When those studies that provide the best scientific evidence are reviewed, there is evidence that increasing dietary fructose consumption can significantly increase fasting plasma triglyceride and cholesterol concentrations....It appears that the magnitude of the deleterious effects vary [sic] depending on such factors as age; sex; baseline glucose, insulin, and triglyceride concentrations; the presence of insulin resistance; and the amount of dietary fructose consumed. Finally, not all studies are consistent in these findings, however, the positive data cannot easily be dismissed and may be of substantial clinical importance. This is particularly true given the fact that: 1) these deleterious changes occur in the absence of any beneficial effect on lipoprotein metabolism, and 2) these abnormalities in lipoprotein metabolism appear to be greater in those individuals already at an increased risk of coronary artery disease.98 Since the FDA’s 1986 report, reviewers have cited the studies that USDA conducted in the 1980s as among the few controlled studies to investigate the impact of added sugars on triglycerides. Those reviewers have noted that, in people with “carbohydrate sensitivity,” diets containing roughly 20 percent of calories from added sugars raise triglyceride and insulin levels more than diets containing similar amounts of starch. (Those “carbohydrate-sensitive” people probably now would be called “insulin-resistant.”) For example, according to one review: These two studies, by Hallfrisch et al and Reiser et al, provide considerable insight into the role of dietary fructose in lipoprotein metabolism. Together they indicate that individuals who are carbohydrate sensitive are very responsive to even small increases in dietary fructose (as little as 7.5% of total energy). Secondly, they suggest that the deleterious effects of dietary fructose observed in these studies was relatively 19 dose dependent. Finally, they indicate that even individuals who are not carbohydrate sensitive, will respond in an adverse manner at the highest intake of dietary fructose (20% of total energy).99 Others reached a similar conclusion: Individuals with hypertriglyceridemia, hyperinsulinemia, or both may be more sensitive than others to any harmful effect of high intakes of fructose or sucrose. For such people there is a particular need for sound evidence on which to base advice on consumption of these sugars. Existing evidence comes largely from studies at one center [USDA’s Human Nutrition Research Center], but the strength of evidence from well-designed studies suggests that this is a real problem and should promote further investigations of this important area.100 In 1984, researchers at Stanford University confirmed USDA’s results in people with high triglyceride levels, a marker for insulin resistance.101 Triglyceride and postprandial insulin levels rose more when researchers switched subjects from a low- (40 percent of calories) to a high-carbohydrate (60 percent of calories) diet that was proportionately higher in sucrose — i.e., when sucrose was increased from 9 percent to 15 percent of calories — than when they increased carbohydrates but held sucrose constant at 13 percent of calories. The FDA’s 1986 report dismisses the evidence linking added sugars to high triglycerides by noting that the levels of sucrose in USDA’s studies are “increased in amounts above those currently consumed in the U.S.”102 In fact, those levels no longer exceed amounts currently consumed in the U.S. The studies at Stanford found increased triglyceride levels in diets containing 15 percent of calories from sucrose. The USDA studies found a rise in triglycerides in diets containing as little as 7.5 percent of calories from fructose. (If, as some researchers suggest, it is the fructose component of sucrose and high-fructose corn syrup that raises triglycerides, diets containing 7.5 percent fructose and 15 percent sucrose should have roughly comparable effects on triglycerides.) Those levels are similar to the added sugar levels now consumed by millions of Americans. The USDA estimates that the average American now gets 16 percent of his or her calories from added sugars, while teenagers average 20 percent of calories from added sugars. However, many Americans — including middle-aged and older people, who have an elevated risk of heart disease — are consuming more than average. 20 For example, according to USDA (two-day) data, 25 percent of adults aged 30 to 39 consume at least 21 percent of their calories from added sugars, 25 percent of adults aged 40 to 49 consume at least 19 percent of their calories from added sugars, 25 percent of adults aged 50 to 59 consume at least 20 percent of their calories from added sugars, and 25 percent of adults aged 60 to 79 consume at least 16 percent of their calories from added sugars.103 Therefore, at least 25 percent of the middle-aged and older population consumes enough sugar to raise triglycerides. Given that median intakes range from 11 to 14 percent of calories for those age groups, considerably more than 25 percent of middle-aged and older Americans may consume enough added sugar (15 percent of calories) to raise triglycerides.104 A recent pilot study supports the notion that not all carbohydrates have the same impact on triglycerides.105 In people with hypercholesterolemia, a low-fat, low-fiber “convenience food diet,” in which most of the sugars came from cookies, sweetened yogurt, and fruit juice, raised triglycerides more than a low-fat, high-fiber “plant food diet,” in which most of the sugars came from fruit. A larger study is under way. The prevalence of insulin resistance in the United States is uncertain, because it is not measured in clinical practice and there are no widely accepted standards. However, some experts estimate that roughly 25 percent of apparently healthy people are insulin resistant.106 A recent study in Italy estimates that the insulin-resistant segment of the population may include 66 percent of people with glucose intolerance, 94 percent of people with diabetes, 84 percent of people with high triglycerides, 88 percent of people with low HDL cholesterol, and 20 percent of normal-weight subjects with no metabolic disorders.107 Furthermore, the incidence of insulin resistance is likely to rise as the population ages and obesity rates rise. It is clear that the prevalence of insulin resistance is sufficiently great as to result in high intakes of sugars posing a public health problem. (ii) Elevated blood triglycerides appears to be an independent risk factor for coronary heart disease. The role of high blood-triglyceride levels in promoting heart disease has been an issue of great debate, with one researcher even calling the debate a “war.” The National Cholesterol Education Program (NCEP) has been ambivalent on triglycerides, stating: It is not clear whether high triglycerides alone increase your risk of heart disease.108 21 Elevated serum triglycerides are positively correlated with risk for CHD (coronary heart disease) in univariate analysis, but they lose some or most of their ability to predict CHD in multivariate analysis.109 In the view of some workers, the statistical methods used to assign independent relationships to CHD risk among the different lipid fractions are of limited value because of high intercorrelations among various lipoprotein fractions and the greater variability in triglyceride measurements. . . . Nonetheless, the 1992 NIH Consensus Conference indicated that triglyceride reduction should be part of the therapy of certain dyslipidemias that carry an increased risk for CHD.110 Numerous experts are more emphatic than the NCEP and have concluded that triglycerides are, indeed, an independent risk factor for heart disease. For example, in a 1996 nested case-control study of blood samples collected prospectively from 574 men in the Physicians’ Health Study, there was a 40 percent increase in the risk of myocardial infarction for every 100 mg/dL increase in nonfasting triglycerides.111 Men in the highest triglyceride quintile had roughly 2.5 times the risk of those in the lowest triglyceride quintile. “These findings indicate that nonfasting triglyceride levels appear to be a strong and independent predictor of future risk of MI, particularly when the total cholesterol level is also elevated,” concluded Meir Stampfer and colleagues at Harvard Medical School and elsewhere. [emphasis added] In a recent study published in the American Heart Association’s journal Circulation, Danish researchers examined the relation between fasting triglycerides and risk of ischemic heart disease (IHD) in middle-aged and elderly white men.112 According to the researchers: Compared with the lowest third level and adjusted for age, body mass index, alcohol, smoking, physical activity, hypertension, non-insulin-dependent diabetes mellitus, social class, and LDL and HDL cholesterol, relative risks of IHD (95% confidence interval) were 1.5 (1.0 to 2.3; P=.05) and 2.2 (1.4 to 3.4; P<.001) for the middle and highest third of triglyceride levels, respectively. When triglyceride levels were stratified by HDL cholesterol levels (triglyceride third multiplied by HDL cholesterol third), a clear gradient of risk of IHD was found with increasing triglyceride levels within each level of HDL cholesterol, including high HDL cholesterol level, which are thought to provide protection against IHD. CONCLUSIONS: In middle-aged and elderly white men, a high level of fasting triglycerides is a strong risk factor of IHD independent of other major risk factors, including HDL cholesterol. [emphasis added] In an accompanying editorial, Antonio M. Gotto, of Cornell Medical School, noted the difficulty in proving whether triglycerides is an independent risk factor for heart disease.113 He wrote: 22 However, the current evidence makes a compelling argument for including TG in the lipoprotein profile in the evaluation of patient risk for coronary disease. . . . The growing attention to hypertriglyceridemia and increased CHD risk is encouraging to veterans of the “triglyceride wars” and congruent with another trend in CHD risk management, namely, the concept of global risk assessment, in which TG and other risk factors are considered in the context of patients’ global risk for developing CHD. Ronald Krauss, head of molecular medicine at the Lawrence Berkeley Laboratory in California, said that the Danish findings support those scientists, including himself, “who have been absolutely convinced that triglycerides are a part of the missing equation ... above and beyond cholesterol” in predicting the risk of heart disease.114 Krauss is former chairman of the American Heart Association’s nutrition committee. A 1998 study, from the University of Maryland School of Medicine, of 350 patients with arteriographically defined coronary artery disease (CAD) concluded that triglycerides was an independent risk factor for heart disease.115 After adjusting for a variety of factors, “multiple logistic regression analysis revealed the following independent predictors of CAD events: . . . [triglycerides] > 100 mg/dl (RR 1.5, 95% CI 1.1% to 2.1%.” [emphasis added] The researchers, led by Michael Miller, director of preventive cardiology, concluded that triglyceride levels previously considered “normal” are predictive of new coronary events. In a separate paper, Miller