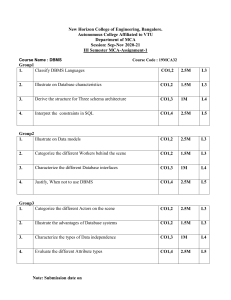
Contract Manufacturing in Biotechnology
advertisement

Contract Manufacturing in Biotechnology Positioning your company for success by selecting the right partner and by knowing and preparing for the ‘previously’ unexpected! Contract Manufacturing and Technology Transfer Dinner Meeting ISPE San Francisco / Bay Area Chapter Vacaville, CA September 10, 2009 Disclaimer The views expressed in these slides and accompanying discussion are my own and do not necessarily reflect the views of Genentech. What ‘Contract Manufacturing’ are we discussing? Internal External Transfers API / Bulk Fill & Finish Devices Services Clinical Commercial Products CMO Pharma Businesses Today’s Focus Contract Manufacturing – a Very Viable Option • Why companies consider utilizing a CMO? • Provides flexibility in your supply chain • Provides surge capacity • Act as supplement to existing internal capacity • It’s ready now – (18 months vs 4-5 years) • Minimizes need for capital investment • Allow company for focus on other capabilities • Provide assistance in development, scale-up, and regulatory areas • Why companies consider providing CMO services? • It’s the business model for the true CMO • Need to fill excess capacity • Opportunity to learn while utilizing new capacity • Train staff • Start-up new facility • Gain valuable experience Successful Contract Manufacturing begins during Selection Contract Manufacturing Process 1. Define 2. Identify Outsourcing Requirements Identified • • • • 3. Evaluate RFP Issued 4. Negotiate Term Sheet Issued 5. Transfer Agreements Signed Identifies gaps and risks to address • In the contract • During tech transfer Set expectations and flush out assumptions • CMO • Your own company Calibrates overall project schedule • Identify early watch outs Establishes working relationships and trust 6. Manage FDA Approval Kind of like a marriage! Selecting the Right Partner… There are differences between ‘dedicated’ CMOs and ‘gap filling’ Pharma companies On the positive side, sure you get this: • Dedicated CMO • Lower cost • Speed • Reliability (failure puts them out of business!) • Experience in wide variety Manufacturing situations • Gap filling Pharma / Biotech company • Experience in Manufacturing • Strong Development and Scale-up expertise • Strong Quality Systems • Strong Regulatory history and expertise Selecting the Right Partner… Understanding more than just the ‘upside’ But you also get this!!!! • Dedicated CMO • Thinly staffed to handle ‘upsets’ • Penny pinching – it’s all about the money • Negative Regulatory perception of some CMOs • Scheduling challenges – Multiple companies at the table • Increased support needed to fill expertise gaps • Gap filling Pharma / Biotech company • Manufacturing experience in one ‘platform’ process • Systems not built to ‘act as a CMO’ • Battle for ‘the best’ resources vs internal projects • Difficulty doing things ‘your way’ Do your homework during Selection Don’t judge a book by it’s cover… • Don’t … • Shortcut this stage • Assume CMO knows best (but don’t discount their experience) • Underestimate cultural fit • Do … • Develop a structured selection process • Cross-functional governance • Stage-gates • Standard evaluation criteria, deliverables • Conduct thorough due diligence of the CMO w/ cross-functional team • Publicly available information • Site visits • Facilitated technical discussions • Friends in the industry • Tease out and consider ways to align incentives Key Evaluation Criteria • • • • • • • • Technical and technology transfer maturity Manufacturing capabilities and infrastructure (Make) Operational capabilities and infrastructure (Plan, Source, Deliver) Regulatory compliance Quality Systems Economic attractiveness Business profile • Management Expertise • Business Stability • Cultural Fit • Risks Strategic fit Made your selection? What you need to know, expect, and prepare for no matter who you choose! • Tight timelines from day 1 • The project end points, ie. when the product needs to be released, will drive the schedule • Back-scheduling will occur from there and all required activities must fit in that time period • Quick engineering decisions for long lead items • Long lead items are critical path from the day the contract is signed. • More engineering will be needed than anticipated. • Dedicated staff • This is not a hobby or side job! • Full time dedicated staff are required in all key functional areas • Dedicated senior project lead with decision making authority a must • Quality organization (RQC) alignment • No two Quality organizations are exactly alike!! • Time must be spent up front to document and align expectations and philosophies Did you really think it would be easy? There’s more you need to expect and prepare for… • Relationship building • Booze cruises and wine tasting are seriously important (seriously!) • Time and effort must be put towards building relationship – including nights