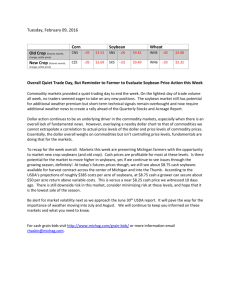
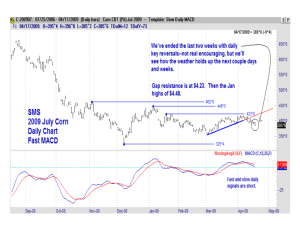
AN ABSTRACT OF THE THESIS OF Animal Sciences presented on
advertisement

AN ABSTRACT OF THE THESIS OF Jonathan D. Albro for the degree of Master of Science in February 3, 1992 Animal Sciences presented on Title: EFFECT OF SOYBEAN PROTEIN SUPPLEMENTS WITH LOW QUALITY ROUGHAGE ON PERFORMANCE AND DIGESTIVE CHARACTERISTICS OF WEAIT BEEF STEERS . Abstract approved:_Redacted A for Privacy Two experiments were conducted to compare whole soybeans (WSB), extruded soybeans (ESB), and soybean meal+barley (SBM+BAR) as supplemental protein sources for growing beef steers consuming low quality mature grass hay (6.5% CP). In Exp. 1, a 23 d digestion study, 4 ruminally cannulated steers were assigned to the following treatments in a 4 X 4 Latin square design. 1) Control, no supplement; 2) 1.5 kghd-Id-1 of WSB; 3) 1.36 kihd-1d-1 of ESB; 4) 1.48 kghd-Id-1 of 62%:38% SBM+BAR. Apparent DMD was increased by supplementation (P <.10), but NDF digestibility was not changed. No differences in digestibility were observed among supplement treatments. In situ rate and extent of supplement CP disappearance did not differ among supplements but extent of DM disappearance was greater for WSB than ESB (P <.10). In situ rate of forage NDF disappearance was decreased by protein supplementation (P =.10). In Exp. 2, 40 British X exotic weanling steer calves were stratified by weight (average BW, 250 kg) and allotted randomly to two replications of the 4 treatments above (8 pens, 5 animals/pen). Forage DMI was not affected by treatment. Average daily gain and feed efficiency were increased by supplementation (P <.05). Supplement source had no effect on intake or ADG, but ESB tended to exhibit better feed efficiency than WSB (P =.10). In conclusion, WSB and ESB (full fat soybeans) appear to be as effective as soybean meal protein supplements for growing beef cattle. In addition, full fat soybeans at the above levels can be incorporated into diets for cattle consuming low quality roughage without deleterious effects on fiber digestion or subsequent performance. (Key words: Protein Supplements, Beef Cattle, Soybeans). EFFECT OF SOYBEAN PROTEIN SUPPLEMENTS WITH LOW QUALITY ROUGHAGE ON PERFORMANCE AND DIGESTIVE CHARACTERISTICS OF WEANED BEEF STEERS by Jonathan D. Albro A THESIS submitted to Oregon State University in partial fulfillment of the requirements for the degree of Master of Science Completed February 3, 1992 Commencement June, 1992 APPROVED: Redacted for Privacy Associate Professor of Animal Sciences in charge of major Redacted for Privacy Hea'of Dkaydrg;;tZa? sc6ces Redacted for Privacy Dean of G duate'Ssloor Date thesis is presented Typed by February 3, 1992 Jonathan D. Albro ACKNOWLEDGEMENTS The most appreciation is expressed to my family. The never ending support and encouragement from my mother Janis Albro, my sisters Laurie Albro and Lisa Albro Peterson, and from my brother-in-law Kevin Peterson were the best tools that I've had in graduate school. The many family get togethers were definitely worth the travel and expense. A great deal of gratitude is also expressed to my aunt, Beverly Clark, who offered a place for me when I first arrived in Corvallis and who has always had good advice about graduate school. To my major professor, Dr. Dale Weber. Dale has been an exceptionally good advisor and a good friend. enjoyed working and teaching for him. To Dr. Tim DelCurto, who helped design experiments and analyze data. certainly gone the extra mile for me. I have Tim has A special thanks is given to Drs. Cheeke, Hannaway, and Christensen for serving on my committee and being interested in my progress. I would like to thank all those people who work behind the scene. Thanks to Roger Miller and Farm Services for grinding hay, supplying tools, and machinery. Appreciation is given to Marvin Martin for helping with research when I wanted and to Deloras Martin for handling most of my paperwork. To Mark Keller in the nutrition lab, who