Name: Chem 332 Analytical Chemistry Exam I
advertisement

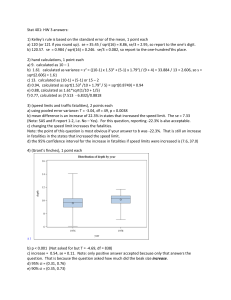
Name: Chem 332 Analytical Chemistry Exam I Show all work for partial credit 1. (10 points) Massachusetts limits the amount of lead in drinking water to .015 mg/L. Express this concentration in Molarity, ppm, and ppb. .015 mg = .000015g .000015g x 1 mole/207.2 g = 72.4 x10-9M 1 liter water = 1000 g .000015g/1000g = 15x10-9 15x10-9 x106 ppm = .015 ppm 15x10-9 x109 ppb = 15 ppb 2. A. (5 points) I am making a 0.1M NaCl (molar mass 58.44) solution for an experiment. To measure the mass of the NaCl, I will use the bulk balance that is only accurate to ±0.01g. I place a piece of paper on the balance and set the tare to read 0.00. I then put the NaCl on the balance until it reads 5.84 g. What it the uncertainty in this mass? 5.84±.01 g -0.00±.01 = 5.84 Subtraction error = sqrt(.012 + .012) = .014 5.84±.014g B. (5 points) The volume of the solution I am making is 1L. To measure this volume I will use a large graduated cylinder that can measure volume to ± 10 mL. What is the uncertainty in my final calculated molarity? (5.84g/58.44 g/mol)/1L = .1M Will ignore the error in the molar mass This is a division problem relative error = sqrt((.014/5.84)2 + (10/1000)2) =.0102 .0102 X .1 M = .001M .100±.001M 3. Statistics Below is the data for % á-acids in hops from our class: % á-acids in hops Toluene Extract Methanol Extract 8.94 10.78 7.43 8.56 7.96 10.44 7.52 6.84 4.02 7.34 7.43 9.46 7.46 7.99 A. (10 points) What is the average, standard deviation, and 95% Confidence interval for the % á-acids in the Toluene extract? Average : 7.25 Standard Deviation: 1.56 CI = 7.25 ±ts/sqrt(n) t=2.45 7.25± 2.45(1.56)/sqrt(7) 7.25±1.41 5.84-8.66 B. (5 points) Could the 4.02 value in the set of toluene extract data be rejected using the Q test? (You can use the Grubbs test instead if you want) Q Grubbs Gap = 7.43-4.02 =3.41 |7.25-4.02|/1.56 Interval = 8.94-4.02=4.92 2.07 Q = .693 >1.938 > .51, reject Reject C. (No points, but needed for next questions) What is the average, and standard deviation for the % á-acids in the Methanol extract? Average = 8.77 s = 1.51 D. (5 points) Use the t-test to determine if the mean you get for the % á- in the Toluene is statistically different than the mean you get for the % á- for the methanol extract. (Use a 99% level for statistical significance) spooled = sqrt((6(1.562) + 6(1.51)2)/12) = 1.53 t=|8.77-7.25|/1.53 sqrt(7x7)/(7+7) = 1.86 < T table (3.169) so not statistically different 4. (10 points) Ag+ at 10-100 ppb can be used to disinfect swimming pools. One way to maintain an appropriate concentration of Ag+ is to add a slightly soluble silver salt to the pool. Calculate the ppb of Ag+ in a saturated solution of AgBr (Ksp = 5.0x10-13) 5.0x10-13 = [Ag+][Cl-] =X2 X=[Ag+] = sqrt(5.0x10-13) = 7.1x10-7 M For grins (and 3 points extra credit) an Olympic sized pool has a volume of 2.5x106 liters. How much silver is there in a pool of this size? 2.5x106 x 7.1x10-7 = 1.7675 moles 1.7675 moles x 107.9 g/mole = 191 g silver (2 points extra credit) The current price of silver is $21.43/ounce. How many $ of silver are in this pool?) 191 g x 1lbs/453.59 g x 16 oz/lbs x $21.43/oz = $144.38 5A. (3 points) Calculate the pH of a .5M solution of Hypochlorous acid, HOCl? (Ka 3.0x10-8) Ka=[A-][H+]/[HA] 3.0x10-8 = X2/.5-X; will assume .5-X ~ .5 X = sqrt(.5(3.0x10-8) = .000122 Assumption that .5-.0001~.5 is good pH=-log(.000122) =3.91 5B. (3 points) What is the Kb of NaOCl? KW =KA@KB 1x10-14=3.0x10-8@KB KB =1x10-14/3x10-8 = 3.33x10-7 5C. (4 points) What is the pH of .5M NaOCl? KB = [HB+][OH-]/[B] 3.33x10-7=X2/.5-X; will assume that .5-X ~ .5 3.33x10-7=X2/.5; 3.33x10-7@.5=X2 X=sqrt(3.33x10-7@.5) = .00041 Assumption that .5-.0004~.5 is good pOH = -log(.0004) = 3.39; pH = 14-3.39 = 10.61