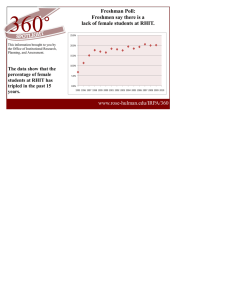
Document 11681654
advertisement

IACUC USE ONLY ACUP #: __________-__________-__________ FINAL APPROVAL Certification of review and approval by the Institute Animal Care and Use Committee: Submission Date: _________________ Name: Approval Date: ___________________________________________ IACUC Chair _________________ Expiration Date: _________________ Signature: ___________________________________________ Date: Initial Submission Renewal Modification ___________________________________________ PLEASE TYPE I. GENERAL INFORMATION Principal Investigator1: Jameel Ahmed Department: Mailing Address: Applied Biology and Biomedical Engineering CM186 / 5500 Wabash Avenue / Terre Haute, IN 47803 Telephone: (812) 872-6033 Fax: Emergency Contact Name and Number2: Grant/Project Title3: (812)877-8025 Email: ahmed@rose-hulman.edu Jameel Ahmed (812)877-4831 (Home) Neural-Vascular Interactions in the Retina II. SPONSORSHIP OF PROJECT Funded 1 Pending New Proposal Not Externally Funded A. Funding Agency / Source of Funds: National Eye Institute, National Institutes of Health B. Dates of Project: From: 12/01/03 To: 11/30/06 All correspondence from the IACUC will be sent to the PI. Emergency Contact is the individual who will be contacted regarding an animal’s health or disposition when morbidity requires action. 3 NIH and RHIT require that the grant and protocol titles match. The information in this protocol must agree with your grant pages. 2 Rev 2-2003 RHIT ACUP Form - 2/16 - III PERSONNEL Provide the following information for ALL INDIVIDUALS to be involved with study. This includes the Principal Investigator (PI), co-investigators, technicians, and specific students involved directly with the animal care and/or study procedures. All personnel listed must have completed the Animal Research Training Program (ARTP) by project start date. Please attach curriculum vita (CV) for PI and Co-PI(s). Name Position Jameel Ahmed Rita Strack (student) (Graduate student)** Years of Experience With the: Species Techniques PI Animal Care surgical assistant 2 4 7 n/a Date Completed ARTP 2/2002* 2/2002* *Rose-Hulman animal training course completed. The ARTP was not in place at time of training. ** Graduate student unidentified as of yet. This person’s role will be to monitor life support instrumentation during surgery. IV. RATIONALE FOR ANIMAL USE, RESEARCH ALTERNATIVES, AND REFERENCES CITED [A] Specify animals to be used for the coming year. Enter the total number of animals to be used in each Pain Classification Column. General Information Species Common Name Genus Rattus norvegicus Type A (USDA C) Pain or distress will not be induced; animals will be used only for collections, post-mortem dissections, injections or similar non-stressful procedures that only cause minor discomfort. Long-Evans rats Size Age Sex 300 g adult eithe r Pain Classification Categories Type B (USDA D) Pain or distress could be induced or there is a potential for the procedure to be painful, but will be relieved by appropriate drugs. Pain Classification Type Ty Type B C pe A 60 (over 3 years) Type C (USDA E) Pain or distress will be induced and will not be relieved; this category includes experiments where drug administration would interfere with the results. [B] Explain your rationale for animal use. [The rationale should include reasons why non-animal models cannot be used.] The fundamental focus of this study is to study functional interactions between the neurons of the retina Rev 2-2003 RHIT ACUP Form - 3/16 - and the vasculature that supplies these neurons with nutrients. These interactions are quite complex, involving several cell types and several chemical messengers, some of which may remain unidentified. This type of complex interrelationship cannot be duplicated in non-animal models. [C] Justify the appropriateness of the species selected. [The species selected should be the lowest possible on the phylogenetic scale.] Rats have a vascular retina that is similar in many respects to the human retina. Mouse eyes would be significantly smaller, multiplying the technical difficulties in making blood flow recordings. Results obtained from non-mammalian species would not be readily applicable to human physiology, decreasing the clinical relevance of the research. [D] Justify the number of animals to be used. [The number of animals should be the minimum number required to obtain statistically valid results.] It is difficult to determine the exact number of animals required for this study, due to the fact that the measurement techniques have yet to be well characterized (characterization of the techniques is part of the proposal). 