Lab – Molar Quantities
advertisement

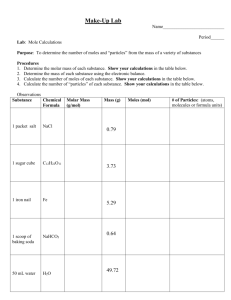
Lab – Molar Quantities Pre-Lab 1. Give a value for the following terms: a. Couple b. Tetrad c. Dozen d. Score e. Gross f. Century g. Ream h. Mole 2. What is the molar mass of each of the following substances? a. Helium f. Potassium fluoride b. Iron g. O2 c. Carbon Dioxide h. Al2O3 d. Sulfur i. C6H12O6 e. Silicon Purpose To mathematically practice the relationship between mass, # of moles and # of particles of elements and compounds Part I Procedure 1. Find the mass of each of the first three samples, enter in the data table. 2. Using the molar mass of the sample, calculate the number of moles in the sample. Show ALL work below the table. 3. Using Avogadro’s number (6.02 x 1023 particles/mole), Calculate the # of particles in the sample. Show ALL work below the table. 4. Assume a 5.00 gram sample for the remaining substances. Do similar calculations as you did with the other samples. Show ALL work below the table. Substance Mass (g) # of moles # of particles Sodium chloride Copper Zinc Substance Water Lead Sugar (C12H22O11) Mass (g) 5.00 g 5.00 g 5.00 g # of moles # of particles Questions 1. List the elements (copper, zinc, lead) in order from smallest mass to greatest mass. 2. List the elements in order from least # of moles to greatest # of moles. 3. List the elements in order from least # of particles to greatest # of particles. 4. Is the number of particles in a sample directly proportional to the mass of the sample OR to the # of moles of sample? Which one? Defend your answer. 5. Explain why a 5.00 gram sample of water does not have the same # of particles as a 5.00 gram sample of sugar. 6. How many grams of sugar would you need to have the same number of particles as 5.00 grams of salt? Show calculations for credit. Watch sig. Figs. and units.