AHRQ Grant Application Submission, Review, and Award Process
advertisement

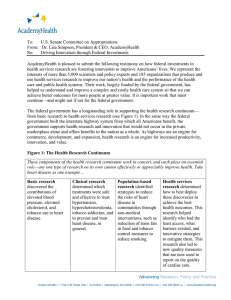
AHRQ Grant Application Submission, Review, and Award Process Development, Receipt, and Assignment of Applications Applicant Investigator develops and submits grant application to NIH/CSR CSR assigns application to NIH Institute or AHRQ CSR Assignment Office AHRQ assigns application to Initial Review Group Myths, Facts, and Suttons Law Francis D. Chesley, Jr., M.D. Director, Office of Extramural Research, Education and Priority Populations June 4, 2007 Facts #1 Research and program priorities matter Application process must be understood – Electronic Grant Application has arrived! Concept papers are an important option Peer review is a step away from funding Avoid common pitfalls DSR Referral Officer assigns to appropriate AHRQ Program IRG members review and evaluate Site visit made if necessary Study Section Review for Scientific Merit CSR sends computerized notice of assignment to applicant CSR sends computerized notice of review results to applicant CSR computes percentile ratings IRG reviews and assigns priority scores or designates noncompetitive DSR informs program that summary statement is available SRAs prepare Summary Statements Site visit report PO mails summary statements to investigators NIH Councils (Duals) Public Affairs notified AHRQ Review for Program Relevance and Funding Determinations Award Negotiation and Issuance PO reviews and prepares/sends recommendation memo to DSR/GMS DSR schedules funding meeting Applications selected for funding Signed “paylists” received by GMO Final review and negotiations Congressional liaison notified Award issued Award received by institution Investigator begins work (30 days) Applicant Responsibilities Know PHS Form 398 and 424 R&R Know the Funding Agency and Staff Know Agency Research Priorities Know the Grant Mechanisms Know the Grant Process and Key Changes Understand Agency Research Budget – Mentorship! Top Myths AHRQ is not funding research AHRQ is not funding K award AHRQ does not fund investigatorinvestigator- initiated grants An “unscored” unscored” application is the kiss of Facts #2 FY07 new research grant budget: $45 million K award success rate: 30% – $1 million for new grants – $5.7 million total What is an investigatorinvestigator-initiated grant? death 1 Fact #3: Sutton’ Sutton’s Law R01: R03: R13: R36: F31/F32: K02/K08: $2 million $1.7 million $1.4 million $0.5 million $0.5 million $1 million Budget for targeted initiatives is approx. $31 million AHRQ EE-grants Transition! ¾ Electronic Grant Application Receipt ¾ Use of the SF424 (R&R) Grant Application AHRQ will require electronic submission of all competing grant applications via Grants.gov using the SF 424 Research and Related (R&R) application. ________________________________________ Paper No More, Use 424 (R&R) Electronic submission is here to stay! AHRQ Transitions to Electronic Grant Application Submission AHRQ transitioning to electronic grant submission through Grants.gov – Grants.gov - Web portal that serves as the single access point for all Federal grant programs. – Grants.gov provides the interface for 26 agencies to announce $350 billion in annual grant awards and for all grant applicants to find and submit applications to those funding announcements. www.grants.gov Transition complete for R01, R03, R13, R18, R36 Getting Started One time registrations for Grants.gov (http://grants.gov) http://grants.gov) and eRA Commons (era.nih.gov/commons) systems must be completed before application submission. For up to date general information on electronic submission, the SF 424 (R&R), and Grants.gov, visit the AHRQ Electronic Submission of Grant Applications Web Site: http://www.ahrq.gov/path/egrants.htm Contact Information “Opportunity is missed by most because it is dressed in overalls and looks like work. work.” Thomas Edison AHRQ WEBSITE www.ahrq.gov Francis D. Chesley, Jr., M.D. (301) 427427-1521 Francis.Chesley@ahrq.hhs.gov 2 Questions ? 3