and weekends • Need to build strong relationships while times are good to effectively work together when times are bad • Face to face time is critical – phone calls and video cons alone will not suffice • Early and often QC Activity • Method Transfers must start early with face to face interactions • Raw material release will be on the critical path • QC needs to be ready for quick turnarounds for process testing • Procurement and Raw Material challenges • Challenges will be abundant when acquiring others raw materials • Pricing, availability, and delivery concerns • Vendor approvals and audits required • Start early and plan for additional resources • Shipping and logistics - Don’t overlook these area’s • Critical to bring in the raw materials, cell banks, and for shipping samples and final bulk. • Requires management well beyond transfer! Maybe you shouldn’t have outsourced after all! You’re stuck now, but you still need to expect and prepare for… • Meetings, Meetings, Meetings • Strong project governance / management is essential • Documented transfer agreements • Quality Agreement • Joint Service Agreement • Time spent in meetings is part of the game • High senior management engagement • Expect more senior management engagement than with internal products • Prepare staff to manage effectively in a high visibility project • Surge in activity to impact all your systems • Change control, documentation management, etc. will see big upswing really quickly • Increased complexity when third party approvals are required • Data availability • More process data will be viewed by more people than ever before • Data will be expected real time – without review at times • Validation disagreements • Validation policies, procedures, practices, experiences, and expectations vary • Expect and work early to identify gaps And yes, there is even more.... You need to understand, expect, and prepare for… • Travel Budgets • Estimate your travel requirements than multiply by 3x • Expect staff to be away from the shop more than expected • Changes in manufacturing kg requirements • Estimating clinical and commercial product demand is far from an exact science • Build flexibility into your manufacturing schedule to prepare for changes • Meets specifications? • Be aware and prepare for the fact that ‘meeting specifications’ is not enough - scope creep • This needs to be controlled early • Maintain schedule at what cost • Must be diligent to maintain RQC levels while confronting business drivers • ‘Business’ is now part of daily life • Approximately 40% of all marriages end in divorce • Constant work will be needed throughout the length of the contract and beyond to ensure your contact manufacturing experience does not end in an nasty divorce!! (don’t forget the ‘kids’!) How to Prepare for Success… • Success begins with selection – get the right people involved early • While the type of CMO you choose does not necessarily matter, it is critical to understand the weaknesses of all options up front • Expect and plan for the ‘previously’ expected • Successful Contract Manufacturing is a lot like a successful marriage. By selecting well and by understanding and accepting your partners weaknesses up front, you will be better prepared for success ahead. ------------------------------------------------------------------------------------- Some Quotes to consider “Marriage is that relation between man and woman in which the independence is equal, the dependence mutual, and the obligation reciprocal.” Louis K Anspacher “Many marriages would be much better if the husband and wife clearly understood that they are on the same side.” Zig Zagler Thank You! Dan Moskey Associate Director, Vacaville Technology Genentech, Inc. dmoskey@gene.com Appendix • Governance Structure • Low Overhead Project Management Establish an Effective Governance Structure • • Governance = People + Process • Project and management teams • Stage review process • Decision-making process Benefits • Minimizes time wasted negotiating through certain conflicts • Ensures best possible minds are contributing to the solution • Minimizes fire drills • No surprises for management • No “plausible deniability” • Leverages resources Executive Steering Committee Management Committee Decisions Leaders Project Team Recommendations Team Leaders Core Team Extended Team (ad hoc) Sub-Teams Leaders Leaders Leaders Leaders Leaders Quality Technical Engineering Validation Supply Chain QC Establish a “low-overhead” project management approach early • • • Communications • Meeting standards (attendance, frequency, format) • Meeting minutes template • Meeting schedule • ADI log (Actions, Decisions, Issues) • Metrics / dashboards • Team roster and roles/responsibilities • Software standards Document management • Document management systems/tools • Project scheduling responsibilities, processes, and systems/ tools Risk assessment and management • Methodology • Risk assessment tool and processes