stated: Convincing evidence of a link between elevated triglyceride levels and CHD has been reported in a meta-analysis of patients whose plasma triglyceride levels were measured in the fasting state. Further evidence has come from several angiographic studies that have examined the relationship between plasma triglyceride levels and the progression of coronary artery disease....In an 18-year follow-up study, incidence and severity...correlated with plasma triglyceride level. At a triglyceride level of 100 mg. dl-1, which current guidelines would consider to be low risk, patients had a reduced chance of survival from coronary events.116 While weight loss and exercise may be the most potent weapons against insulin resistance and high triglycerides, avoiding heavy consumption of added sugars also appears to be an effective weapon. Nutrition labeling should make it easier for people who are insulin-resistant or who for other reasons have high triglyceride levels to reduce their intake of added sugars. Although it is unclear whether naturally occurring sugars in fruit and milk products raise triglycerides in those 23 people, it is clear that those people should limit their intake of added sugars before they cut back on fruit and low-fat milk products, which products may help lower the risk of cancer, heart disease, stroke, and osteoporosis. (e) Added sugars contribute to obesity. In June 1995, the Dietary Guidelines Advisory Committee told the Secretary of Health and Human Services and the Secretary of Agriculture that “Many Americans are overweight and gain weight as they grow older...the number of overweight people has increased.”117 Between NHANES II (1976-1980) and NHANES III (1988-1991), overweight increased from 8 percent to 11 percent in children, from 6 percent to 11 percent in adolescents, and from 25 percent to 33 percent in adults.118 By the updated NHANES III (1988-1994), those figures had risen to 14 percent of children, 12 percent of adolescents, and 35 percent of adults.119 Using the World Health Organization’s definition of overweight (BMI>25), a definition recently adopted by the U.S., the prevalence of overweight is 55 percent.120 Obesity is more prevalent among the poor and minorities, especially women, than among their middle- or high-income counterparts.121 Overweight is a serious public health problem, according to the Advisory Committee and others, because “Both overweight and adult weight gain are linked to high blood pressure, heart disease, stroke, diabetes, certain types of cancer, arthritis, breathing problems, and other illness.”122 Foods that are high in added sugars appear to be contributing to the nation’s epidemic of obesity because they are often high in calorie density. A recent review of clinical studies suggests that diets rich in calorie-dense foods promote obesity.123 The review states “...when the fat content was controlled but the energy density varied, subjects ate a constant weight of food; therefore, the greater the energy density, the greater was the energy intake.”124 Calorie-dense foods are typically high in fat and/or sugar. For example, an Entenmann’s Chocolate Fudge Cake has 34 grams of added sugars and a caloric density of 3.6 (310 calories per 3 oz.).125 A Cinnabon contains 49 grams of added sugars and a caloric density of 3.2 (670 calories per 7.5 ounces). An order of Burger King Cini-minis with icing has 38 grams of added sugars and a caloric density of 4.0 (530 calories per 4.7 ounces). 24 Added sugars may contribute to obesity simply because they comprise a large fraction of the excess caloric intake consumed by millions of Americans. “I think 18 percent sugar intake is very high in this country and it contributes very significantly to the caloric load that we’re eating,” observed obesity expert Xavier Pi-Sunyer at a recent meeting of the Dietary Guidelines Advisory Committee.126 Furthermore, sweetened foods are highly palatable. Studies suggest that a heightened preference for fatty sweets may contribute to obesity among some segments of the population.127 This evidence is supported by a recent British study that found higher intakes of “high-fat sweet products” such as cake, cookies, and chocolate among women with a higher BMIs.128 Interestingly, this positive association becomes inverse if individuals with low energy intakes—that is, individuals reporting presumably invalid data—are included. The apparent inverse association between BMI and fatty sweets is “due to the reduced reporting of these products by obese women,” conclude the authors. The British results also suggest that studies reporting an inverse or null relationship between added sugars intake and BMI may be flawed by invalid data, especially from overweight individuals. Those studies may also have been unable to detect positive relationships between BMI and added sugars because they failed to examine specific categories of high-sugar foods — such as fatty sweets or soft drinks — or because they failed to examine relationships for particular segments of the population, such as women, men, children, the overweight, etc. Several lines of evidence suggest that soft drinks, by far the largest source of added sugars in the average American’s diet, may increase the risk of obesity. A review of the literature and a clinical study indicate that people do not compensate for the calories consumed in liquid foods as well as they do for the calories consumed in solid foods.129 These results are particularly disturbing, considering that 46% of added sugars come from liquids (soft drinks, fruit drinks, and tea). In addition, a recent analysis of NHANES-III found that overweight boys and girls consume a greater percentage of their calories from soft drinks, but not other beverages, than do normal-weight children.130 An analysis of 1994 CSFII data found that school-age children who consume non-diet soft drinks ingested more calories than did nonconsumers of soft drinks.131 25 While soft drinks are the largest source of added sugars, the growing consumption of fruit drinks may also be contributing to the rising incidence of overweight and obesity in the U.S. Among children aged 2 to 17, the consumption of fruit drinks rose by approximately 50 percent between the 1989-91 and the 1994-95 CSFII surveys.132 Those beverages, which typically contain 5 percent or 10 percent fruit juice mixed with water, additives, and added sugars, are now the third-largest source of added sugars in the average American’s diet.133 Additional suggestive evidence that added sugars and other carbohydrates contribute to obesity comes from USDA’s surveys. Carbohydrate intake (including added sugars) increased from 195.6 g per day in 1977-78 to 208.6 g in 1987-88 and to 255.4 g in 1994-96. Added sugars increased from 57 g in 1977-78134 to 80.4 g in 1996,135 according to two different USDA dietary surveys. In addition, USDA’s sugars-disappearance data show that the availability of caloric sweeteners increased from 126 pounds per year in 1977-78 to 132 pounds in 1987-88 to 149 pounds per year in 1994-96. Thus, the increased intake of added sugars and other carbohydrates appears to have fueled the increasing rates of obesity. (In contrast, fat intakes have remained roughly constant over the past two decades, according to USDA’s dietary surveys and disappearance data.) Regardless of whether added sugars contribute to weight gain, nutritionists and weight-loss experts routinely advise individuals already overweight to consume fewer calories — starting with cutting back on empty-calorie foods such as sugary soft drinks (as well as separated fats). For instance, the National Institutes of Health recommends that people who are trying to lose or control their weight should drink water instead of soft drinks with sugar.136 Some parties argue that it is counterproductive to urge people to cut back on added sugars because high sugar intakes are not associated with obesity. Furthermore, they argue that the socalled “fat-sugar seesaw” will lead people who consume less sugar to consume more fat. In fact, correlations between sugar intakes and obesity are often confounded by age — that is, people who consume more sugar are younger, so they have a lower incidence of obesity.137 Many of those young people will become obese as they grow older. A recent study indicates that the few Americans who consume the recommended number of servings from the food groups in USDA’s Food Guide Pyramid appear to consume less added sugars than others.138 Furthermore, the “seesaw” is partly due to 26 the nature of percentages. As the percentage of one contributor goes up, others must go down. When researchers have attempted to examine fat and added-sugars intake without adjusting for calories, the two are positively correlated: that is, they rise in tandem (though that approach also has drawbacks).139 (f) Added sugars contribute to tooth decay. It is generally recognized that added sugars is one of several important factors that promote tooth decay (dental caries). Citing its own 1986 report on sugars, the FDA accepted that fact in 1993.140 The Surgeon General’s Report on Nutrition and Health stated: Frequent consumption of sugars, especially sucrose, promotes formation of dental plaque, the key predisposing cause of both caries and periodontal disease. . . . Evidence exists that sugars as they are consumed in the average American diet contribute to the development of dental caries, suggesting that the general public should reduce its sugar consumption.141 The National Academy of Sciences–National Research Council, in its landmark report Diet and Health, concluded: The committee does not recommend increasing the intake of added sugars, because their consumption is strongly associated with dental caries, and, although they are a source of calories for those who may need additional calories, they provide no nutrients.142 [emphasis added] Caries rates have declined significantly in recent decades, thanks to such preventive factors as fluoride-containing toothpaste, fluoridated water, and tooth sealants. Nevertheless, new information published subsequent to the NLEA 1993 regulations demonstrates that caries remains a problem for some sub-groups. A large survey in California found that children (ages 6 to 8, 15) of lesseducated parents have 20 percent higher rates of decayed and filled teeth.143 A national study found that African-American and Mexican-American children (6 to 18 years old) are about twice as likely to have untreated caries in their permanent teeth as their white counterparts.144 For people in such high-risk groups, prevention is particularly important. The single largest source of added sugars, regular soft drinks, is not a sticky food, but it can promote decay because it bathes the teeth of frequent consumers in sugar-water for long periods of time, not just at meal time. An analysis of data from 1971-74 found a strong correlation between the 27 frequency of between-meal consumption of soft drinks and dental caries.145 Those researchers took into account the consumption of other sugary foods and other variables. To prevent tooth decay, even the Canadian Soft Drink Association recommends limiting between-meal snacking of sugary and starchy foods, avoiding prolonged sugar levels in the mouth, and eating sugary foods and beverages with meals. Unfortunately, most consumers of soft drinks and other foods