20 animals are requested for the first year, and this seems a reasonable number to characterize the measurement techniques. Currently 20 animals each are requested for the last 2 years of the proposal. More animals may be requested in later years if there appears to be a greater variability associated with the measurement than is expected. [E] Consideration of Alternatives for Pain Classifications B and C If any procedures fall into Classifications B or C, causing more than momentary or slight pain or distress to the animals, describe your consideration of alternatives, including methods that (1) refine existing tests by minimizing animal distress, (2) reduce the number of animals necessary for the pain category, and your determination that alternatives are not available. Please also delineate the methods and sources used in your search for alternatives. A search for alternatives was done on the ALTWEB database. Search words included rats and retina and blood flow and (refine or reduce or replace). No alternatives were identified using this search. [F] Describe Pain Management 1. Describe Pain Management Procedure [Classification B] All experiments proposed here will be terminal, acute experiments. Animals will be anesthetized at the beginning of the experiment and maintenance doses will be given continually over the course of the experiment. Animals are not expected to awaken after initial anesthesia. Anesthesia will be induced by one of two methods. The first is subcutaneous injection of urethane anesthetic (1.6 g/kg), which is a slow, but extremely long-lasting anesthetic. Animals anesthetized with this technique will take roughly 1-2 hours to reach surgical levels of anesthesia. An alternative form of induction anesthetic will be 100 mg/kg of a 50-50% mixture of ketamine and xylazine injected intraperitoneally, followed by infusion of urethane anesthetic (200 mg/kg loading dose, 100-200 mg/hr maintenance dose). This will result in much faster induction of anesthesia. Regardless of the initial anesthetic used, the depth of anesthesia will be monitored using cardiovascular parameters as well as by using the pinch test on a forelimb. As the initial anesthetic wears off, sodium pentobarbital (5%, given in 0.05 ml doses) will be injected to maintain surgical depth of anesthesia. During surgery, the animal will be placed on a heating blanket and body Rev 2-2003 RHIT ACUP Form - 4/16 - temperature will be kept at normal levels. Also during surgery, the ECG and oxygen saturation will be monitored continually. 2. Provide justification if no Pain Management Procedure(s) is used [Classification C] N/A [G] References Cited 1. Describe the literature review procedure used for this project. A pubmed review was conducted and the results are included as part of the attached research plan portion of the grant proposal. 2. List 2 or 3 literature references most directly related to the project. Ahmed J., Pulfer, M.K. & Linsenmeier, R.A. (2001) Measurements of blood flow through the retinal circulation of the cat during normoxia and hypoxemia using fluorescent microspheres. Microvasc. Res. 62(2) 143-53. Falsini B, Riva CE, & Logean E. (2002) Flicker-evoked changes in human optic nerve blood flow: relationship with retinal neural activity. Invest Ophthalmol Vis Sci. Jul;43(7):2309-16. Khoobehi B, Shoelson B, Zhang YZ, & Peyman GA.(1997) Fluorescent microsphere imaging: a particle-tracking approach to the hemodynamic assessment of the retina and choroid. Ophthalmic Surg Lasers Nov;28(11):937-47. Saszik, S.M., Wu, J., Robson, J.G. & Frishman, L.J. (2001) The scotopic threshold response of the dark-adapted electroretinogram (ERG) of the mouse. Investigative Ophthalmol. Vis. Sci. Supplement 42(4):S180 [H] Housing 1. Describe the primary housing for the animals. Include Building and Room #. Animals will be housed in the vivarium in O109B. Animals will be housed in pairs in 33x15x13 cm (L x W x H) cages. 2. Location of experiments. M112 (Myers’ Hall) [I] Transportation Rev 2-2003 RHIT ACUP Form - 5/16 - If animals will be transported, describe the methods, the containment, the route and elevator(s) to be utilized. Animals will be transported in a small cage with a wire lid which will in turn be placed in an unsealed cardboard box for the trip to Myers’ Hall. Animals will be transported via the 1st floor hallways in Olin, Hadley and Moench Halls. On dry days when the temperature is greater than 65.2º F, Moench Hall will be exited via the southeast exit on the ground floor, through the parking lot and into Myers’ Hall. Otherwise, the stairs will be taken to the ground floor of Moench Hall and the animal will be transported into Myers Hall via the northeast exit of Moench Hall. V. DESCRIPTION OF EXPERIMENTAL DESIGN AND ANIMAL PROCEDURES Briefly explain the experimental design and specify all animal procedures. This description should allow the IACUC to understand the experimental course of an animal from its entry into the experiment to the endpoint of the study. Specifically address the following items for