Chemistry 112 Third Hour Exam Name:____________ Please show all work for partial credit
advertisement
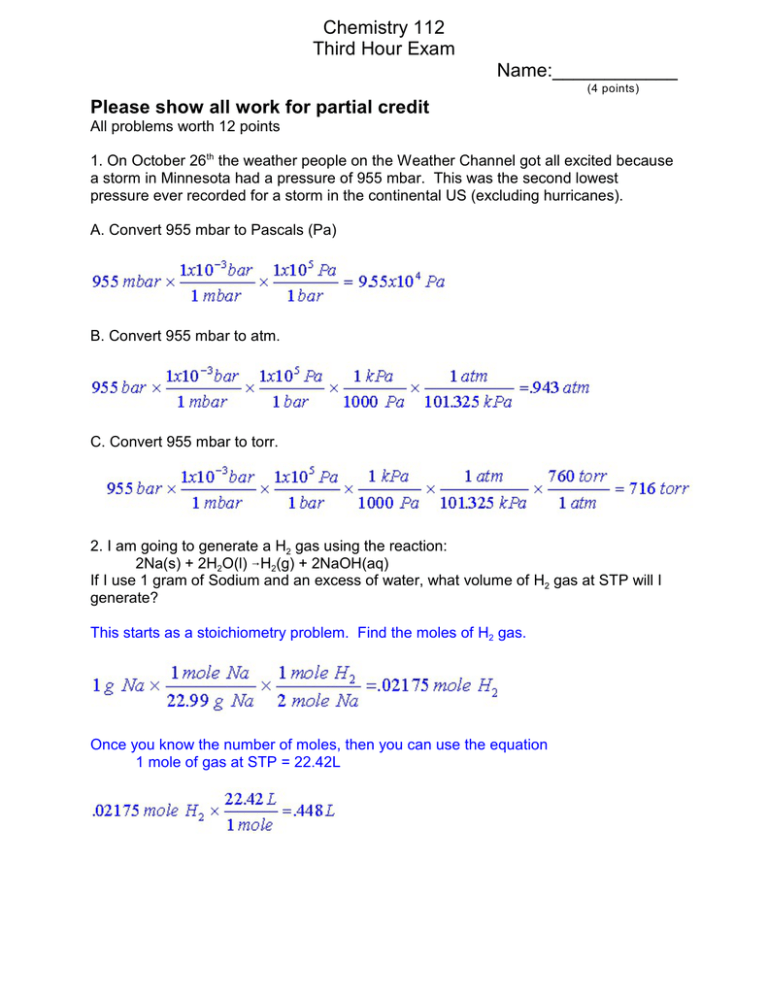
Chemistry 112 Third Hour Exam Name:____________ (4 points) Please show all work for partial credit All problems worth 12 points 1. On October 26th the weather people on the Weather Channel got all excited because a storm in Minnesota had a pressure of 955 mbar. This was the second lowest pressure ever recorded for a storm in the continental US (excluding hurricanes). A. Convert 955 mbar to Pascals (Pa) B. Convert 955 mbar to atm. C. Convert 955 mbar to torr. 2. I am going to generate a H2 gas using the reaction: 2Na(s) + 2H2O(l) 6H2(g) + 2NaOH(aq) If I use 1 gram of Sodium and an excess of water, what volume of H2 gas at STP will I generate? This starts as a stoichiometry problem. Find the moles of H2 gas. Once you know the number of moles, then you can use the equation 1 mole of gas at STP = 22.42L 2 3A. (8 points) Give a least four of the postulates that make the kinetic molecular theory of gases. 1. A gas is composed of tiny particles 2. The tiny particles are in constant motion. 3. The volume of the particle is so small compared to the volume that it is moving around it that its volume can be ignored. 4. The kinetic energy of the particle is directly proportional to temperature. 5. When particles collide there is no attraction or repulsion between the particles 6. Pressure results from particles colliding with a surface. 3B. (4 points) Which two of these postulates are wrong, and how do they get changed to reflect the behavior of real gases under non-ideal conditions. #3 and #5 from the above list are incorrect. At high pressure or low temperatures the volume of the particles has to be taken into account (The b factor in the equation in 4B.), and attractive or repulsive forces between the particles must be included (The a factor in equation 4B). 4A. (4 points) Calculate the pressure for 100 moles of an ideal gas when T=150K, and V = 20 L PV=nRT; P=nRT/V =100 moles x .08206 L@atm/K@mol x150K /20L =61.5 atm 4B (8 points) Now calculate the pressure for 100 moles of Cl2 gas when T=150K, and V = 20 L using the van der Waals equation: Cl2 gas has coefficients of 6.49 atm@L2/ mol2 for a, and .0562 L/mol for b. When I picked the numbers for this problem, they looked reasonable, especially when the answer to 4A gave me a nice high pressure. So I didn’t try out the answer to 4B until I was making the key, and then was surprised to find that this set of numbers gives an impossible pressure. Sorry about that, but I did warn you! 3 5. Define