high in added sugars (and other carbohydrates) violate each of those precepts. In summary, substantial scientific evidence indicates that diets high in added sugars contribute to a variety of health problems and health-related conditions. We grant that the proof that diets high in added sugars cause health problems does not attain the same level of certainty as, say, the evidence that saturated fat causes heart disease. Nevertheless, we do not believe that the NLEA compels the FDA to prove beyond a shadow of a doubt that diets high in added sugars have adverse health consequences before the agency requires better food labeling. The existing evidence and expert opinion is sufficient to impel FDA to help consumers — including not just those who consume average amounts of added sugars, but also those who consume larger amounts — maintain “healthy dietary practices,” as stated in the NLEA, and protect the public health simply by ensuring that consumers have useful information on food labels (as opposed to sterner measures, such as limiting the sugars content of soft drinks). (2) Contrary to the FDA’s 1993 conclusion, there are ways to enforce regulations requiring disclosure of added sugars. In January 1993 one of the reasons the FDA gave for not listing “added sugars” on the food label was: There is currently no analytical methodology that would allow the agency to distinguish between sugars that are added to a food and those that are naturally occurring. Therefore, FDA would be unable to evaluate the accuracy of claims about the levels of added sugars in foods.146 New analytical techniques, as well as older techniques, can often distinguish added sugars from natural sugars. Furthermore, the FDA’s professed inability to measure added sugars has not prevented the FDA from promulgating and enforcing other regulations the enforcement of which 28 depends upon an ability to assess the levels of added sugars, natural sugars, and other ingredients. (a) Analytical methods can distinguish added sugars from naturally occurring sugars in many foods. In the case of many manufactured foods, it is a simple matter to measure added sugars. For instance, many foods contain only added sugars; so the total measured sugars content is a direct measure of added sugars. Hard candies, soft drinks, ice pops, and many other foods contain sugars that are entirely, or almost entirely derived, from added sugars. Also, many foods that contain added sugars contain natural sugars that are easily distinguished by normal analytical methods, such as liquid chromatography. Such foods include flavored milks (e.g., chocolate milk), pudding mixes, and many popular flavors of frozen desserts (e.g., vanilla ice cream) and yogurts (e.g., vanilla). The dairy ingredients provide significant amounts of sugar, but that sugar is lactose (a disaccharide made up of galactose and glucose). The added sugars are usually sucrose, glucose, and fructose. Some foods, such as sweetened breakfast cereals, contain mostly added sugars, along with small amounts of naturally occurring sugars. In many cases, one could determine how much naturally occurring sugars is present in equivalent unsweetened versions of those products (or in the ingredients of which those products are made) and determine the amount of added sugars by subtraction. The most difficult foods to analyze are those that contain both fruit (or fruit juice) and added sugars, because fruit contains varying levels of sucrose, fructose, and glucose. Since the FDA’s promulgation of nutrition-labeling rules in 1993, new analytical methods have been developed or refined that provide increasing ability to distinguish in many foods added refined sugars from naturally occurring sugars. Those methods are particularly adept at identifying the presence of added sugars in products that purport not to contain them. One method uses high-pressure liquid chromatography (HPLC), gas chromatography, or capillary gas chromatography (“cap-GC”) to measure a food’s content of various sugars. That method can identify “marker” peaks of minor constituents (oligosaccharides, phytochemicals, etc.) 29 in refined sugars (such as invert sugar and HFCS) and in fruit (or fruit juice). Quantifying the levels of those minor constituents may enable one to determine the amount of added sugars in foods that contain naturally occurring sugars. One study detected 5 percent added sugars (including highfructose syrup and beet and cane invert syrup) in apple juice and orange juice.147 In a study of pineapple juice, liquid or cap-GC detected 10 percent added HFCS, cane invert syrup, and beet invert syrup.148 Chromatographic methods are economical. A second approach is based upon the different levels of carbon and hydrogen isotopes that occur in different foods or in the same foods grown in different geographic regions.149 That method relies, in part, on the fact that most plants produce glucose by one of two enzymatic pathways that result in different levels in the glucose of two carbon isotopes, 12C and 13C (pineapple, which uses both pathways, is an exception). Corn (hence, corn sugar and HFCS) and sugar cane (hence cane sugar and cane invert sugar) utilize a metabolic pathway (C4) that results in a 13C/12C ratio that is relatively high compared to most fruits (oranges, apples, cherries, and others) consumed in the United States and to sugar beets, which use a different pathway (C3). Chemists can isolate and quantify the sugars from a food, then use combustion and mass spectrometry to measure isotope ratios. That method can ascertain added sugars to within an accuracy of about ±5-40 percent, depending on the food. It is ideal for foods that contain fruit and are sweetened by either corn sweeteners (HFCS, corn sugar, corn syrup) or cane sugar (cane sugar, invert sugar). It has been used to detect adulteration of orange and apple juices with cane sugar and HFCS.150 Measuring 13C/12C ratios is of no use when beet sugar or invert beet sugar (C3) is present (possibly mixed with cane sugar) in a food containing fruit (also C3). To determine the amounts of added sugars in those situations, one can take advantage of a second isotopic difference: Deuterium/ hydrogen (D/H) ratios vary in constituents of plants grown at different latitudes.151 That method often can detect beet sugar present in a food that contains C3 fruit or fruit juice. According to the U.S. General Accounting Office, in the best situations, beet sugar can be detected if it comprises 10% to 20% of fruit juice.152 However, the sensitivity of using D/H ratios is greatly reduced when a sugar or fruit ingredient is not obtained from a limited geographic region, but is composed of a mixture of ingredients grown at different latitudes. 30 To maximize the utility of isotope analyses, it is sometimes appropriate to measure both D/H and 13C/12C ratios. By measuring both ratios, and by knowing the expected ratios in pure fruit(s), one can sometimes estimate accurately the amounts of cane and beet sugars present in a food.153 Isotopic analyses (especially D/H ratios) can be expensive, but such analyses would only be used for a modest number of enforcement actions in cases in which the FDA or a state agency suspected that labeling was erroneous. Food manufacturers, because they know the recipes for the foods they make, know what fractions of the sugars in their products are added and naturally occurring and could provide accurate labels without resorting to isotopic (or other) analyses. (b) The enforcement of several existing FDA regulations requires distinguishing added sugars from naturally occurring sugars. Though the FDA rejected in 1993 the listing of added sugars on nutrition labels because, among other reasons, the agency did not have techniques for measuring amounts of added sugars, the agency enforces four sets of regulations — including two that were adopted in 1993 — that can only be enforced by measuring levels of added sugars. (i) Sugars in ingredient lists. The FDA currently requires the accurate listing of ingredients in descending order by weight on the ingredient label.154 Verifying the accuracy of ingredient listings requires determining the amounts of sucrose, glucose, fructose, corn syrup, HFCS, honey, lactose, maltose, and other sweeteners that are added to foods as distinguished from the sucrose, glucose, fructose, lactose, and maltose that occur naturally in foods. (ii) Claims such as “no added sugar.” The FDA now enforces regulations that allow the use of the terms “no added sugar,” “without added sugar,” or “no sugar added” only if no sugars or ingredients containing added sugars — including jam, jelly, or concentrated fruit juice — have been added to the food.155 The enforcement of those regulations requires the ability to measure added sugars as distinct from naturally occurring sugars. 31 (iii) Percentage of fruit or vegetable juice. The FDA now enforces regulations requiring the disclosure of the percentage of fruit or vegetable juice in a beverage, such as “contains 50 percent juice.”156 If the beverage contains 100 percent juice and also contains a non-juice sweetener, the regulations permit a label declaring that the beverage is “100% juice with added sweetener.”157 The enforcement of those regulations requires the ability to distinguish added sugars from naturally occurring sugars (for instance, the FDA must ensure that “with added sweetener” is disclosed on labels of the relevant products). (iv) Standards of identity. The FDA has established numerous “standards of identity” (recipes) that specify a minimum or maximum added-sugars content of certain foods. The FDA now enforces standards of identity for 22 different groups of foods,158 including some that contain both added sugars and naturally occurring sugars. For example, for canned applesauce, the FDA’s regulations distinguish between “sweetened” and “unsweetened” on the basis of whether a nutritive carbohydrate sweetener is added159 even though apples contain sugar. To ensure that “unsweetened” applesauce has not been sweetened, FDA must be able to determine whether sugars have been added. Orange juice must be labeled either as “sweetener added” or “______ added,” with the name of the sweetener, if any amount of a sweetener — defined as sugar, invert sugar, dextrose, dried corn syrup, or dried glucose syrup — has been added even though orange juice itself contains sugar.160 For fruit jelly, sweeteners may be added to fruit-juice ingredients provided that the fruit-juice ingredients are at least 45 percent by weight.161 For fruit preserves and jams, sweeteners may be added to fruit ingredients provided that the fruit ingredients are at least 45 percent or 46 percent by weight, depending upon the product.162 To enforce all of those standards the FDA must be able to distinguish between naturally occurring and added sugars. In sum, even though it said in 1993 that it cannot distinguish by analytical methods added sugars from naturally occurring sugars, the FDA still apparently enforces numerous regulations requiring knowledge of added-sugars content, including regulations for net weight of ingredients, added sugars, fruit juices, and standards of identity. The FDA sometimes enforces those regulations by, in part, simply asking manufacturers to provide recipes,163 invoices for ingredient purchases, and 32 other records. When reliable analytical methods are not