the Experimental Group(s) and Control Group(s), as applicable: • • • • • • • • • • • • Injections or inoculations (substances, e.g., infectious agents, adjuvants, etc.; dose, sites, volume, route, and schedules). Blood withdrawals (volume, frequency, withdrawal sites, and methodology). Surgical procedures (provide details of survival and non-survival surgical procedures in Section VI.). Experimental timeline (include timeframe and duration of each relevant activity of the project) Radiation (dosage and schedule). Methods of restraint (e.g., restraint chairs, collars, vests, harnesses, slings, etc.). Include how animals are restrained for routine procedures like blood withdrawals. Prolonged restraint must be justified with appropriate oversight to ensure it is minimally distressing. Describe any sedation, acclimation or training to be utilized. Animal identification methods (e.g., ear tags, tattoos, collar, cage card, implant, etc.). Other procedures (e.g., survival studies, tail biopsies, etc.). Resultant effects, if any, that the animals are expected to experience (e.g., pain or distress, ascites production, etc.). Other potential stressors (e.g., food or water deprivation, noxious stimuli, environmental stress) and procedures to monitor and minimize distress. If a study is Classification B, indicate any nonpharmaceutical methods to minimize pain and distress. Experimental endpoint criteria (e.g., tumor size, percentage body weight gain or loss, inability to eat or drink, behavioral abnormalities, clinical symptomatology, or signs of toxicity) must be specified when the administration of tumor cells, biologics, infectious agents, radiation or toxic chemicals are expected to cause significant symptomatology or are potentially lethal. List the criteria to be used to determine when euthanasia is to be performed. Death as an endpoint must always be scientifically justified. Veterinary care (indicate desired plan of action in case of animal illness, e.g., initiate treatment, call investigator prior to initiating treatment, euthanize). [A] Experimental Group(s) Rev 2-2003 RHIT ACUP Form - 6/16 - Surgical Procedures: In all animals, cannulae will be inserted into the trachea, and into the femoral artery and vein. The chest will also be opened to facilitate intraventricular (into the heart) injection of microspheres. In some experiments, surgery will be done to expose the side of the eye to facilitate attachment to an eyering, which is used to immobilize the eye for injection of pharmacological agents into the vitreous. Other Procedures: Particle tracking method for measuring retinal blood flow The technique proposed here is a modification of the technique presented by Khoobehi et al. (1997). Their technique will be modified for use with rats (the Khoobehi study examined rhesus monkeys), along with using a microscope with attached video camera in place of a scanning laser ophthalmoscope (not available at RHIT). A schematic drawing of the apparatus for making recordings for the particle tracking approach is shown in Figure 1. For these experiments an anesthetized animal will be placed on its side and its head will be immobilized in a modified head holder via earbars. A custom-made plano-concave (flat on one side, curved on the other) lens will be placed over the eye with a thick solution of methylcellulose (artificial tears) to provide a tear film. The eye position can be slightly adjusted using two sutures placed through the superficial layers of the sclera, or by moving the eyering if the eye is attached to one. Once in place, a stereo microscope with attached video camera will be centered over the eye, and settings will be adjusted to give a clear image of retinal vessels. A VCR will be used to record control and experimental images over time for later digitization and analysis. CCD Camera Monitor VCR Stereo Microscope Emitted light Fluorescence Emission Filter Light Source Computer with imageacquisition card Plano-concave lens Rat eye Figure 1: Recording system for making particle tracking measurements Once the imaging system has been aligned, fluorescently-labeled polystyrene microspheres, 2 µm in diameter Rev 2-2003 RHIT ACUP Form - 7/16 - will be injected into a vein. Excitation of the fluorescent dyes will be done using a home-made LED-based light source. LEDs with wavelengths appropriate to excite the fluorescent dyes used to label the microspheres will be used. Intensity of the excitation light will be altered using optical filters and be adjusted to be the minimum required for adequate imaging of particles. This excitation light will be focused onto the retina using a parabolic mirror. A hole in the center of the mirror will allow reflected light to be sent into the microscope. An emission filter will remove the excitation wavelength from the reflected beam, allowing visualization of the fluorescent