Centennial Honors College Western Illinois University Undergraduate Research Day 2012
advertisement

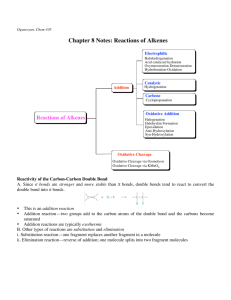
Centennial Honors College Western Illinois University Undergraduate Research Day 2012 Poster Presentation Oxidative Cleavage of Alkenes Using an In-Situ Generated Iodonium Ion: A pH Dependent Investigation Amanda Ellison Faculty Mentor: T. K. Vinod Chemistry Oxidative cleavage of alkenes is a synthetically important transformation emphasized during the first term of a sophomore undergraduate organic chemistry curriculum. The practical difficulties with the ozonolysis procedure make it an inconvenient experiment for undergraduate students to carry out. We have recently described a facile and operationally convenient catalytic procedure for oxidative cleavage of alkenes. In situ formed [hydroxy(4-carboxyphenyl)iodonium]ion, from the oxidation of 4-iodobenzoic acid, has been shown to facilitate the cleavage of a variety of alkenes in presence of Oxone as a co-oxidant. Optimization of this convenient procedure using 1-phenyl-1cyclohexene as a protypical substrate to develop a useful undergraduate experiment at different pHs will be described.