available, the agency could enforce the regulations we request here in the same manner. (3) Contrary to the FDA’s 1993 conclusion, consumers would not be misled by information about added sugars. In 1993 the FDA said that “in some fruits canned in heavy syrup, added sugars may represent only about 50 percent of total sugars. Disclosure of only the added sugars could be misleading to consumers who are concerned with total sugar intake.”164 Several points should be made about that argument.165 First, CSPI is not urging that only added sugars be disclosed. It is reasonable to continue to show total sugars along with added sugars. Second, another Federal agency, the USDA, obviously does not believe that consumers are now being misled by the Pyramid’s quantitative daily dietary recommendations for added sugars. Indeed, the USDA believes that its recommendations will help consumers follow the guidance of the USDA-HHS Dietary Guidelines for Americans, which presents “choosing a diet moderate in sugars” as one of its seven guidelines. That guideline clearly refers to added, not naturally occurring, sugars. The FDA should help consumers comply with HHS’s and USDA’s recommendations by requiring disclosure of added sugars. Third, more and more academic experts are recognizing the importance of distinguishing natural from added sugars. That is reflected in the broad support for the goals of this petition (see Exhibit 1). Also, at the March 9, 1999, meeting of the Dietary Guidelines Advisory Committee, Alice Lichtenstein of the Jean Mayer USDA Human Nutrition Research Center on Aging at Tufts University said, “I think that there needs to be a mechanism for distinguishing sugar that comes from fruit and milk from other kinds of sugar. . .”166 Fourth, survey research indicates strong consumer support for labeling of added sugars. In July 1999, CSPI commissioned a nationally representative telephone survey of 776 randomly selected primary or joint grocery shoppers.167 The survey found that 54 percent of respondents preferred to have the label indicate “both the total amount of sugar and the amount of sugar used to 33 make processed food,” as compared to 30 percent who preferred to have labels indicate “only the total amount of sugar in a serving.” The remaining respondents did not know or preferred something else. Considering the complete absence of public discussion of sugars labeling, it appears quite remarkable that more than half the respondents favor specific labeling of added sugars. (When asked what term should be used on labels to indicate the sugars used to manufacture foods, 44 percent preferred “added sugar,” 27 percent preferred “refined sugar,” 21 percent did not know, and 8 percent preferred some other term .) Far from misleading consumers, disclosing the amount of added sugars would enable consumers to evaluate foods that contain naturally occurring sugars (many of which foods, such as fruit, are usually accompanied by various nutrients and whose consumption is associated with a lower risk of cancer, osteoporosis, stroke, and other diseases) versus foods higher in added sugars (which are often high in empty calories and calorie density and may contribute to tooth decay, obesity, and heart disease). For example, such a disclosure would tell consumers how much sugars has been added to yogurt, ice cream, puddings, frozen fruit bars, sorbet, canned or frozen fruit, fruit snacks, juice drinks (beverages, cocktails, etc.), jams, jellies, breakfast cereals, cereal bars, blueberry (or other fruit) muffin, and raisin (or other fruit) cookies, and would apprise consumers of the percentage of the recommended daily limit (%DV) of added sugars that servings of those foods provide. Many of those foods carry label claims such as “made with fruit” or “real fruit juice,” which appeal to consumers who want to follow advice to eat more fruit to reduce the risk of cancer and other health problems. In fact, many of those products contain far more added sugars than fruit. Yet, in most cases, consumers have no way of determining how much added sugars the foods contain and how those amounts compare to the recommended intake.168 (4) The FDA’s conclusion in 1993 that naturally occurring sugars and added sugars have the same physiological impact ignores the adverse health impact of diets high in added sugars. In 1993 the FDA gave as one of its reasons for rejecting mandatory disclosure of added sugars that “There is no scientific evidence that the body makes any physiological distinction between added sugars and those naturally occurring in a food.”169 The FDA’s observation, while 34 correct, ignores the large body of scientific evidence, discussed above in sections III.C.(1)(b) and (c), that foods high in added sugars squeeze healthier foods out of the diet, thereby having different nutritional consequences from foods that contain naturally occurring sugars. Elsewhere in its 1993 decision not to require added-sugars labeling, FDA recognized that foods rich in naturally occurring sugars have a more important role in a healthy diet than foods rich in added sugars. However, the FDA erroneously assumed that it could make that critical distinction clear to consumers without requiring added sugar labeling. The agency stated that: While FDA is not distinguishing, on the nutrition label, between added and naturally present sugars, the agency does intend to include information about this distinction in the consumer education program that it is preparing. This information will help consumers: (1) Use the information on the nutrition label to differentiate between sugar-containing foods with high versus low levels of other important nutrients, (2) use the ingredient statement to distinguish foods with naturally occurring versus added sugars, and (3) appreciate the important role in the total daily diet of foods, such as fruits and dairy products, with naturally occurring sugars.170 While the FDA may have had good intentions, it is clear that any consumer- education efforts have failed. The annual per capita consumption of added sugars continued to climb by eleven pounds — from 144.4 pounds in 1993 to 155.6 pounds in 1998 — in the short time since it issued its labeling regulations. The continued climb in soft-drink consumption and the concomitant decline in milk consumption indicates that the FDA has failed in getting the public to appreciate the important role that foods such as low-fat dairy products play in the diet. Furthermore, no matter how vigorous a consumer-education program FDA mounted, the public would still be unable to figure out how much added versus naturally occurring sugars are in foods that contain both (see Exhibit 5). (5) The FDA’s conclusion in 1993 that there is no consensus on a daily reference value for added sugars ignored important information, which has been buttressed by new information. In 1993 the FDA gave as one reason for not establishing a DRV for added sugars that there was no consensus on whether there should be one and, if so, what it should be.171 However, it did acknowledge that there was some support for a DRV, namely the WHO’s recommendation of 10 percent added sugars. The FDA also notes that setting a DRV for total sugars would be inconsistent 35 with dietary guidelines that recommend consuming more fruits and dairy products, which contain naturally occurring sugars. That reason is irrelevant to this petition, which specifically asks the FDA to establish a DRV for added, not total, sugars. Importantly, the FDA failed to acknowledge that USDA’s Food Guide Pyramid — which was first issued in April 1992 and revised in 1996 — (by interpolation) recommends limiting daily consumption of added sugars to 40 grams a day for a diet of 2,000 calories, with larger or smaller amounts and percentages of calories from added sugars considered appropriate for people who consume more or fewer calories. The FDA also did not acknowledge an earlier influential report, Dietary Goals for the United States, which was published in 1977 by the Senate Select Committee on Nutrition and Human Needs.172 That report’s third goal stated: Reduce the consumption of refined and other processed sugars by about 45 percent to account for about 10 percent of total energy intake. While the Senate committee was not itself an expert scientific body, it received testimony from a large number of expert witnesses. Further, it prepared its recommendation with the close assistance of several key consultants, including D. Mark Hegsted, a professor of nutrition at the Harvard School of Public Health who subsequently became the chief of human nutrition at the U.S. Department of Agriculture, and Philip Lee, the director of the Health Policy Program at the University of California at San Francisco who later became Assistant Secretary for Health at HHS. The FDA also did not acknowledge that numerous nations, especially technologically advanced nations rather similar to the U.S., have adopted nutrition guidelines. According to one survey, 82 out of 100 sets of dietary guidelines from 30 countries (including governmental and private health organizations) analyzed through 1991 said “eat less [added] sugar”; 74 made the recommendation for everybody, and eight for people at high risk (meaning obese or diabetic).173 Twenty-three of the reports set targets for added sugars, the average being 10 percent or less of calories. 1991 United Kingdom, Department of Health, COMA 1981, 1987 Sweden, National Food Administration 10% 1982 Norway, Ministry of Health 1986 Netherlands, Ministry of Health 36 10% 10% or less 0-10% 1987 Australia, Department of Health 12% 1987 Finland, Nutrition Board 10% or less 1980, 1989 Scandinavia, Nordic Council of Ministers 10% or less 1989 Poland, National Institute less than 10% 1989 Singapore, National Advisory Committee less than 10% Furthermore, in 1992, Consumer Reports surveyed 94 nutrition professionals — scientists, clinicians, registered dietitians, and educators — who had served on federal advisory boards relating to nutrition, or on nutrition committees of professional organizations.174 Sixty-eight of them completed a comprehensive 18-page questionnaire. “Half of them recommended reducing the average intake of sugars to 5 percent [of calories] from the current average of 11 percent.” Finally, this petition is supported by a letter from more than two dozen nutrition, publichealth, dental, medical, obesity, osteoporosis, and nutrition-education experts and more than three dozen health and citizen organizations who endorse the recommendation for setting a DRV of 40 grams. While those people do not constitute an expert committee, they reflect broad new support among health experts for establishing a DRV for added sugars and listing added sugars on food labels. Thus, two expert agencies (USDA, WHO), a Senate committee advised by scientists, numerous foreign nations, and numerous academic experts have all endorsed a recommendation that the average person limit intake of added sugars to about eight to ten percent of calories. The most sophisticated and well-substantiated of those recommendations is USDA’s, because it is based on Americans’ dietary patterns and recognizes that the sugar allowance may increase with increased caloric intake/expenditure. The time has now come for the FDA to enable consumers to follow that recommendation. D. Consumers need a disclosure of both the