excitation wavelengths by the optical system. Light from the light source will be transmitted to the mirror via a fiber optic light pipe. Fluorescent microsphere method for measuring blood flow One of the techniques that will be used to make blood flow measurements will utilize the fluorescent microsphere method. The PI has previously used this technique in retinal blood flow studies in the cat. In addition, radioactivity-labeled microspheres have been used extensively to make retinal blood flow measurements in the rat. In the microsphere method, a large number of labeled spheres (≈1 million), usually 15 µm in diameter, are injected into the left ventricle of the heart. There, they mix with the cardiac output and are distributed to the peripheral vascular beds. Once in the peripheral vasculature, they become trapped in the capillaries (they are too big to pass through these small vessels). By determining the percentage of microspheres that become embedded in the capillaries of an organ of interest, the percent of the cardiac output that is supplied to that organ can be determined. Using a reference blood sample that is withdrawn at a known rate, this percentage can be converted to an absolute flow rate (in ml/min). The microsphere method is the only method that has yielded reliable absolute measurements of retinal blood flow. In each experiment, depending on the health of the animal, up to 3 blood flow measurements will be made using spheres with a different fluorescent label for each measurement. After sacrifice of the animal, retinas will be removed and fixed in 4% formalin. Once trapped, the number of spheres can now be counted in a couple of ways. The easiest method involves removal of the tissue of interest, chemical digestion of the tissue, and release of the label into the digesting solution. The total amount of label in the solutions can be measured using a fluorescence meter. This total fluorescence in the tissue sample is compared with the total fluorescence in the reference sample to yield an absolute blood flow. A second approach to counting the number of spheres in the retina is to make retinal wholemounts and examine them using a fluorescence microscope with filter sets that are tuned to the different labels used. Retinal wholemounts are made by making cuts in the fixed eyecup, allowing it to lie flat on a microscope slide. A few drops of glycerol are placed in the retina and a coveslip is placed over the wholemount. Weights are placed over the retinal wholemount to help flatten it. Once flattened, the coverslip is sealed with nail polish or some other sealant. For counting of spheres, a computerized microscopic stage is used to make a grid scan of the retinal wholemount, taking non-overlapping images at each point in the grid. From these images, a composite picture of the whole retina with microspheres can be constructed. This is repeated using different filter sets matched to Rev 2-2003 RHIT ACUP Form - 8/16 - each of the fluorescent labels used on the microspheres. The actual number of spheres embedded in the retina can then be counted directly, either by hand or by using image processing algorithms. This second approach requires an alteration in the way that reference sample is analyzed. This is done by taking multiple sub-samples of known volume from the reference samples and examining each sub-sample in the same way as the retinal wholemounts. By looking at all of the sub-samples, an accurate estimate of the number of spheres in the reference sample can be made. Retinal stimulation Photic stimulation will be done using an LED stimulator that has been constructed in-house (Sundseth et al., 2002). Stimulation will be controlled using LABVIEW software. Flicker stimulation will be done while the retina is largely under rod-dominated conditions. Control blood flow measurements will be made while the retina is exposed to a mean level of illumination. Flicker stimuli will be modulated about this mean level at temporal frequencies above 4 Hz. Microsphere blood flow measurements during retinal stimulation will be made after 1 minute of stimulation. Electroretinogram recording Scotopic electroretinograms will be recorded bilaterally using either DTL fibers under contact lenses or by using chlorided silver wire loops on the corneas. In both cases, corneas will be dilated using 0.1% atropine. Methylcellulose will be used to maintain hydration of the corneas. Electroretinograms will be taken under fully dark-adapted conditions. Use of pharmacological agents to isolate the effect of spiking neurons Specific aim #4 relies on using tetrodotoxin (TTX) to remove the spiking component from the retinal response. TTX will be injected into the vitreous of the rat. The electroretinogram will be monitored for characteristic changes in the ERG that accompany blockage of spiking activity. The procedures