was often more excited about results than I was. The College of Veterinary Medicine deserves thanks, especially to Dr. Wayne Schmotzer for performing cannulation surgery on 4 steers for my study. I would like to finally thank my fellow graduate students. The most gratitude is expressed to Tom Dill who taught me how to play the graduate school game. To Anne Ayers who has been a very special friend, and who gave me free haircuts. To Marc Horney and Michelle Stamm, they always gave me a good laugh. Marc never turned me down and Michelle always did more than was expected. To Steve Brandyberry for helping on weigh days and with rumen evacuations, and to his wife, Kelly Brandyberry who helped a great deal with lab work at Burns. The list could certainly continue. the Animal Sciences Department. I thank everyone in I have enjoyed very much being a part of Oregon State University. J.D.A. TABLE OF CONTENTS Page REVIEW OF LITERATURE 1 INTRODUCTION THE ROLE OF SOYBEANS AS LIVESTOCK SUPPLEMENTS CLASSIFICATION FEEDING RAW SOYBEANS FATS AND RUMEN FERMENTATION FATS AND FIBER DIGESTION PROTEIN SUPPLEMENTATION AND LOW QUALITY ROUGHAGE PROTEIN SOLUBILITY AND FORAGE UTILIZATION PROTEIN DEGRADABILITY OF SOYBEAN PRODUCTS VOLATILE FATTY ACID PRODUCTION AND ABSORPTION INDIVIDUAL VFA ABSORPTION AND METABOLISM LIPID CONTAINING FEEDS AND VFA PERFORMANCE STUDIES AND SOYBEAN SUPPLEMENTS ECONOMICS SUMMARY 1 2 4 . . 6 9 13 . . . . . COMPARISON OF WHOLE SOYBEANS, EXTRUDED SOYBEANS OR SOYBEAN MEAL/BARLEY ON DIGESTIVE CHARACTERISTICS AND PERFOMANCE OF WEANED BEEF STEERS CONSUMING MATURE GRASS HAY INTRODUCTION MATERIALS AND METHODS RESULTS AND DISCUSSION IMPLICATIONS 16 18 20 23 25 26 28 30 31 33 33 35 42 50 LITERATURE CITED 60 APPENDIX 67 LIST OF FIGURES Figure 1. Page INFLUENCE OF SOYBEAN PROTEIN SOURCE ON RUMINAL VALERATE CONCENTRATIONS (Exp 1) 51 INFLUENCE OF SOYBEAN PROTEIN SOURCE ON RUMINAL AMMONIA CONCENTRATIONS (Exp. 1) 52 . 2. LIST OF TABLES Table 1. 2. 3. 4. 5. 6. Page CHEMICAL COMPOSITION OF GROUND MATURE GRASS HAY AND TREATMENT SUPPLEMENTS (Exp. 1) 53 INFLUENCE OF SUPPLEMENTAL WHOLE SOYBEANS (WSB), EXTRUDED SOYBEANS (ESB), AND SOYBEAN MEAL+BARLEY (SBM+BAR) ON DM INTAKE AND APPARENT DIGESTIBILITY (Exp. 1) 54 INFLUENCE OF SUPPLEMENTAL WHOLE SOYBEANS (WSB), EXTRUDED SOYBEANS (ESB), AND SOYBEAN MEAL+BARLEY (SBM+BAR) ON RUMINAL KINETICS (Exp. 1) 55 INFLUENCE OF SUPPLEMENTAL WHOLE SOYBEANS (WSB), EXTRUDED SOYBEANS (ESB), AND SOYBEAN MEAL+BARLEY (SBM+BAR) ON RUMEN pH AND VFA CONCENTRATION (Exp. 1) 56 IN SITU DEGRADATION OF TREATMENT SUPPLEMENTS AND INFLUENCE OF SUPPLEMENT SOURCE ON DEGRADATION OF GROUND MATURE GRASS HAY (Exp. 1) 57 INFLUENCE OF WHOLE SOYBEANS (WSB), EXTRUDED SOYBEANS (ESB), AND SOYBEAN MEAL+BARLEY (SBM+BAR) ON DM INTAKE AND PERFORMANCE OF WEANED BEEF STEERS (Exp. 2) 7. CHEMICAL COMPOSITION OF FEEDSTUFFS (Exp. 2) 58 . . . . 59 LIST OF APPENDIX TABLES Table A.1 A.2 A.3 A.4 Page INFLUENCE OF SUPPLEMENTAL SOYBEAN SOURCE ON INTAKE AND DIGESTIBILITY (Exp. 1) 67 INFLUENCE OF SUPPLEMENTAL SOYBEAN SOURCE ON RUMINAL KINETICS (Exp. 1) 68 INFLUENCE OF SUPPLEMENTAL SOYBEAN SOURCE ON RUMINAL VFA CONCENTRATIONS (Exp. 1) 69 INFLUENCE OF SUPPLEMENTAL SOYBEAN SOURCE ON RUMINAL pH AND AMMONIA CONCENTRATION (Exp. 1) . . . 71 . . . 73 A.5 IN SITU DEGRADATION OF SUPPLEMENTS (Exp. 1) A.6 INFLUENCE OF SUPPLEMENTAL SOYBEAN SOURCE ON IN SITU DEGRADATION OF FORAGE SOURCE (Exp. 1) 75 INFLUENCE OF SUPPLEMENTAL SOYBEAN SOURCE ON FEED INTAKE (Exp. 2) 79 INFLUENCE OF SUPPLEMENTAL SOYBEAN SOURCE ON PERFORMANCE (Exp. 2) 83 FEED COST AND COST/UNIT OF GAIN (Exp. 2) 91 A.7 A.8 A.9 . EFFECT OF SOYBEAN PROTEIN SUPPLEMENTS WITH LOW QUALITY ROUGHAGE ON PERFORMANCE AND DIGESTIVE CHARACTERISTICS OF WEANED BEEF STEERS REVIEW OF LITERATURE INTRODUCTION Nutritional supplementation of beef cattle has been explored throughout this century. Early studies in the 1920's and 1930's reported animal performance by simply comparing different feed sources available to researchers and producers at the time. Studies dealt with animal