amount of added sugars and the “%DV” to help them gauge their added-sugars intake against recommended levels. (1) Consumers need a disclosure of the amount of added sugars. Consumers need a disclosure of the amount of added sugars in foods so they can monitor — and in many cases — reduce their intake. Furthermore, as noted above, a nationally representative survey indicates that consumers want that information. Without added-sugars labeling, consumers 37 cannot figure out how much added versus naturally occurring sugars are in foods — including fruit muffins, fruit drinks, fruit snacks, frozen fruit bars, cereal bars, ice cream, yogurt, frozen yogurt, and puddings — that contain both (see Exhibit 5). FDA suggests, in its 1993 decision to require only total sugars on the label, that its education program will help consumers “use the information on the nutrition label to differentiate between sugar-containing foods with high versus low levels of other important nutrients” and “use the ingredient statement to distinguish foods with naturally occurring versus added sugars.”175 Using the nutrition label, consumers would only be able to distinguish between foods that contain low or high amounts of vitamins A and C, calcium, and iron. That information would not help them determine how much added versus naturally occurring sugars a food contains. Using the ingredient label, consumers would only be able to estimate very roughly how much added sugar a food contains. A nutritionist armed with a calculator might be able to estimate the added-sugars content based on the ingredient list, but it is naive to expect the average consumer to make those estimates, especially when several different added sugars (for example, sucrose, invert sugar, corn syrup) are scattered among a long list of ingredients. Clearly, a line in the Nutrition Facts label listing the amount of added sugars and %DV would be a far stronger tool than the current ingredient list for helping consumers ascertain the added-sugars content of foods. Even if an occasional consumer were able to figure out the amount of added sugars, the absence of a %DV would prevent the consumer from knowing how that amount of added sugars fit into an overall diet. (2) Consumers need a disclosure of the “%DV”. Nutrition Facts labels disclose not only the absolute quantities of key nutrients, but a “%DV” to help consumers determine how much of a day’s worth of several nutrients a serving of the food supplies. Without a %DV for added sugars, it is difficult, if not impossible, for the public to compare the added-sugars content of a food to the recommended daily limit. It is likely that the absence of that information has contributed to the rising intakes of added sugars in the U.S. If the FDA were only to require added-sugars disclosures in grams, but not %DV, it would fail to give consumers the information they need. Few Americans outside of USDA’s Beltsville 38 Human Nutrition Research Center know how much added sugars is appropriate in a healthful diet. While consumers could use declarations of added-sugars contents to compare two foods, without a %DV they could not determine how a quantity like 20 or 30 or 40 grams of added sugars fits into a total daily diet. CSPI requests that the FDA establish a Daily Reference Value (“DRV”) for “added sugars” of 40 grams and to require a mandatory disclosure of added sugars in both grams per serving and % Daily Value, i.e., the percentage of that DRV. As discussed in section III.B. above, the figure of 40 grams is based on USDA’s advice to consumers — who ingest 2,000 calories per day and consume recommended levels of a variety of healthful foods and consume 30 percent of their calories from fat — that they “try to limit” their consumption of added sugars to 10 teaspoons per day. In the chart at the bottom of some nutrition labels that provide recommendations for several nutrients in the context of a 2,500-calorie diet, the DRV should be, interpolating USDA’s recommendations, 60 grams (15 teaspoons). We recognize that, as with fat, sodium, and other nutrients, there is no absolute level of added sugars below which there is assurance of health and safety and above which there is harm or risk. The DRV of 20 grams for saturated fat, for instance, was not dictated by studies showing that 20 grams was the highest safe level. In fact, there is a gradient: the less saturated fat one consumes, the greater the benefit, with no known lower limit. Rather, the 20-grams figure reflects a compromise between saturated-fat’s atherogenicity, current levels of consumption, and the practicality of reducing consumption. In the case of added sugars, the USDA based its recommendation largely on broad nutritional concerns, not the causation of a specific disease. The USDA recognized that the more added sugars one consumes, the greater the likelihood that a diet would not contain adequate levels of healthful foods and the nutrients contained therein. Of course, increasing consumption of added sugars might also contribute to obesity (and its sequelae), dental caries, and heart disease (due to increased blood triglycerides). The USDA recommendations are particularly credible, because they were based solely on nutritional concerns and arrived at outside of the politicized regulatory process. They should be adopted by the FDA for setting the DRV at 40 grams for a 2,000-calorie diet. Any DRV proposed 39 by the FDA that was higher than 40 grams would be highly suspect as being influenced by commercial pressures. E. Nutrient-content and health claims about added sugars should be held to the same standards as claims about fat, saturated fat, cholesterol, and sodium. In addition to adding “added sugars” to the nutrition label and establishing a DRV for added sugars, the FDA should make corresponding changes to its nutrient-content and health-claim regulations so that added sugars are treated in the same fashion as fat, saturated fat, cholesterol, sodium, and calories. The FDA’s regulations now require that foods (other than “meal products” and “main dish products”) containing more than 20 percent of the DRV for fat, saturated fat, cholesterol, or sodium must comply with two particular labeling requirements. First, no health claim may be made for such foods (unless the FDA has permitted the claim based on a finding that such a claim will assist consumers in maintaining health dietary practices).176 That ban should also be applied to foods containing added sugars in excess of 20 percent of the DRV. Second, the FDA now regulates when a food can claim to be “low” in fat, saturated fat, cholesterol, and sodium.177 In January 1993, the FDA explained that applying “low” to various nutrients “should assist consumers in assembling a prudent daily diet and in meeting overall dietary recommendations to limit the intake of certain nutrients.”178 At that time the FDA decided that: (a) “low” fat means less than 5 percent of the DRV for fat, (b) “low” saturated fat means less than 5 percent of the DRV for saturated fat, (c) “low” cholesterol means less than 6.8 percent of the DRV for cholesterol, and (d) “low” sodium means less than 5.8 percent of the DRV for sodium.179 Applying the same rationale to added sugars would mean that a food could say it is “low” in added sugars only if it contains less than 5 percent of the DRV — 2 grams — of added sugars per serving. Third, the FDA now regulates when a food can claim to be reduced in sugar or have less sugar than another food.180 As discussed above in section III.C.(1), public health concerns focus on added sugars, not naturally-occurring sugars, and so the provision dealing with reduced sugar or less sugar should be amended to allow such claims for added sugars provided that such foods that are not 40 “reduced” or “lower” in total sugar bear a disclosure indicating that they are not reduced or lower in total sugar. Fourth, if there is a claim characterizing the level of any nutrient for a food that contains fat, saturated fat, cholesterol, or sodium exceeding 20 percent of the DRV, then there must be a label stating “see nutrition information for ____ content,” with the blank filled in with the identity of the nutrient(s) exceeding the specified level.181 The FDA explained, in January 1993, that a slightly different version of that requirement182 will ensure that “if a nutrient content claim is made, the label must provide the consumer with the facts that bear on the advantages asserted by the claim and with sufficient information to understand how the product fits into a total dietary regime.”183 As discussed above in section III.C.(1) there is now scientific evidence about the public health impact of added sugars analogous to that for fat, saturated fat, cholesterol, and sodium, and so this provision should be expanded to include foods that provide more than 20 percent of the DRV for added sugars, i.e., 8 grams per reference serving. Finally, FDA’s current regulations provide that a food may be labeled as “healthy” only if it is low in fat and saturated fat, is not high in sodium or cholesterol, and is a good source of vitamin A, vitamin C, calcium, iron, protein, or fiber.184 That provision should be expanded to require that a healthy food not be high in added sugars, i.e., that it not exceed 8 grams of added sugars per serving (16 grams of added sugars in a “meal product” and 12 grams of added sugars in a “main dish product”). Clearly, it would be inappropriate for a low-fat — but high-sugar — ice cream, cake, or cookie to carry a “healthy” label, even if it supplies 10% of the DV for vitamin A or C, calcium, iron, protein, or fiber.185 IV. STATEMENT OF LEGAL GROUNDS A. In 1990 Congress decided that to assist consumers in maintaining healthy diets, the FDA should ensure that its nutrition-labeling regulations are consistent with new researchand other information. Section 2(a) of the NLEA186 provides that the Secretary may require food-labeling information both for nine specific nutrients (including sugars) and for any additional specific nutrients if the Secretary determines that providing such information “will assist consumers in maintaining healthy 41 dietary practices.” The NLEA does not require the FDA to prove a direct effect of a nutrient on the prevalence of a particular disease or health problem. By not including such a requirement in the NLEA, Congress showed particular wisdom, given the complexity of nutrition science and the difficulty in identifying the exact causes of conditions, such as obesity, that are affected by a multitude of factors. The House of Representatives Committee on Energy and Commerce’s report on the NLEA explains that that statutory provision gives the Secretary “the discretion to take new information into account and the ability to require that the nutrition label of foods be consistent with new research and other information.”187 As discussed above in section III, a considerable body of new (since the FDA’s 1993 decision) research and other information on added sugars makes it essential that the agency fulfill its mandate to “assist consumers in maintaining healthy dietary practices” by taking the actions requested in this petition. It is well established that an agency, faced with new developments or in light of reconsideration of the relevant facts and its mandate, may alter its past interpretation and overturn past administrative rulings and practice. The Supreme Court has said that agencies must be given ample latitude to “adapt their rules and policies to the demands of changing circumstances.”188 “[T]his kind of flexibility and adaptability to changing needs...is an essential part of the office of a regulatory agency. Regulatory agencies do not establish rules of conduct to last forever; they are supposed, within the limits of the law and fair and prudent administration, to adapt their rules and practices of the Nation’s needs in a volatile, changing economy. They are neither required nor supposed to regulate the present and the future within the inflexible limits of yesterday.”189 B. In 1993 the FDA decided that using a “%DV” disclosure best complied with the Congressional mandate to provide nutrition information in a way that facilitates the public’s understanding. Section (2)(b)(1)(A) of the NLEA190 directs that the FDA’s “regulations shall require the required information to be conveyed to the public in a manner which enables the public to readily observe and comprehend such information and to understand its relative significance in the context of a total daily diet.” The House Committee report states “one way that this could be accomplished would be to include information about the recommended daily intake on the label.”191 42 In 1993 the FDA, relying in part on focus-group discussions that it conducted, decided that “DRVs provide an appropriate approach to accomplishing the statutory mandate.”192 As discussed above in section III.D.