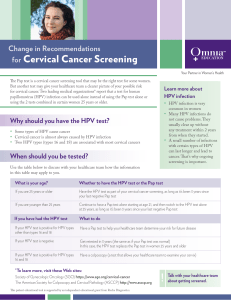
Cervical cancer screening practices in guidelines on genital human

Cervical cancer screening practices in the U. S. since the release of national guidelines on genital human papillomavirus (HPV) testing:
Results of a national clinician survey,
2004
2005 AcademyHealth Annual Research Meeting
KL Irwin 1 , D Montaño 2 , D Kasprzyk 2 , L Carlin 2 , C Freeman 2 , R
Barnes 1 , N Jain 1
1 U.S. Centers for Disease Control and Prevention, Atlanta, Georgia
USA
2 Battelle Memorial Institute, Seattle, Washington, USA
Cervical cancer and genital human papillomaviruses (HPV)
• Genital HPV are very common sexually transmitted viruses
– ~ 6 million new infections per year in US
– ~ 20 million Americans are currently infected
• Most infections are transient, asymptomatic, and clear without medical intervention.
• Persistent infection with oncogenic HPV types may cause:
– Cervical cancer precursor lesions or
C ervical I ntraepithelial N eoplasia (CIN)
– Invasive cervical cancer
• In 2004, US women experienced:
– ~ 4000 deaths from cervical cancer
– > 3 million abnormal Pap tests, most HPV-related
– > $1 billion in Pap test screening, follow up, and treatment costs
New tests for oncogenic types of HPV
• In the late 1990s, highly sensitive and specific
DNA tests for oncogenic HPV were developed.
• Use of new liquid-based Pap test methods facilitates collection of HPV test specimens.
• FDA recently approved HPV tests for various reasons, including
– managing patients with abnormal Pap tests
– an adjunct to Pap tests to screen women aged
30+ for cervical cancer
Indication 1:
HPV testing to manage patients with abnormal Pap results
• Women with abnormal Pap tests benefit from colposcopy = magnified visualization of cervix
– requires a speculum-aided pelvic examination, special equipment, and trained colposcopist
– Permits cervical biopsy that provide pathologic diagnosis that determines treatment
• Colposcopy is a costly, painful procedure that is in short-supply in the U.S., especially in communities with highest cervical cancer incidence.
Indication 1 (continued)
• In 2000, FDA approved HPV tests to guide colposcopy triage of patients with the most common Pap test abnormality ASC-
US ( A typical S quamous C ells, U ndetermined S ignificance)
• In 2001, several organizations issued guidelines endorsing
HPV tests as an option to guide such triage, including:
– American College of Obstetricians and Gynecologists
– Centers for Disease Control and Prevention
– American Cancer Society
• With this option, one orders HPV test if Pap result is ASC-US:
– HPV-infected women = high risk of developing CIN recommend prompt colposcopy
– HPV-uninfected women = low risk of developing CIN recommend repeat Pap test
• HPV tests not recommended for higher grade Pap results because colposcopy is advised regardless of HPV test results
Indication 2:
HPV test as Pap test adjunct to guide follow-up Pap interval
• In 2003, the FDA approved HPV tests as an adjunct to Pap tests to screen women aged 30+ to guide optimal follow up Pap test intervals because infection in women aged 30+ often represents persistent infection that increases risk of CIN.
• In 2003, two organizations issued guidelines that endorsed
HPV tests as an option to screen women aged 30+:
– American Cancer Society
– American College of Obstetricians and Gynecologists
• This option would advise for women with normal Pap tests:
– If HPV test positive = high risk of progression to CIN repeat
Pap test and HPV test in 6-12 months
– If HPV test negative = low risk of progression to CIN less frequent follow up Pap screening intervals (every 3 years)
• This option could reduce frequent screening of women at low risk for developing HPV-related abnormalities and enhance follow up of women at high risk for these abnormalities.
2004 national survey of US clinicians:
Selected questions
• What guidelines are being used to guide cancer screening practices and management of abnormal Pap tests?
• How are HPV DNA tests being used for:
– managing patients with abnormal Pap tests
– cervical cancer screening
• How are HPV test results influencing colposcopy and Pap test follow up practices?
• What are patient notification and consent when ordering
HPV tests?
• How do HPV test use and test results influence patient counseling and education messages?