performance and nutrition, but they were not focused on any certain aspect such as protein or energy supplementation. In the last 30 years protein supplementation of beef cattle has been investigated more closely; and it has been shown to increase overall performance in beef cattle (Clanton and Zimmerman, 1970). Protein supplementation has increased reproductive efficiency (Clanton, 1982), live weight. gain, and milk yield in lactating cows (Lee et al., 1985). Beef cow body condition and weight losses during winter have been minimized due to protein supplementation (DelCurto et al., 1990b). The evidence for improved performance due to protein supplementation is well documented by the above researchers and numerous others. 2 THE ROLE OF SOYBEANS AS LIVESTOCK SUPPLEMENTS Soybeans are a very versatile food source for both humans and livestock. For livestock, soybeans are a very popular protein supplement because of a favorable amino acid balance, especially that of lysine which is often the first limiting amino acid in many livestock feedstuffs. in the U.S Soybeans are normally in adequate supply and are usually economical to feed. Soybeans have the potential for providing both energy and protein to ruminants (Illg and Stern, 1990). Proximate chemical composition of soybeans varies due to variety and growing conditions; but average figures report that soybeans contain 40% protein, 20% lipid, 35% carbohydrate, and 5% ash on a dry matter basis (Snyder and Kwon, 1987). The lipid fraction of soybeans is primarily composed of unsaturated fatty acids. The following table lists the amino acid and fatty acid content of soybeans. 3 Amino Acidsa % of DM Full fat soybeans Soybean meal Fatty Acidsb Isoleucine 1.77 2.27 Myristic C14 1 Leucine 3.11 4.01 Palmitic C16 11 Lysine 2.51 3.16 Stearic C18 4 Methionine 0.58 0.72 Oleic C18:1 25 Phenylalanine 2.07 2.42 Linoleic C18:2 51 Threonine 1.61 1.99 Linolenic C18:3 Tryptophan 0.60 0.68 Valine 1.98 2.40 Histidine 1.09 1.43 aIllg and Stern (1990) bSonntag (1979) % of Lipids 9 4 CLASSIFICATION Soybean protein is available in several forms. The terminology that is used to classify different soybean feedstuffs can be somewhat confusing. Raw soybeans are mature soybeans that have been harvested with no further processing following the threshing. Raw soybeans may be whole, cracked, or ground and should be referred to as such. Extruded soybeans are soybeans that have been processed through a mechanical extruder. The extruder is a screw device that rotates within a barrel to cause friction and high temperatures for a short period of time. The heated material is then forced through a restricted opening at the end of the barrel (Snyder and Kwon, 1987). Heat caused by the extrusion process is intense enough to destroy trypsin inhibitors and urease (Cheeke and Shull, 1985). Extruded soybeans are still considered full fat soybeans since little oil is lost during processing. Soybean meal is simply what is left of the soybean after the oil has been extracted, either mechanically or chemically. Mechanical extraction is performed by an expeller or screw press. Expellers subject the soybeans to high pressure which forces out the oil. Expellers are commonly used in small extraction plants throughout the world that are capable of handling a variety of oil-bearing seed crops (Snyder and Kwon, 1987). On a larger scale, solvent extraction is most commonly used. Many different 5 solvents are adequate if they are non-toxic and if they do not react with any soybean components. Today, hexane is the most commonly used solvent for extracting oil from soybean. Soybean meal is the major protein supplement used for swine and poultry diets in the U.S. (Cheeke and Shull, 1985), and it is also a traditional component of many winter range supplements for beef cattle (Hibberd and Martin, 1990). 