(2), a DRV for added sugars would help consumers choose a more healthful diet by ensuring that the information is “conveyed to the public in a manner which enables the public to readily observe and comprehend such information and to understand its relative significance in the context of a total daily diet,” as the NLEA states. C. Congress directed the FDA to prohibit nutrient-content and health claims on food labels unless they are made in accordance with regulations issued by the Secretary. Section 3(a) of the NLEA193 provides that a food that makes a claim regarding either the level of a nutrient or the relationship of a nutrient to a disease or health-related condition shall be deemed to be misbranded unless the claim complies with a regulation issued by the Secretary. Two sections of the Federal Food, Drug, and Cosmetic Act (“FFDCA”) provide the general legal basis for FDA’s regulation of nutrient-content and health claims.194 Section 403(r)(1)(A)195 of the FFDCA prohibits any claim that characterizes the level of any nutrient that is of the type listed on the Nutrition Facts label unless such claim uses terms defined in regulations issued by the Secretary. That statutory provision gives the FDA ample power to amend its nutrient-content-claim regulations.196 Section 403(r)(1)(B) of the FFDCA197 deals with health claims198 and bars a claim about the “relationship of any nutrient...to a disease or a health-related condition” if, as stated in section 403(r)(3)(A)(ii) of the FFDCA,199 the food contains “any nutrient in an amount which increases to persons in the general population the risk of a disease or health-related condition which is diet related, taking into account the significance of the food in the total daily diet. . .”200 [emphasis added] Finally, the FDA has general authority to promulgate regulations to prevent the misbranding of food under sections 201(n), 403(a), and 701(a) of the FFDCA,201 and the agency in May 1994 relied in part on such authority to issue regulations governing when the term “healthy” may be used on a food label.202 In sum, those statutory provisions give the FDA ample power to amend its nutrient-content 43 and health-claim regulations203 to include added sugars. V. CONCLUSION For the reasons stated above, the FDA should initiate a rulemaking to establish a daily reference value for added sugars, to require nutrition labeling of added sugars, and to make corresponding changes to its regulations governing nutrient-content and health claims. VI. ENVIRONMENTAL IMPACT The action requested is subject to a categorical exclusion under 21 C.F.R. §§ 25.30(k) and 25.32(p) and therefore does not require the preparation of an environmental assessment. VII. ECONOMIC IMPACT No statement of the economic impact of a revision of this rule is presented because none has been requested by the Commissioner.204 VIII. CERTIFICATION The undersigned certifies that, to the best knowledge and belief of the undersigned, this petition includes all information and views on which the petition relies, and it includes representative data and information known to the petitioner which are unfavorable to the petition. Respectfully submitted, Michael F. Jacobson, Ph.D. Executive Director Benjamin Cohen Senior Staff Attorney 44 Bonnie Liebman, M.S. Director of Nutrition 1 47 Fed. Reg. 53917 (November 30, 1982) and 47 Fed. Reg. 53923 (November 30, 1982). The FDA determined that corn sugar, corn syrup, invert sugar, and sucrose are generally recognized as safe as direct human food ingredients. See 21 C.F.R. §§ 184.1854-184.1865. 2 47 Fed. Reg. at 53920 and 47 Fed. Reg. at 53927. 3 Caloric sweeteners include cane and beet sugar, high-fructose corn syrup, glucose, dextrose, edible syrups (sorgo, maple and sugarcane syrup, edible molasses, and edible refiner’s syrup), and honey. USDA, Economic Research Service. Food Consumption, Prices, and Expenditures, 1970-97 (1999) at 76. USDA, Economic Research Service, Sugar and Sweetener. Publication SSS-225, May 1999, at 87 (Table 59). 4 Petitioner Center for Science in the Public Interest, a nonprofit organization based in Wash- ington, D.C., is supported by approximately one million members who subscribe to its Nutrition Action Healthletter. CSPI has been working to improve the nation’s health through better nutrition and safer food since 1971. 5 See Exhibit 1 for a letter to the FDA from organizations, researchers, and nutritionists who support the thrust of this petition. 6 Frazao E, ed. Economic Research Service, USDA. America’s Eating Habits: Changes and Consequences (1999). Agriculture Information Bulletin No. 750, at 878. 7 21 U.S.C. § 343(q)(2). 8 This petition is submitted pursuant to section 4(e) of the Administrative Procedure Act, 5 U.S.C. § 553(e), and 21 C.F.R. §§10.25 and 10.30. 9 This voluntary disclosure is authorized by 21 C.F.R. § 101.9(c)(6)(iv). 10 In 1993 the FDA explained that stripped fruit juice is a juice-derived, rather than sugar- derived, sweetening ingredient “whose nutrient profile has been diminished to a level below the normal nutrient range for the juice.” 58 Fed. Reg. at 2922-23. We believe that stripped juices, as defined in 21 C.F.R. §102.33(f), are included in the FDA’s current definition of added sugars. We note that a more encompassing definition — which includes oligosaccharides from corn 45 syrup — is provided in USDA and HHS’s Dietary Guidelines for Americans (1995) at 33-4. 11 12 If all the sugar in the food is “added sugars,” there is no need for a “Total Sugars” line. This provision has separate parts dealing with food, “a meal product,” and “a main dish product.” For each of those parts we apply the same percentage of the DRV — 20 percent, 40 percent, and 30 percent respectively — for added sugars as is now used for fat, saturated fat, cholesterol, and sodium. 13 We apply a standard of 20 percent of the DRV for added sugars, which is the same standard currently used for fat, saturated fat, cholesterol, and sodium in this provision. 14 We apply a standard of 5 percent of the DRV for added sugars, as the current standard applies 5 percent of the DRV for fat and saturated fat, 5.8 percent of the DRV for sodium, and 6.8 percent of the DRV for cholesterol. 15 We apply a standard of 6 percent of the DRV, as that is the standard applied for “low” for calories. 21 C.F.R. § 101.60(b)(3)(i). 16 17 18 P. L. 101-535. Dietary Goals for the United States, second edition, December, 1977, at 27-34. World Health Organization. Diet, Nutrition, and the Prevention of Chronic Diseases. Tech. Rep. Series 797, 1990, at 113. 19 USDA, Center for Nutrition Policy and Promotion. USDA’s Food Guide Pyramid (April, 1992). That pamphlet, revised slightly in 1996 [hereafter referred to as Pyramid], lists the content of added sugars in each of 28 different foods (see Exhibit 3). Those foods are a sample of the information the USDA has collected about the amounts of added sugars and other nutrients in about 6,000 foods. 20 Ibid. at 17. 21 USDA, Center for Nutrition Policy and Promotion. The Food Guide Pyramid (October, 1996). 22 58 Fed. Reg. 2070-2964 (January 6, 1993). 23 CSPI suggested a DRV of 50 grams based on a 1986 FDA study estimating that the average daily per capita consumption of added sugars was 53 grams. 46 24 58 Fed. Reg. at 2221. 25 58 Fed. Reg. at 2221. 26 Glinsmann WH, et al. “Evaluation of health aspects of sugars contained in carbohydrate sweeteners. Report from FDA’s Sugars Task Force, 1986.” J Nutr. 1986;116 (11S):S1-S216. 27 Woteki CE, Welsh SO, Raper N, et al. “Recent trends and levels of dietary sugars and other caloric sweeteners.” In Metabolic Effects of Utilizable Dietary Carbohydrates. Reiser S., Ed. (New York and Basil: Marcel Dekker Inc., 1982), 1-27. 28 USDA, Economic Research Service. Food Consumption, Prices, and Expenditures, 1970- 97 (1999) at 76. USDA, Economic Research Service, Sugar and Sweetener. Publication SSS-225, May 1999, at 87 (Table 59). 29 Cleveland LE, et al. Pyramid Servings Data: Results from USDA’s 1996 Continuing Survey of Food Intakes by Individuals (USDA Agricultural Research Service, Beltsville Human Nutrition Research Center, 1997) at 26 (Table 6). “Added sugars” includes “white sugar, brown sugar, raw sugar, corn syrup, honey, molasses, and artificial sweeteners containing carbohydrate that were eaten separately or used as ingredients in processed or prepared foods such as breads, cakes, soft drinks, jams, and ice cream.” 30 The 20-teaspoon figure is inflated by about 0.5 teaspoons due to sugars that are consumed by yeast in bread and rolls. That assumes that 75% of sugars in bread are eliminated by yeast or Maillard reaction, that 100 g of bread is made with 1.3 teaspoons of sugars, and that the average consumption of yeast breads and rolls is 50 g/d/person. Personal communication, Linda Cleveland, Agricultural Research Service, USDA, July 7, 1999, and <http://www.barc.usda.gov/bhnrc/ foodsurvey/pdf/Csfii3yr.pdf> [accessed July 7, 1999]. 31 Michael Jacobson, Liquid Candy (Washington, D.C.: CSPI, 1998). Diet sodas, which provide no calories, constitute only 4% of soft-drink consumption by teenage boys and 11% by teenage girls. Footnote 17. 32 Personal communication, from Shanthy Bowman, USDA/ARS, July 29, 1999. 33 USDA, Human Nutrition Information Service. “USDA’s Food Guide: background and development.” Misc. Pub. No. 1514, Sept., 1993, at 14. 47 34 Krebs-Smith SM, Cleveland LE, Ballard-Barbash R, et al. “Characterizing food intake patterns of American adults.” Am J Clin Nutr. 1997;65(4suppl):1264S-8S. 35 Bingham SA. “The use of 24-h urine samples and energy expenditure to validate dietary assessments.” Am J Clin Nutr. 1994;59(1suppl):227S-31S. 36 United Fresh Fruit and Vegetable Association. Fruit and Vegetable Consumption: Consumer Attitudes vs. Behavior, 1995, as cited in Kantor LS, A Dietary Assessment of the U.S. Food Supply: Comparing Per Capita Food Consumption with Food Guide Pyramid Serving Recommendations. Washington, D.C.: USDA, Economic Research Service, Agricultural Economic Report No. 772, Dec. 1998, at 5. 