Clinician survey methods
• Express mailed surveys to 5386 primary care clinicians in specialties that commonly provide Pap testing.
• Nationally-representative random samples (n=760-826) drawn from national clinician registries of:
– Physicians:
– Family/general practice
Midlevel providers:
Physician assistants
– Adolescent medicine
– Internal medicine
– Obstetrics/gynecology
Certified nurse midwives
Nurse practitioners
• Cover letter noted results will inform new clinician training and decision supports tools and patient education materials
• Survey required 20-40 minutes, $50 cash sent with first mailing
• Analyses weighted to account for differences by clinical specialty in sampling and non-response
Survey Disposition
5386 Surveys mailed
736 Retired or ineligible 746 Refused or no response
565 Undeliverable or deceased
3339 Completed surveys
Adjusted response rate by specialty
Midlevel provider
Nurse midwives
Nurse practitioners
Physician assistant
%
95
96
86
Physician
Adolescent med
Ob/Gyn
Family/Gen practice
Internal med
5386 surveys mailed
Overall response rate after adjusting for respondents who were deceased, retired, ineligible, or did not have current address = 82%
%
79
81
68
59
Characteristics of Pap test providers
(n=2980)
Value Range (%)
Clinician characteristics:
• Female (%)
• Mean years in practice
46
16
(32-99)
(11-21)
Practice characteristics:
• Practice in private office setting (%) 74
•
• Use liquid-based Pap method (%)
Mean number Pap tests last yr
78
479
• Mean number abnormal Paps last yr 53
• Have on-site colposcopy (%) 46
Patient characteristics:
• Mean % patients who are female
• Mean % patients who are white
• Mean % patients private insured
67
68
52
(37-87)
(58-92)
(110-1397)
(10-158)
(20-98)
(59-100)
(56-71)
(33-67)
Sources used to guide cervical cancer screening or abnormal Pap management decisions among 2930 Pap test providers
• >50% reported using guidelines or materials of:
– American College of Obstetrics/Gynecology
– Their clinical specialty organization
– Centers for Disease Control and Prevention
– American Cancer Society
• < 50% reported using guidelines or materials of:
– US Preventive Services Task Force
– American Society for Colposcopy & Cervical
46
Pathology 40
– HPV test manufacturer
– Health plans or insurance companies
%
36
23
%
73
78
63
58
Percent reporting ever using HPV tests for patients with Pap test abnormalities of any type
100
90
80
70
60
50
40
30
20
10
0
OB/GYN CNM NP FP PA IM ADOL Total
HPV test use by type of screening Pap test abnormality*
•
• Pap test abnormality
• Borderline result “ASC-US” reporting usual/always use
% range (%)*
98 (96-100)
• Higher grade results**:
• Atypical squamous cells of
• undetermined significance –
• cannot exclude high grade lesion**
79
• Low grade intraepithelial neoplasia** 63
(72-84)
(36-82)
• High grade intraepithelial neoplasia** 61 (31-78)
•
* Among clinicians reporting HPV tests for any borderline or abnormal
Pap test result. Range across 7 clinical specialties.
•
**This use NOT approved by FDA or recommended by guidelines of U. S. clinical organizations. Internal and adolescent medicine physicians most commonly reported use for high grade lesions.