6 FEEDING RAW SOYBEANS Feeding raw soybeans has not been a very common practice in the beef cattle and swine industries; however the dairy industry feeds a significant amount. Increased potential in milk production has required an increased energy content of dairy rations. Supplemental lipid for dairy cattle increases the energy density of the diet, allowing animals to consume required energy without consuming excessive amounts of fermentable carbohydrates (Illg and Stern, 1990). Historically this energy requirement has been met with high amounts of cereal grains. The negative effects of excess starch feeding such as acidosis and grain bloat, along with an increased availability of feed-grade fats has led to an interest in the use of fat sources to increase the energy content in rations. Soybeans, however are not recommended as an energy supplement alone because excessive intake may cause ammonia toxicity (McCormick et al., 1983). In some areas of the U.S., producers have considered using whole soybeans in livestock diets as an alternative source for protein (Erickson and Barton, 1987). The use of whole soybeans in cattle diets may be one way to reduce protein cost, salvage weather damaged soybeans and/or obtain a greater return on the soybean crop (Mader, 1988). Whole soybeans in cattle diets have also shown gains comparable to those fed soybean meal, cottonseed meal and linseed meal, 7 (Morrison 1956; Edwards et al., 1969). Raw soybeans have not been popular in nonruminant rations because of the presence of trypsin enzyme inhibitors. Trypsin inhibitors in soybeans bind to the digestive enzyme trypsin and render it inactive (Snyder and Kwon, 1987). Deleterious effects of trypsin inhibitors include decreased growth rates, poor protein digestion, pancreatic hypertrophy, and sulfur amino acid deficiencies (Liener and Kakade, 1980). Most of these effects occur in poultry or other nonruminants and the greatest effects are observed in young animals. Older animals seem to be less susceptible to trypsin inhibitors. Raw soybeans have been fed with success to gestating swine as a protein supplement resulting in similar pig survival rates compared with sows fed soybean meal (Crenshaw and Danielson, 1985). The effects of soybean trypsin inhibitors on ruminants are not fully understood. It has been shown that bovine trypsin can be inactivated by trypsin inhibitors (Liener and Kakade, 1980), but the feeding of raw soybean products has not produced deleterious effects in mature ruminants. Apparently, trypsin inhibitors are destroyed by rumen fermentation. Van Dijk et al. (1983) compared extruded soybeans with raw soybeans by feeding them to early lactating dairy cattle. No advantage to feeding extruded soybeans over raw soybeans was found. In young ruminants such as dairy calves, soybean meal has been the major source 8 of protein in starter rations. Only limited amounts of raw soybeans have been fed because of fear of its decreased utilization. Abdelgadir et al. (1984) reported that starter dairy calves had poorer growth performance on raw soybeans than on heated soybeans. Other studies with young pre- ruminant calves have not linked poor growth performance to trypsin inhibitors in raw soybeans alone (Kakade et al., 1976). 9 FATS AND RUMEN FERMENTATION Other factors that are involved in feeding full fat soybeans, either raw or extruded soybeans, are the negative effects that the lipid content of soybeans can have on performance and digestive characteristics of ruminants. Fat may be added at levels of 3 to 4% in finishing diets for cattle to control dust, hold the feed together, increase the caloric density, or to protect protein from ruminal degradation. Levels of fat in ruminant diets above 5% have generally decreased animal performance. High levels of fat in the rumen can interfere with rumen microbial function. In a review by Shirley (1986), addition of fat in ruminant diets has produced conflicting effects ranging from improved ether extract digestibility to depressed digestibility of nutrients other than fat. In some studies, addition of fats to diets of finishing cattle gave increased gain; however, a number of investigators found that fat additions to diets resulted in no