developed for use by Frishman and Saszik ( Saszik et. al., 2001) for injecting agents into the vitreous of the mouse will be modified for use in the rat. Blood flow measurements will be made after all signs of spiking activity are removed from the ERG. Experimental Design: Experiments for specific aims #3 and #4 will utilize both techniques for retinal blood flow measurements that are developed in specific aims #1 and #2. The use of both techniques in the same experiment will allow the drawbacks of both techniques to be mitigated. The lack of reference standard and limited sampling area of the particle tracking method will be offset by the microsphere methods, while the variability inherent in the microsphere method will be slightly offset by the particle tracking method. Figure 2 shows the experimental time line for the experiments in specific aim #3. Microsphere injections with associated reference sample withdrawals s will be made during control conditions and during flicker stimulation of the retina as shown in figure 2. Particle tracking measurements will be made at these times and at other times Rev 2-2003 RHIT ACUP Form - 9/16 - during the experiment. Sphere1 Sphere2 eye 1 Sphere3 Flicker Flicker eye 2 Time Figure 2: Generalized experimental timeline for flicker experiments Figure 3 shows the generalized experimental timeline for the TTX experiments of specific aim #4. Microsphere injections will be made under normal conditions, during flicker stimulation of the retina and during flicker stimulation of the retina after injection of TTX. Particle tracking measurements will be made at different stages throughout the recording period. Sphere 1 Sphere 2 Sphere 3 eye eye Flicker TTX Flicker ERG monitoring Time Figure 3: Timeline for blood flow measurement during TTX experiments. Sample Experimental Timeline (actual starting time may differ): 7:00 AM: Subcutaneous injection of urethane anesthetic 8:45 AM: Injection of ketamine/xylazine (if urethane is not used) 9:00 AM: (Assuming sufficient depth of anesthesia) Surgery to insert tracheal, arterial and venous cannula; maintenance dose of urethane started; periodic injection of pentobarbital as needed. 10:00 AM: Insertion of earbars 10:15 AM: Chest surgery 10:45 AM: Eye surgery (if necessary) 11:15 AM: IV injection of pancuronium for paralysis; connection to ventilator 11:30 AM-3:30PM: Blood flow/ERG measurements (length will be variable, 4 hours would be a long experiment) Rev 2-2003 RHIT ACUP Form - 10/16 - 5:00: Euthanasia followed by enucleation of eyes. Methods of Restraint: Animals will be restrained by hand during injection of anesthetics. Animal Identification Methods: Animals will be identified using cage cards. Veterinary Care: Veterinary Care will be provided by Dr. Holscher, the institute veterinarian. Dr. Holscher will be called when any health problems arise. [B] Control Group(s) N/A VI. SURGERY If proposed, complete the following: [A] Identify and describe the surgical procedure(s) to be performed. Include preoperative procedures (e.g., fasting, analgesic loading), and monitoring and supportive care during surgery. Include the aseptic methods to be utilized. The anesthesia protocol for these experiments is given in section IV-F. After induction of anesthesia, cannulae will be placed in the femoral artery and vein of the rat. The arterial cannula will be connected to a pressure transducer to allow monitoring of systemic blood pressure. A tracheostomy will be performed and the animal will be intubated to facilitate later ventilation of the animal. Xylocaine gel anesthetic will be placed in the ear canals of the animal. Once prepared, the animal will be placed in a modified stereotaxic instrument, and ear bars will be inserted into the anesthetized ear canals for stabilization of the head. If vitreal injections of drugs are required, surgery will be done to expose the eye and if necessary the eye will be placed on an eyering using suture. If no vitreal injections are required the eye will be wetted with artificial tears and closed. During retinal stimulation, eyelids will be retracted using a speculum. Whether or not eye surgery is done, pupils will be dilated using 1% atropine and corneal hydration will be maintained using methylcellulose. After general surgery, including eye surgery if necessary, is complete, the animal will be paralyzed with pancuronium bromide (induction to effect, maintenance dose of 0.2 mg/kg-hr) and artificially ventilated. Once ventilated, the chest will be opened and the heart exposed to facilitate injections of microspheres. Rev 2-2003 RHIT ACUP Form - 11/16 - Intravitreal injections of TTX In experiments requiring intravitreal (into the chamber of eye) injection of TTX, the method developed by Saszik and Frishman (Saszik et al. 2001) will be used. A small hole in the eye just behind the limbus ( a line that runs along the midline of the eye) will be made using a 30G needle. 