Percent Composition (from formula)
advertisement

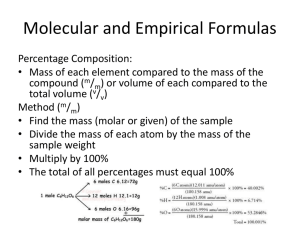
Percent Composition (from formula) Percent composition refers to what percent of the total mass of a compound comes from a specific element. Going from a molecular formula to a percent (or %) composition is easy and is what I will work on here. Going in the opposite direction, that is from a % composition to a molecular formula can be much more difficult, and is the subject of a different series of tutorials. Percent Composition (from formula) Just like any % calculation it is going to look like this Part/total x 100% In this case the ‘part’ is the mass of any one element in a compound and the ‘total’ is the total mass of the compound. Putting this together you have: % composition of an element = total mass of one element in compound × 100% total mass of compound Percent Composition (from formula) Let’s start with the compound Ca(OH)2. What is the % composition of Ca, O, and H in this compound? Well, from the formula you know that 1 mole of this compound contains 1 mole of Ca, 2 moles of O and 2 mole of H. Thus one mole of this compound would contain 1 mole x 40.08 g or 40.08 g of Ca 2 moles x 16.00 or 32.00 g of O 2 moles x 1.008 or 1.016 g of H And your molecular mass, is simple the total of these masses or 1(40.08) + 2(16.00) + 2(1.008) = 74.096 g Percent Composition (from formula) % composition of Ca is then: (40.08g/74.096g) x 100% = 54.09% % composition of O is: (32.00g/74.096g) x 100% = 43.19% And % composition of H is: (2.016g/74.096g) x 100% = 2.72% The sum of the %’s should = 100 so this is a quick check 54.09 + 43.19 + 2.72 = 100.00% so you are good! Percent Composition (from formula) Let’s try another example. What is the % composition of all of the elements in the compounds NaHCO3? Percent Composition (from formula) NaHCO3 Mass of Na = 1 x 22.99g = 22.99g Mass of H = 1 x 1.008g = 1.008g Mass of C = 1 x 12.01g = 12.01g Mass of O = 3 x 16.00g = 48.00 g TOTAL = 84.008g % comp of Na = 22.99g/84.008g x 100% = 27.37% % comp of H = 1.008g/84.008g x 100% = 1.20% % comp of C = 12.01g/84.008g x 100% = 14.30% % comp of O = 48.00g/84.008g x 100% = 57.14% (Check -sum of %s is 100.01, close enough!) Percent Composition (from formula) Finally try one more example, what is the % composition of all the elements in Iron(III) nitrite? Percent Composition (from formula) The correct formula here is Fe(NO2)3 Percent Composition (from formula) Fe(NO2)3 Mass of Fe = 1 x 55.85g = 55.85g Mass of N = 3 x 14.01g = 42.03g Mass of O = 6 x 16.00g = 96.00g TOTAL = 193.88g % comp of Fe = 55.85g/193.88g x 100% = 28.81% % comp of N = 42.03g/193.88g x 100% = 21.68% % comp of O = 96.00g/193.88g x 100% = 49.52% (Check - sum of %s is 100.01, close enough!)