improvement in gains and others actually observed a depression in weight gains (Shirley, 1986). Rumen microbes can modify dietary lipids very quickly and extensively as the lipids pass through the rumen (Byers and Schelling, 1988). Most fatty acids in conventional diets are found in the esterified form which is generally unavailable. Rumen microbes act to hydrolyze these esterified forms to free fatty acids and glycerol. This 10 hydrolysis is known as lipolysis and is performed mainly by rumen lipolytic bacteria. Protozoa have little if any lipolytic activity (Palmquist and Jenkins, 1980). Hydrolysis can be affected in several ways. First, it is a rate limiting process, in other words, only a certain amount of fatty acid can be hydrolyzed at once. This may serve to prevent the buildup of excessive amounts of free polyunsaturated fatty acids which may interfere with fiber digestion or inhibit biohydrogenation (Byers and Schelling, 1988). Lipids found in plant seeds such as soybean seeds are mainly in the form of triglycerides. Microbes can hydrolyze glyceride lipids and hydrogenate unsaturated fatty acids when limited levels of fat are present in diets. High levels may delay the action of microbes (Shirley, 1986). Two major properties of fat may influence their effect on digestion in the rumen: esterification. These are unsaturation and Unsaturated fats (usually of plant origin) are more toxic to rumen microbes than are saturated fats (usually of animal origin) (Henderson, 1973). Soybean lipid is predominantly composed of the polyunsaturated fatty acids linoleic and oleic acids. Plant oils are hydrolyzed much more extensively in the rumen than are animal oils. One reason for this may be because some plant oils may already be in the free form (unesterified) before they are fed. Hydrolysis of plant oils in oil seeds can be extensive during storage and most C1 -C6 fatty acids (volatile fatty 11 acids) are usually present in the free form (Byers and Schelling, 1988). Biohydrogenation is the next step following hydrolysis in rumen fatty acid metabolism. Hydrolysis is required first and fatty acids that escape hydrolysis usually escape biohydrogenation and subsequent rumen fermentation (Palmquist and Jenkins, 1980). Biohydrogenation is basically a multistep process which incorporates the addition of hydrogen (H) to fatty acids with double bonds (unsaturated fatty acids). The double bonds are converted to single bonds forming saturated fatty acids. The saturation is not always complete and a variety of fatty acids, both fully and non-fully saturated do result (Byers and Schelling, 1988). The process of biohydrogenation is facilitated mainly by rumen bacteria. Protozoa are also fairly active but not as important as bacteria (Moore and Christie, 1984). Other researchers, however have reported that protozoa are very important in biohydrogenation. Byers and Schelling (1988) reported that biohydrogenation is less complete with low protozoa numbers on high grain diets than with diets containing less grain. This may be because hydrolysis is also lower on high grain diets and if hydrolysis is lower, then biohydrogenation will also be lower. The most common of the saturated fatty acids formed by biohydrogenation is stearic acid (Palmquist and Jenkins, 12 1980). Perry and Macleod (1968) observed that feeding unsaturated fatty acids resulted in little deposition of linoleic acid (predominant fatty acid in soybeans) in milk fat, but that stearic and oleic acids increased. This indicates the efficiency of biohydrogenation of linoleic acid by rumen microbes. Biohydrogenation of unsaturated fatty acids is one way that microbes can dispose of excess H ions from the reducing environment of the rumen (Byers and Schelling, 1988). This could be why some studies involved in feeding lipids have reported an increase in ruminal pH (Larson and Shultz, 1970). To summarize fatty acid metabolism in the rumen: Through the process of lipolysis and biohydrogenation, a high proportion of polyunsaturated fatty acids in the diet are converted to a range of saturated fatty acids (Noble, 1984). These fatty acids are then passed on to the omasum, abomasum, and small intestine where further metabolism and absorption occur. 