the following terms: State function A property of a system that depends only on the state of the system, not the path taken between states. Intensive Variable A property that does not depend on the amount of material in a system. First law of Thermodynamics The energy of the universe is constant. -or- Energy cannot be created or destroyed. Constant pressure calorimeter A coffee cup calorimeter. A calorimeter that measures ÄH at atmospheric pressure and assumes that this pressure does not vary during the time of the experiment so the pressure is constant. qP Heat measured at a constant pressure = ÄH Internal Energy E = the sum of all the kinetic and potential energy of all the particles in a system. 6. I have an unknown substance. When 100 g of this material at 73oC is placed into 1000 g of water at 25oC, the equilibrium temperature for the mix of water and material is 26oC. What is the specific heat capacity of this unknown material. (The Specific Heat Capacity of water is 4.18 J/oC@g.) Heat energy gained by water = S.H.C x grams x ÄT =4.18 x 1000 x (26 - 25) = 4180 J The heat lost by the unknown substance = -4180 J -4180 = Unknown heat capacity x 100g x (26-73) -4180/(100 x -47) = X = .889 J/oC@g 4 7. Given the following data 2O3(g) 6 3O2(g) O2(g) 6 2O(g) NO(g) + O3(g) 6 NO2(g) + O2(g) ÄH = -427 kJ ÄH = +495 kJ ÄH = -199 kJ calculate ÄH for the reaction NO(g) + O(g) 6NO2(g) Start with the last equation because it have both a product and a reactant we want NO(g) + O3(g) 6 NO2(g) + O2(g) ÄH = -199 kJ This equation does not contain O so I will next use equation 2 But this equation has O on the wrong side so I will reverse it. 2O(g) 6O2(g) ÄH = -495 kJ But this is not quite right because it has 2 O’s instead of 1, so I have to divide by 2 O(g) 6½ O2(g) ÄH = -247.5 kJ At this point the net equation is: NO(g) + O3(g) + O(g) 6½ O2(g) + NO2(g) + O2(g) NO(g) + O3(g) + O(g) 6 NO2(g) + 3/2 O2(g) So I will now reverse the first equation 3O2(g) 62O3 ÄH = +427 kJ But I need to divide it by 2 so it will cancel out the unwanted terms from our equation. 3/2 O2(g) 62/2 O3 ÄH = +213.5 kJ Adding all three equations together we have NO(g) + O3(g) + O(g) 3/2 O2(g) 62/2 O3 + NO2(g) + 3/2 O2(g) NO(g) + O(g) 6 NO2(g) ÄH= -233 kJ 8. UV -B light has a frequency of about 1x1015 Hz. What is the wavelength of this light? c=ëí ; c/í=ë =3x108 m/sec /1x1015sec-1 =3x10-7 m = 300 x10-9 m = 300 nm What is the energy of a single photon of this light? E=hí =6.626x10-34 J@s x 1x1015 sec-1 =6.63x10-19 J What is the mass of a photon of this light? Mass = h/ëc =6.626x10-34 J@sec/(3x10-7m × 3x108 m/sec) =7.36x10-36 kg