1.5 µl of solution containing the pharmacological agent will be injected into the eye through the hole using a glass pipette tip (~20 µm) on a microsyringe. Initial doses of agents will be chosen to produce a vitreal concentration similar to what is currently used in cat and monkey (Robson & Frishman, 1995; Sieving, et al., 1994). For rats, a vitreal volume of 39 µl will be assumed (Bush et al., 1995). To assess the efficacy of these agents, dark-adapted ERG responses will be monitored until the response stabilizes. If no change occurs in the ERG, the injection will be repeated. [B] Who will perform surgery and what are their qualifications and/or experience? Jameel Ahmed will perform the surgery. Dr. Ahmed has been involved in doing surgery on rats, cats and monkeys since 1991. His experience includes monitoring of the effects of barbiturate and urethane anesthetics. [C] Where will surgery be performed and postoperative care provided (building and rooms)? Surgery will be performed in M112. Since these are acute experiments, there will be no postoperative care. [D] If survival surgery, describe postoperative care required, frequency of observation, and identify the responsible individual(s). Include detection and management of postoperative complications during work hours, after hours, weekends and holidays. N/A [E] If non-survival surgery, describe how humane euthanasia is enacted and how death is determined. At the end of the experiments, euthanasia will be carried out using an overdose of sodium pentothal (5%). Death will be ensured by monitoring blood pressure and ECG activity and by direct observation of the heart. [F] Are paralytic agents used during surgery? Yes or No . If yes, please describe how ventilation will be maintained and how pain will be assessed. Rev 2-2003 RHIT ACUP Form - 12/16 - Ventilation will be maintained using a rodent ventilator connected to the tracheal cannula. After paralysis, monitoring of blood pressure and heart rate will be used as measures of pain. [G] Has major survival surgery been performed on any animal prior to being placed on this study? [Major survival surgery penetrates and exposes a body cavity or produces substantial impairment of physical or physiologic functions (such as laparotomy, thoracotomy, craniotomy, joint replacement, or limb amputation).] Yes or No . If yes, please explain: No [H] Will more than one major survival surgery be performed on an animal while on this study? or No . Yes If yes, please justify: N/A VII. ANESTHETICS, ANALGESICS, SEDATIVES, TRANQUILIZERS, OR OTHER PHARMACOLOGIC AGENTS List the anesthetics, analgesics, sedatives, tranquilizers, or other pharmacologic agents to be used. Include the name of the agent(s), the dosage, route and schedule of administration. If information is provided in Section IV above, please cross-reference. Describe tracking and security of controlled drugs (Drug Enforcement Agency requirements). Drugs used 1. Ketamine (anesthetic) 2. Xylazine (sedative) 3. Urethane (anesthetic) 4. Sodium Pentobarbital (anesthetic) 5. Xylocaine gel (topical anesthetic) 6. Pancuronium Bromide (paralytic) 7. Methylcellulose (corneal wetting agent) 8. Atropine (applied topically to dilate the pupil) 9. Tetrodotoxin (Na+ channel blocker) See section IV for drug dosage information. Dr. Ahmed has both federal and state controlled substance permits for ketamine and sodium pentothal. Drugs are stored in a drug safe in M112. All use of controlled substances are marked in a controlled substances log book that is kept in M112. VIII. METHOD OF EUTHANASIA OR DISPOSITION OF ANIMALS AT END OF STUDY Indicate the proposed method of euthanasia. If a chemical agent is used specify the dosage and route of administration. If the method(s) of euthanasia include those not recommended by the American Veterinarian Medical Association (AVMA) Panel Report on Euthanasia (e.g., decapitation or cervical Rev 2-2003 RHIT ACUP Form - 13/16 - dislocation without anesthesia), provide scientific justification why such methods must be used. Indicate the method of carcass or tissue disposal if not described in Section IX below. At the end of the experiments, euthanasia will be carried out using an overdose of sodium pentothal (5%). Overdose with barbiturate anesthetics has been approved by the AVMA (200 report) as the most desirable form of euthanasia in small animals. Death will be ensured by monitoring blood pressure and ECG activity and by direct observation of the heart. After euthanasia and procurement of the eyes, carcasses will be placed in a freezer in O109C until they are picked up with other biohazard waste. IX. HAZARDOUS MATERIALS and BIOLOGICAL AGENTS Are hazardous materials and/or biological agents (radioactive, hazardous chemicals, drugs, recombinant DNA, etc.) to be used in