13 FATS AND FIBER DIGESTION The addition of fat in ruminant diets has been shown to decrease fiber digestibility by numerous researchers. Davendra and Lewis (1974) summarized 4 theories to explain this effect. 1.) Fat may physically coat fiber particles in the rumen, causing a barrier to microbial attachment. This could be beneficial from a protein standpoint. Research has shown that lipid coating of protein supplements such as oil coating soybean meal is an effective method for reducing ruminal protein degradation (Glenn et al., 1977; Davenport et al., 1987); 2.) Rumen microbial populations may be modified because of toxic effects that certain fats have on certain microorganisms; 3.) Inhibition of microbial activity from surface active effects of fatty acids on cell membranes; 4.) Reduced cation availability (for cation exchange) from formation of insoluble complexes with long chain fatty acids. The last effect could be caused by the amount of minerals in the diet which may directly affect cations available for microbial function or indirectly by affecting rumen pH (Palmquist and Jenkins, 1980). Most research supports the theories of fat having a negative effect on microbial activity (Henderson, 1973; Palmquist and Jenkins, 1980). The depression in digestibility from feeding fat has been shown to be reversed by numerous researchers by the addition of metal cations, especially that of calcium. 14 Divalent cations such as calcium (Ca") react with fatty acids to cause the formation of insoluble soaps that do not reduce ruminal digestibility of fiber (Palmquist and Jenkins, 1982). Other alkaline metal minerals capable of forming these soaps are the divalent forms of barium and magnesium (Ba" and Mg") (Palmquist and Jenkins, 1980). Preformed calcium soaps have also been the form of fat added to diets to increase energy content of rations (Jenkins and Palmquist, 1984). The formation of insoluble soaps remove fatty acids from solution so they are no longer available to bind with rumen microbes. These soaps eventually escape rumen fermentation and are passed to the lower gut where they are broken down and absorbed. One could think of these soaps as a way to protect fats to increase their escape of rumen fermentation. Several factors may limit the formation of soaps in the rumen when fat and minerals are fed separately. Two of these include type and amount of mineral fed and rumen pH (Jenkins and Palmquist, 1982). Fats can have positive effects on rumen function just by reducing the level of readily available carbohydrate needed for energy (Byers and Schelling, 1988). Supplementing with readily available carbohydrate sources such as corn or other cereal grains has been shown to decrease forage intake and digestibility (Sanson and Clanton, 1989). The levels and types of fats fed, along with mineral balance are very important in determining the 15 extent to which fat will impact fiber digestion. 16 PROTEIN SUPPLEMENTATION AND LOW QUALITY ROUGHAGE Protein supplementation of low quality roughage increases forage intake and utilization (DelCurto et al., 1990a). Increasing forage dry matter intake will result in a greater substrate flow to the rumen, which in turn may enhance microbial growth. As the forage dry matter component in the diet increases, saliva production increases and many rumen characteristics improve such as maintained rumen pH and improved microbial attachment to feed particles (Sniffen and Robinson, 1987). If the microbial population is active, this leads to improved rumen fermentation and decreases retention time of the forage. Some protein supplementation studies have not found differences in performance or forage intake and utilization. Weston and Hogan (1968), reported that supplemental protein did not increase intake of mature ryegrass hay in sheep but that grinding