37 USDA, America’s Eating Habits, p. 153. 38 Kantor LS, Lipton K, Manchester A, et al. Economic Research Service, USDA. “Estimat- ing and addressing America’s food losses.” FoodReview Jan-Apr., 1997;20(1):2-12. 39 Ibid. at 7, Table 1. 40 USDA, America’s Eating Habits, at 91. 41 The 1982 comment was made in the FDA’s proposals to affirm that sucrose, corn sugar, corn syrup, and invert sugar are generally recognized as safe (“GRAS”). 42 43 58 Fed. Reg. at 2221. We do not argue here that the substantial increases in consumption since previous FDA safety reviews warrants revocation of GRAS status, reducing the added-sugars content of certain foods, or restricting the production of certain foods. However, at the very least, that increased consumption warrants greatly expanded educational programs, including disclosures on labels of the amount of added sugars in a serving and the percentage of a Daily Value. 44 45 USDA, America’s Eating Habits, at 87. Personal communication, Shanthy Bowman, USDA/ARS, based on CSFII 1994-96 1-day data, July 30, 1999. 46 The Advisory Committee was appointed because section 301 of the National Nutrition Monitoring and Related Research Act of 1990, P. L. 101-445, directs the Secretary of Health and Human Services and the Secretary of Agriculture to jointly issue at least every five years a reported entitled Dietary Guidelines for Americans, that contains nutritional and dietary information and guidelines for the general public that are based on the preponderance of scientific and medical 48 knowledge current at the time of publication. 47 Report of the Dietary Guidelines Advisory Committee on the Dietary Guidelines for Ameri- cans (1995) at 16. 48 Ibid. at 16. 49 Food, Nutrition and the Prevention of Cancer: A Global Perspective (Washington, D.C.: World Cancer Research Fund/American Institute for Cancer Research, 1997). 50 Gillman MW. “Protective effect of fruits and vegetables on development of stroke in men.” JAMA. 1995;273:1113-7. 51 Appel LJ. “A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group.” N Engl J Med. 1997;336:1117-24 (1997). 52 Baron JA. “Calcium supplements for the prevention of colorectal adenomas. Calcium Polyp Prevention Study Group.” N Engl J Med. 1999;340:101-7. 53 Glinsmann et al. at S15. 54 55 Ibid. at S112. Life Sciences Research Office, Federation of American Societies for Experimental Biology. Evaluation of the Health Aspects of Sucrose As A Food Ingredient. 1976 at 13. 56 Testimony by Rachel Johnson, Dietary Guidelines Advisory Committee, meeting, March 9, 1999, at 354. <http://www.usda.gov/cnpp/DG2000/March9.htm> [accessed June 24, 1999]. Personal communication, Shanthy Bowman, Agricultural Research Service, USDA, March 26, 1999. 57 Earlier studies also found that higher intakes of sugars are associated with a lower intake of vitamins, minerals, and other nutrients. Lewis CJ, et al. “Nutrient intakes and body weights of persons consuming high and moderate levels of added sugars.” J Am Diet Asso. 1992;92:708-13; Gibney M, et al. “Consumption of sugars.” Am J Clin Nutr. 1995;62(1 Suppl):178S-94S. Those studies minimized the impact of added sugars because the authors failed to separate the naturally occurring sugars in fruit from the added sugars in soft drinks, pastries, etc. For instance, had the studies excluded fruit sugar, people consuming high levels of added sugars probably would have been more likely to get less than the RDA for vitamin C. Furthermore, Gibney et al. argue that the most nutritious diets are those with intermediate 49 levels of sugar, because the people who eat the least sugar also have lower nutrient intakes. In fact, that observation does not exonerate added-sugars’ impact on nutrient density. It simply raises questions about whether the group with the low-sugar intakes was consuming large quantities of fat, and/or reporting inaccurate food intakes. 58 Johnson testimony, at 364. 59 Krebs-Smith, et al. 60 Dietary Guidelines Advisory Committee meeting, March 9, 1999, at 372. <http:// www.usda.gov/cnpp/DG2000/March9.htm> [accessed June 24, 1999]. 61 Ibid. at 375. Standing Committee on the Scientific Evaluation of Dietary Reference Intakes. Food and Nutrition Board. Institute of Medicine. Dietary Reference Intakes for Calcium, Phosphorus, Magnesium, Vitamin D, and Fluoride (Washington, D.C.: National Academy Press, 1997). 63 Appel. 64 Baron. 65 Rimm EB, et al. “Vegetable, fruit, and cereal fiber intake and risk of coronary heart disease among men.” JAMA. 1996;275:447-51; Pietinen P, et al. “Intake of dietary fiber and risk of coronary heart disease in a cohort of Finnish men. The Alpha-Tocopherol, BetaCarotene Cancer Prevention Study.” Circulation. 1996;94:2720-7; Wolk A, et al. “Longterm intake of dietary fiber and decreased risk of coronary heart disease among women.” JAMA. 1999;281:1998-2004. 66 Salmeron J, et al. “Dietary fiber, glycemic load, and risk of non-insulin-dependent diabetes mellitus in women.” JAMA. 1997;277:472-7; Salmeron J, et al. “Dietary fiber, glycemic load, and risk of NIDDM in men.” Diabetes Care. 1997;20:545-50. 67 Kushi L. “Dietary antioxidant vitamins and death from coronary heart disease in postmenopausal women.” N Engl J Med. 1996;334:1156-62. 68 Giovannucci E, et al. “Multivitamin use, folate, and colon cancer in women in the nurses’ health study.” Annals of Internal Medicine. 1998;129:517-24. 69 Rimm EB, et al. “Folate and vitamin B6 from diet and supplements in relation to risk of coronary heart disease among women.” JAMA. 1998;279:359-64. 70 Boushey CJ, et al. “A quantitative assessment of plasma homocysteine as a risk factor for vascular disease. Probable benefits of increasing folic acid intakes.” JAMA. 1995;274:1049-57. 71 Centers for Disease Control. “Recommendations for the use of folic acid to reduce the number of cases of spina bifida and other neural tube defects.” MMWR. 1992;41(No. RR-14):1-7. 62 72 Rimm 1998. 73 Harnack L, et al. “Soft drink consumption among U.S. children and adolescents: nutritional consequences.” J Am Diet Assoc. 1999;99:436-41. 74 Skinner JD, et al. “Fruit juice intake is not related to children’s growth.” Pediatrics. 50 1999;103:58-64. 75 Guenther PM. “Beverages in the diets of American teenagers.” J Am Diet Assoc. 1986;86:493-9. 76 Goulding A, Cannan R, Williams SM, et al. “Bone mineral density in girls with forearm fractures.” J Bone Miner Res. 1998;13:143-8. 77 Wyshak G, Frisch RE, Albright TE, et al. “Nonalcoholic carbonated beverage consumption and bone fractures among women former college athletes.” J Orthopedic Res. 1989;7:91-9. 78 Johnson testimony at 364. 79 World Cancer Research Fund. 80 Rimm 1996. 81 Law MR, Morris JK. “By how much does fruit and vegetable consumption reduce the risk of ischaemic heart disease?” Eur J Clin Nutr. 1998;52:549-56. 82 Gillman. 83 Appel. 84 Salmeron, JAMA, Diabetes Care. 85 Standing Committee on the Scientific Evaluation of Dietary Reference Intakes. 86 Muñoz KA, Krebs-Smith SM, Ballard-Barbash R, et al. “Food intakes of US children and adolescents compared with recommendations.” Pediatrics. 1997;100:323-9; 1998;101:952-3. 87 World Cancer Research Fund at 225, 383. 88 Ibid. at 540. 89 Glinsmann et al. at S13. 90 Ibid. at S90. 91 Reaven GM. “Role of insulin resistance in human disease.” Diabetes. 1988;37:1595-607. Grundy SM. “Hypertriglyceridemia, insulin resistance, and the metabolic syndrome.” Am J Cardiology. 1999;83:25F-9F. 92 Glinsmann et al. at S13. 93 Ibid. at S10. 94 Hallfrisch J, et al. “Blood lipid distribution of hyperinsulinemic men consuming three levels 51 of fructose.” Am J Clin Nutr. 1983;37:740-8. 95 Reiser S, et al. “Blood lipids, lipoprotein, apoproteins, and uric acid in men fed diets con- taining fructose or high-amylose cornstarch.” Am J Clin Nutr. 1989;49:832-9. 96 Liu G, et al. “The Effect of Sucrose Content in High and Low Carbohydrate Diets on Plasma Glucose, Insulin, and Lipid Responses in Hypertriglyceridemic Humans.” Journal of Clinical Endocrinology and Metabolism 1984;59:636-42. 97 Glinsmann et al. at S89. Hollenbeck CB. “Dietary fructose effects on lipoprotein metabolism and risk for coronary artery disease.” Am J Clin Nutr. 1993;58:800S-9S. 99 Hollenbeck. 100 Daly ME, et al. “Dietary carbohydrates and insulin sensitivity: a review of 98 the evidence and clinical implications.” Am J Clin Nutr. 1997;66:1072-85. 101 Liu G, et al. 102 Glinsmann et al. p. S10. 103 Personal communication, Shanthy Bowman, USDA/ARS, July 29, 1999. 104 Researchers have not established a threshold level for sugars’ effects on triglycerides. Considering how small the cited clinical studies are, 7.5 percent fructose is unlikely to be the lowest level that affects blood lipid levels. 105 Gardner CD, et al. “Response of cardiovascular disease risk factors to plant food-based versus convenience food-based approaches for meeting NCEP step one dietary guidelines: pilot study.” Canadian Journal of Cardiology. 1997;13:236B. 106 Reaven GM. 107 Bonora E, et al. “Prevalence of insulin resistance in metabolic disorders: the Bruneck Study.” Diabetes. 1998;47:1643-9. 108 National Cholesterol Education Program, National Heart, Lung and Blood Institute. <http://www.nhlbi.nih.gov/hnlbi/cardio/chol/gp/fabc/fabc.htm> [accessed: July 12, 1999] 109 National Cholesterol Education Program, National Heart, Lung and Blood Institute. <http://www.nhlbi.nih.gov/hnlbi/cardio/chol/prof/atp2/atp_sum.htm> [accessed: July 12, 1999] 110 National Cholesterol Education Program, National Heart, Lung and Blood Institute. <http://www.nhlbi.nih.gov/nhlbi/cardio/chol/prof/atp2/atp.txt> [accessed: July 12, 1999] 111 Stampfer MJ, Krauss RM, et al. “A prospective study of triglyceride level, low-density 52 lipoprotein particle diameter, and risk of myocardial infarction.” JAMA. 1996;276:882-8. 112 Jeppesen J, Hein HO, Suadicani P, et al. “Triglyceride concentration and ischemic heart disease: an eight-year follow-up in the Copenhagen male study.” Circulation. 1998;97:1029-36. 113 Gotto AM. “Triglyceride: the forgotten risk factor.” Circulation. 1998;97:1027-8. 114 Saltus R. “New clue in heart disease risk seen. Triglyceride level called key factor.” Bos- ton Globe, March 24, 1998, A5. 115 Miller M, Seidler A, Moalemi A, et al. “Normal triglyceride levels and coronary artery disease events: the Baltimore coronary observational long-term study.” J Am Coll Cardiol. 1998;31:1252-7. 116 Miller M. “Is hypertriglyceridaemia an independent risk factor for coronary heart disease? The epidemiologic evidence.” Eur Heart J. 1998;19(Suppl H):18-22. 117 Report of the Dietary Guidelines Advisory Committee on the Dietary Guidelines for Ameri- cans (1995) at 9, 13. 118 “Update: Prevalence of overweight among children, adolescents, and adults—United States, 1988-1994.” MMWR. 1997;46:198-202. 119 Ibid. 120 Flegal KM, et al. “Overweight and obesity in the United States: prevalence and trends, 1960-1994.” International Journal of Obesity and Related Metabolic Disorders 1998;22:39-47. See also http://www.nhlbi.nih.gov/nhlbi/cardio/obes/prof/guidelns/ob_home.htm, accessed July 31, 1999. 121 National Center for Health Statistics. Healthy People 2000 Review 1998-99. Hyattsville, MD. DHHS Publication No. (PHS) 99-1256, page 41; National Research Council. Diet and Health: Implications for Reducing Chronic Disease Risk. (Washington, D.C.: National Academy Press, 1989), 116. 