this project? Yes or No If yes, the use of hazardous materials and biological agents requires approval from the Office of Environmental Health and Safety. Attach documentation of approval for the use of these materials. Approval must be obtained before any hazardous materials or biological agents are purchased. Describe the practices and procedures required for the safe handling and disposal of animals and material associated with this study. Also describe methods for removal of all waste and, if applicable, the monitoring of exposure by the personnel involved with this protocol. Urethane anesthetic is a suspected carcinogen. Mixing of the anesthetic will be done by Jameel Ahmed wearing an organic vapor mask and gloves. Once in solution, syringes will be filled while wearing gloves. Hands will be washed. The Office of Environmental Health and Safety will provide the organic vapor mask. Additional safety considerations: N/A X. GENETIC MANIPULATIONS OF ANIMALS (Transgenic and Knockout) Describe any phenotypic consequences of the genetic manipulations to the animals. Describe any special care or monitoring that the animals will require. No genetic manipulations of animals will be done. Rev 2-2003 RHIT ACUP Form - 14/16 - XI. FIELD STUDIES If animals in the wild will be used, describe how they will be observed, any interactions with the animals, whether the animals will be disturbed or affected, and any special procedures anticipated. Indicate if Federal permits are required and whether they have been obtained. N/A XII. SPECIAL CONCERNS OR REQUIREMENTS OF THE STUDY Identify other special circumstances or requirements not previously described in this document: Rev 2-2003 RHIT ACUP Form - 15/16 - XIII. PRINCIPAL INVESTIGATOR CERTIFICATIONS The undersigned, being the Principal Investigator in the research project described on the preceding pages of this document, hereby gives assurance that he/she will comply fully with Federal Law as set forth in the Animal Welfare Act; further that, if the research protocol described herein is approved by the Institute Animal Care and Use Committee, the investigator certifies: 1. I have attended the Animal Research Training Program required investigator training course. 2. I have determined that the research proposed herein is not unnecessarily duplicative of previously reported research. 3. All individuals working on this proposal who are at risk are participating in the Institution's Occupational Health and Safety Program. 4. The individuals listed in Section III are authorized to conduct procedures involving animals under this proposal, have attended the institutionally required investigator training course, and received training in: the biology, handling, and care of this species; aseptic surgical methods and techniques (if necessary); the concept, availability, and use of research or testing methods that limit the use of animals or minimize distress; the proper use of anesthetics, analgesics, and tranquilizers (if necessary); and procedures for reporting animal welfare concerns. 5. For all Pain Classification B and C protocols (see Section IV. [A]): I have reviewed the pertinent scientific literature and the sources and/or databases as noted in Section IV. G. and have found no valid alternative to any procedures described herein that may cause more than momentary pain or distress, whether it is relieved or not. 6. I will obtain approval from the IACUC before initiating any significant changes in this study. 7. I will notify the IACUC regarding any unexpected study results that impact the animals. Any unanticipated pain or distress, morbidity or mortality will be reported to the attending veterinarian and the IACUC. 8. I am familiar with and will comply with all pertinent institution policies, as well as all federal, state, and local regulations. Principal Investigator: Name: Jameel Ahmed Signature: __________________________ Date: _____________ Rev 2-2003 RHIT ACUP Form - 16/16 - XIV. ADDITIONAL APPROVALS Department Head of Principal Investigator: Name: Lee R. Waite Signature: ___________________________ Date: ________ Department Head Environmental Health and Safety: (Required for all studies utilizing hazardous/biological materials) Name: Michael R. Howard Signature: _________________________ Date: ________ Environmental Health and Safety Coordinator Facility Manager certification of resource capability in the indicated facility to support the proposed study: Name: N/A Signature: ___________________________ Date: ________ Facility Manager Attending Veterinarian certification of review, resource capability in the indicated facility to support the proposed study, and consultation on proper use of anesthetics and pain relieving medications for any painful procedures: Name: James Holscher Doctor of Veterinary Medicine Signature: ___________________________ Date: ________