and pelleting the hay did. These researchers concluded that intake was simply limited by a slow passage rate of hay out of the rumen. Palatability may have been a contributing factor in this type of study as well (Grovum, 1988). Other researchers that have failed to show a response to protein supplementation have concluded that protein must not have been a limiting factor in the diet (Rittenhouse et al., 1970; Kartchner, 1980). Reduced intake of forage is often associated with diets of a crude protein content below 8%. The low protein diets 17 starve rumen microbes of nitrogen (N) which will decrease microbial health and activity. This reduction in microbial activity may lead to a decrease in forage digestibility which subsequently may lead to lower forage intake (Van Soest, 1982). Rumen microbial populations require N as ammonia (NH3), peptides, and amino acids for the synthesis of amino acids and microbial protein. Optimum microbial growth requires NH3-N levels of 2 to 5 mg /dl of rumen fluid (Satter and Slyter, 1974). Normally ruminants receive 40 to 80% of their daily protein requirements from microbial protein (Sniffen and Robinson, 1987). Protein supplementation of low quality forage diets elevates ruminal NH3-N to provide bacteria with an optimal environment for growth (Gunter et al., 1990). It has also been shown, that the efficiency of microbial protein production is low regardless of the N source supplied when low quality forage contributes over 90% of the diet (Peterson et al., 1985). 18 PROTEIN SOLUBILITY AND FORAGE UTILIZATION The types of proteins used for supplements can have an influence on fiber fermentation and microbial activity. Proteins low in soluble N content are less degradable in the rumen and by-pass the rumen in greater quantities than proteins of high soluble N content (Chalupa, 1975). Protein low in degradibility may inhibit microbial growth because N would not be available in the rumen. protein can also be detrimental. Too much degradable If there is excess degradable protein for the carbohydrate available, then microbes will waste protein by producing excess NH3 that is absorbed into the bloodstream. (Sniffen and Robinson, 1987). This may be why urea is best utilized with high carbohydrate feeds such as grains (Johnson, 1976). By-pass protein values reported for many feeds have been shown to be inconsistent (Zinn and Owens, 1983). By- pass values for soybean meal alone have ranged from 17 to 61%. The variation may be accounted for by the fact that by-pass values of protein supplements are lower when fed with high roughage diets than with high concentrate diets. High roughage diets will have slower digestion and greater retention times which may allow microbes to degrade more protein over a longer period. With this in mind one could conclude that a higher degradable protein would provide more N for rumen microbes which would subsequently increase 19 forage intake and fiber digestion. On the contrary to this theory, McAllan and Griffith (1987) supplemented steers on low quality roughage with fish meal, soybean meal, and ureacasein. The researchers found that rumen digestibility of the forage was highest when supplemented with a low degradable protein such as the fish meal. DelCurto et al. (1990c), compared a soybean-sorghum supplement with alfalfa hay and alfalfa dehydrated pellets. It was found that in situ protein degradability was lower in the dehydrated pellets and that steers supplemented with the pellets had greater basal forage intakes than steers receiving the other supplements. 