122 Report of the Dietary Guidelines Advisory Committee on the Dietary Guidelines for Ameri- cans (1995) at 9. 123 Roberts SB, et al. “Physiology of fat replacement and fat reduction: effects of dietary fat and fat substitutes on energy regulation.” Nutrition Reviews. 1998;56:S29-41. 53 124 Ibid. quoting: Bell EA, et al. “Energy density of foods affects energy intake in normal weight women.” Am J Clin Nutr. 1998;67:412-20. 125 Based on the cake’s calcium content, we estimate that two grams of the sugar in this product come from the whole milk it contains. 126 Testimony by Xavier Pi-Sunyer, Dietary Guidelines Advisory Committee meeting, June 17, 1999, at 11. 127 Drewnowski A, et al. “Taste responses and food preferences in obese women: effects of weight cycling.” International Journal of Obesity and Related Metabolic Disorders. 1992;16:63948. 128 Macdiarmid JI, Vail A, Cade JE, Blundell JE. “The sugar-fat relationship revisited: differ- ences in consumption between men and women of varying BMI.” International Journal of Obesity and Related Metabolic Disorders. 1998; 22:1053-61. 129 Mattes RD. “Dietary compensation by humans for supplemental energy provided as ethanol or carbohydrate in fluids.” Physiology and Behavior. 1996;59:179-187; DiMeglio D, Mattes RD. “Liquid versus solid carbohydrate (CHO): effects on food intake and body weight.” FASEB Journal. 1999;13:A870. 130 Personal communication, Richard P. Troiano. Am J Clin Nutr (forthcoming). 131 Harnack et al. 132 Morton JF, Guthrie JF. “Changes in children’s total fat intakes and their food group sources of fat, 1989-91 versus 1994-95: implications for diet quality.” Family Economics and Nutrition Review. 1998;11(3):44-57. 133 Personal communication, Shanthy Bowman, July 30, 1999. 134 Woteki, at 18. 135 Cleveland, at Table 6. 136 National Heart, Lung and Blood Institute and Office of Research on Minority Health. “Em- brace your Health! Lose Weight if You Are Overweight.” NIH Publication No. 97-4061, Sept. 1997. 137 Drewnowski A, et al. “The fat-sucrose seesaw in relation to age and dietary variety of 54 French adults.” Obesity Research. 1997;5:511-8. 138 Krebs-Smith, et al. 139 Emmett PM, et al. “Is extrinsic sugar a vehicle for dietary fat?” Lancet. 1995;345:1537-40. 140 58 Fed. Reg. at 2221. U.S. Department of Health and Human Services, Public Health Service. The Surgeon General’s Report on Nutrition and Health (Washington, D.C.: Government Printing Office, 1988), 368. 142 National Research Council, Committee on Diet and Health. Diet and Health: implications for reducing chronic disease risk (Washington, D.C.: National Academy 141 Press, 1989), 15. 143 The Dental Health Foundation. “A Neglected Epidemic: The Oral Health of California’s Children.” (San Rafael, Calif., 1997). 144 Vargas CM, Crall JJ, Schneider DA. “Sociodemographic distribution of pediatric dental caries: NHANES III, 1988-1994.” J Am Dent Assoc. 1998;129:1229-38. 145 Ismail AI, Burt BA, Eklund SA. “The cariogenicity of soft drinks in the United States.” J Am Dent Assoc. 1984;109:241-5. 146 147 58 Fed. Reg. at 2222. Low NH. “Determination of fruit juice authenticity by capillary gas chromatography with flame ionization detection.” JAOAC Int. 1996;79:724-37. 148 Low NH, Brause A, Wilhelmsen E. “Normative data for commercial pineapple juice from concentrate.” JAOAC Int. 1994;77:965-75. 149 Doner LW. “Stable carbon isotope ratios for detecting added sugars in orange and apple juices and added citric acid in lemon juices,” in Linskens HF and Jackson JF. Modern Methods of Plant Analysis (Berlin Heidelberg: Springer-Verlag, 1988), New Series, Vol. 8, 120-33. 150 Carbon-isotope analysis of individual sugars (sucrose, glucose, fructose) can be used to detect as low as 3 percent added C4 sugar in orange juice. That sensitivity is made possible by the use of intermolecular isotope correlations between different components in the fruit (individual sugars and/or acids) to improve the sensitivity of the method. That approach is particularly useful for pineapple. Personal communication, Michele Lees, Eurofins Scientific S.A., Dec. 23, 1998. 151 Guillou C, Remaud G, Martin GJ. “Application of deuterium NMR and isotopic analysis to 55 the characterization of foods and beverages.” Trends in Food Science & Technology. (April 1991) at 85-9. 152 The General Accounting Office study was done because of Congressional concern about the costs and problems associated with the sale of adulterated fruit juice in school meal programs. General Accounting Office. Fruit Juice Adulteration (November 1995) GAO/RCED-96-18 at 17. 153 Lees M, “Jus de fruit - pur,” Analysis Europa. 1996(March-April 1996);20-6. 154 21 C.F.R. § 101.4(a). 155 21 C.F.R. § 101.60(c)(2). 156 21 C.F.R. §101.30. 157 21 C.F.R. §101.30(b)(3). 158 Section 401 of the Federal Food, Drug, and Cosmetic Act (“FFDCA”), 21 U.S.C. § 341, authorizes the Secretary to promulgate regulations fixing and establishing for any food a “standard of identity.” The FDA has established food standards for milk and cream; cheeses and related cheese products; frozen desserts; bakery products; cereal flours and related products; macaroni and noodle products; canned fruits; canned fruit juices; fruit butters, jellies, preserves, and related products; fruit pies; canned vegetables; vegetable juices; frozen vegetables; eggs and egg products; fish and shellfish; cacao products; tree nut and peanut products; beverages; margarine; sweeteners and table syrups; and food dressings and flavorings. 159 If a sweetener is added and the soluble solids content of the finished food is not less than 16.5 percent, the applesauce may be called “sweetened” applesauce. 21 C.F.R. § 145.110(a)(3). 160 21 C.F.R. § 146.140(e)(2). 161 21 C.F.R. §150.140(d)(1). 162 21 C.F.R. §150.160. 163 CSPI interview on November 18, 1998, with Felicia Satchell, Chief of Food Standards Branch, Office of Labeling, Center for Food Safety and Applied Nutrition, FDA. Gross discrepancies between company labels and USDA’s data base for about 6,000 foods might suggest products that the FDA should examine more closely. 56 164 58 Fed. Reg. at 2098. 165 The FDA presented no evidence to support its conclusion. The FDA’s failure to present any evidence in support of this conclusion renders it “arbitrary and capricious” within the meaning of the Administrative Procedure Act, 5 U.S.C. § 706(2)(A). Cf. Menorah Medical Center v. Heckler, 768 F.2d 292 (CA 8 1985)(regulation for reimbursing Medicare health providers for the portion of their malpractice-insurance premiums attributable to Medicare patients is invalid because there was no evidence in the record to support the Secretary’s conclusion that lower malpractice awards for Medicare patients leads to lower malpractice-insurance premiums). 166 Dietary Guidelines Advisory Committee meeting, March 9, 1999, at 372. 167 The survey was conducted by Bruskin/Goldring Research on July 9-11, 1999. 168 Juice drinks, beverages, cocktails, etc. disclose juice content, enabling nutritionists to esti- mate the amount of added sugars. Other foods, including those made with juice, do not provide information about added and naturally occurring sugars. 169 58 Fed. Reg. at 2098. 170 Ibid. 171 58 Fed. Reg. at 2221-2. 172 Dietary Goals for the United States, at 27-34. 173 Cannon G. Food and Health: The Experts Agree. (London: Consumers Association, 1992). 174 “Are you eating right.” Consumer Reports. October, 1992; 644-55. 175 58 Fed. Reg. at 2098. 176 21 C.F.R. § 101.14(e). 177 21 C.F.R. §§ 101.62(b)(2), 101.62(c)(2), 101.62(d)(2), and 101.61(b)(4). In early 1993 the FDA refused to define “low” in connection with sugar because there was no “consensus” on a quantitative recommendation for the daily intake of sugars. Thus, the FDA did not issue a DRV for sugar and therefore, did not define “low” for sugar. 58 Fed Reg. at 2335. 178 58 Fed. Reg. at 2334. 179 21 C.F.R. §§ 101.62(b)(2)(i)(A), 101.62(c)(2)(i), 101.62(d)(2)(i)(A), and 101.61(b)(4)(i). 57 180 21 C.F.R. § 101.60(c)(5). Another portion of this regulation already indicates when the terms “no added sugar,” “without added sugar,” or “no sugar added” may be used. 21 C.F.R. § 101.60(c)(2). 181 21 C.F.R. § 101.13(h)(1). 21 C.F.R. § 101.13(h)(2) deals with disclosure for a “meal prod- uct,” and 21 C.F.R. §101.13(h)(3) deals with disclosure for a “main dish product.” The former uses 40 percent of the DRV of fat, saturated fat, cholesterol, or sodium to trigger the disclosure statement, and the latter uses 30 percent of the DRV to trigger disclosure. We request the same trigger for added sugars in these two provisions, i.e., 16 grams and 12 grams. 182 The current version comes from section 305 of the Food and Drug Administration Modern- ization Act of 1997, P.L. 105-115, which amended section 403(r)(2)(B) of the Federal Food, Drug, and Cosmetic Act, 21 U.S.C. § 343(r)(2)(B). The earlier version said that the disclosure should state “See [appropriate panel] for information about [nutrient requiring disclosure] and other nutrients.” The FDA explained in 1998 that the 1997 statutory change simply referred to how the disclosure is to be made and not the conditions triggering it. 63 Fed. Reg. 26978 (May 15, 1998). 183 58 Fed. Reg. at 2307. 184 101 C.F.R. §101.65(d). 185 The FDA should determine whether additional conforming changes to related labeling regulations need to be made to regulate claims regarding sugar and added sugar in a manner consistent with the agency’s regulations for fat, saturated fat, cholesterol, and sodium claims. 186 21 U.S.C. § 343(q)(2). 187 H.R. Rep. 101-538, 101st Cong. 2d Sess. (1990) at 14. 188 Permian Basin Area Rate Cases, 390 U.S. 747, 784 (1968). 189 American Trucking Assns., Inc. v. Atchison, T. & S.F.R.. Co., 387 U.S. 397, 416 (1967). 190 21 U.S.C. § 343 note. 191 H. R. Rep. 101-538, 101st Cong. 2d Sess. (1990) at 18. 192 58 Fed. Reg. at 2207. 193 21 U.S.C. §§ 343(r)(1). 194 Section 403(r)(2)(B) of the FFDCA, 21 U.S.C. § 343(r)(2)(B), also deals with nutrient58 content claims and provides that if “the Secretary makes a determination that the food contains a nutrient at a level that increases to persons in the general population the risk of a disease or healthrelated condition that is diet-related, the label or labeling of such food shall contain, prominently and in immediate proximity to such claim, the following statement: ‘See nutrition information for _____ content.’” 195 21 U.S.C. § 343(r)(1)(A). 196 21 C.F.R. §§ 101.13 and 101.60. 197 21 U.S.C. § 343(r)(1)(B). 198 The health claim regulations are contained in 21 C.F.R. § 101.14. 199 21 U.S.C. § 343(r)(3)(A)(ii). 200 The House Committee report explains that “By requiring the Secretary to decide this issue in the context of the total daily diet, the bill permits the Secretary to differentiate between different foods which have the same level of a nutrient. For example, a particular level of fat in a frozen dinner might not trigger the provision, whereas the same amount of fat in a snack food product might trigger it.” H.R. Rep. 101-538, 101st Cong. 2d Sess. (1990) at 21. 201 21 U.S.C. §§ 321(n), 343(a), and 371(a). 202 59 Fed. Reg. 24249 (May 10, 1994). The “healthy” regulations are now in 21 C.F.R. § 101.65(d). 203 21 C.F.R. §§ 101.13, 101.14, 101.60, and 101.65(d). 204 21 C.F.R. §10.30(b). 59