Relation of HPV test results on colposcopy or followup Pap advice for women with ASC-US Pap results
100
80
% who usually advise follow-up procedure
60
40
20
0
Colposcopy Pap test
HPV test positive
Colposcopy Pap test
HPV test negative
Percent who report ever using HPV tests as an adjunct to Pap tests by specialty
OB/GYN CNM NP FP PA IM ADOL Total
40
35
30
25
20
15
10
5
0
37.3
26
19.6 19.3
15.7
10.2 11.8
20.8
Specialty
Percentage who reported usually/always using HPV tests as an adjunct to Pap tests by patient age
10
Percentage
20 30 40
Sexually-active
*Women < 30
Sexually-active
Women >= 30
0
*Indication not endorsed by national guidelines
Percentage who reported usually/always using HPV tests as an adjunct to Pap tests by patient age
20
Percentage
40 60 80 100
Sexually-active
*Women < 30
Sexually-active
Women >= 30
0
*Indication not endorsed by national guidelines
Summary
• Most commonly cited guidelines are credible, noncommercial, and consistent with recent scientific evidence
• More than half of Pap test providers use HPV tests for ASC-
US Pap results (a recommended option), especially:
– Obstetrician/gynecologists and nurse midwives
– Clinicians with high volume of Paps and abnormal Pap tests
– Clinicians with on-site colposcopy
• Colposcopy is more likely to be recommended to women with positive HPV tests as intended by guidelines
• However, many report using HPV tests for patients with higher grade Pap abnormalities which is not recommended
• Few report usually using HPV tests as adjunct to Pap tests in women 30+ (a recommended option)
• However, such HPV test use is more common in women
< 30 (not-recommended) than women 30+ (recommended)
Study strengths and limitations
STRENGTHS:
• Large sample size with high overall response rate (82%)
• Stratified sampling design and weighting yielded more generalizable estimates within and across specialty
• Included midlevel providers who provide much cervical cancer screening but are often overlooked in surveys
LIMITATIONS:
• Reported practices may not reflect actual practices
• Survey did not determine:
– If use of HPV tests resulted in more appropriate use of colposcopy or follow up Pap intervals
– reasons HPV tests used for non-recommended reasons
Recommendations
• Interventions are needed to promote HPV testing practices that are consistent with national guidelines to avoid unnecessary:
– patient anxiety, stigma, or psychosocial burden due to STD
– counseling burden by clinicians
– HPV testing costs
• Interventions should be designed with input of key stakeholders:
– national organizations that issue screening guidelines
– laboratories
– health insurers, health plans, and health care purchasers
– HPV and Pap test manufacturers
– women eligible for cervical cancer screening
• CDC, in collaboration with other organizations is:
– Updating clinical training and clinical decision support tools
– Disseminating materials using print, web, and 2005 webcast
– Updating patient and public education web and print materials to promote HPV test demand consistent with guidelines
CDC sponsored webcast to train
U.S. clinicians about
HPV and cervical cancer
Date: August 9, 2005
Time: 1-2 pm Eastern Time
Audience: Primary care clinicians
CME and CEU credits available
For more information: www.phppo.cdc.gov/PHTN/HPV-05
Patient counseling messages used by
2980 clinicians providing Pap tests
Message % Range (%)
•
When collecting Paps, I usually/always:
• Address methods to prevent cancer
• Discuss HPV as cancer risk factor
• Discuss HPV prevention
49
43
43
(43-72)
(32-66)
(30-79)
For patients w/ HPV+ test or HPV-related Pap, I usually/always say:
• Virus is sexually transmitted
• Patient could transmit virus to partners
• HPV is common in sexually active persons
95
91
92
• Monogamy/ ↓ partner # can prevent transmission 79
• Condom use can prevent transmission 84
• Abstinence can prevent transmission 41
(88-99)
(83-97)
(76-93)
(70-86)
(81-95)
(30-63)
Key issues when addressing patients with positive HPV tests or HPV-related
Pap results
• Addressing HPV would: %
– Increase likelihood patient would return for repeat Pap 87
– Assure patients they are getting complete information 83
– Raise patient concerns about partner fidelity 68
• It is somewhat or quite problematic to:
– Indicate when/from whom infection was acquired
– Deal with emotional or relationship issues
– Get enough reimbursement to counsel patients
– Find time to counsel/educate patients
%
85
73
74
54
Reported use of liquid-based cytology
(LBC) and Conventional cytology
LBC and
Conventional Pap
LBC only
Conventional only
0
11.3
21.1
20 40
% Respondents
NON-NBCCEDP
60
67.6
80
Usual HPV test consent practices among 2680 Pap test providers
• When using to manage abnormal Pap results:
– Seek patients consent for test
– Tell patients you are ordering
– Explain purpose of test as relates to Pap
– Explain HPV test detects STD
• When ordering test as adjunct to screening Pap test: %
– Seek patients consent for test 36
– Tell patients you are ordering
– Explain purpose of test in relation to Pap
– Explain HPV test detects STD
59
63
64
%
28
48
58
59
Summary:
HPV test consent, notification, and counseling practices
• About one third explicitly report seeking consent for HPV testing
• About half report notifying patients when they are ordering HPV tests
• About two-thirds report telling patients that test detects sexually transmitted infection
• However, more than half report telling patients about sexually transmitted HPV when:
– collecting Pap tests
– notifying patients with positive HPV tests or HPV-related Pap abnormalities