20 PROTEIN DEGRADABILITY OF SOYBEAN PRODUCTS Ruminal protein degradation of soybean products is extensive. A review by Illg and Stern (1990) reports that in situ protein degradability of raw soybeans (if ground) may be as high as 90% with a disappearance rate of 20%/h. Many processing methods have been used to decrease the protein degradability. Roasting soybeans has reduced crude protein degradation to near 80%, or to a rate of 14%/h which is similar to soybean meal. Stern et al. (1985) compared in situ degradation of ground raw soybeans, soybean meal and whole soybeans that had been extruded at temperatures of 132° or 149° C. It was found in this study that ground raw soybeans were more degradable than processed soybeans. The soybean meal and extruded soybean products revealed a similar amount of N disappearance at 1 h of ruminal digestion but as time of exposure increased to 24 h, the extruded soybeans were more resistant to degradation. Extent of protein degradation over 24 hrs was greater than 95% for soybean meal and ground raw soybeans, while the extruded soybean treatments were at 60 and 77% respectively for extrusion at 132° and 149° C. As mentioned earlier, extruded soybeans are heated during processing and this heat causes considerable denaturation of protein and mallaird product formation which lowers protein degradability. As shown above, the processing method can greatly 21 affect crude protein degradability. The raw soybeans in the research above were ground for in situ analysis and this will increase crude protein degradation by allowing more surface area for microbial attachment. Estimating crude protein disappearance of whole oil seeds by in situ or in vitro methods may be misleading because of normal procedures used to prepare samples (such as grinding). Feeding the whole (unground) seeds may slow the release of nutrients during rumen fermentation (Earleywine, 1989). When the whole seeds are ground for analysis, this factor is removed. Earleywine (1989) suggests that the best analysis may be to estimate a normal level of mastication and process seeds used for analysis accordingly. When ground raw soybeans are fed in performance studies, poorer performance usually occurs when compared to whole soybeans (Davenport et al., 1987; Mader, 1988). These results may be due to the reduced impact of the oil on rumen fermentation due to its slower release from the whole seed (Earleywine, 1989). Protein release in whole soybeans may also be slower resulting in a lower degradability and an increase by-pass of the protein (Mader, 1990, personal communication). Ruminal NH3 levels may be another method used for estimating ruminal degradability of protein (Mielke and Schingoethe, 1981). Ruminal NH3 concentrations in lambs fed whole soybeans were lower than for lambs fed soybean meal 22 (Erickson and Barton, 1987). Davis and Stallcup (1967), reported lower ruminal NH3 levels in steers fed whole soybeans than in steers fed soybean meal which also suggests a lower protein degradibility in whole soybeans. Extruded soybeans have also been shown to have less degradable protein by the use of NH3 assays (Cleale et al., 1985). Davenport et al. (1987) compared low and high levels of ground raw soybeans and soybean meal supplements with corn silage to growing calves. among both treatments. Ruminal NH3 levels were similar This suggests that grinding raw soybeans will make them equally degradable in crude protein to soybean meal. It also suggests that the lipid component of the ground raw soybeans did not protect the protein from ruminal degradation. It was also found in a second trial, that the in situ rate and extent on N disappearance was highest for the ground raw soybean treatment. In the same trial, coating soybean meal with soybean oil did reduce NH3 levels but only for 3 h of incubation. Since soybean products contain a high proportion of degradable protein, it has been suggested that processing methods to reduce degradibility be implemented (Stern et al., 1985); or the inclusion of a relatively undegradable protein source to improve overall protein quality (Davenport et al., 1990). Both of these practices have been shown to improve overall performance in ruminant production schemes. 23 VOLATILE FATTY ACID PRODUCTION AND ABSORPTION With high forage diets, volatile fatty acids (VFA) provide 50 to 85% of the metabolizable energy used by ruminants (Owens and Goetsch, 1988). High roughage diets contain a high amount of cellulose, moderate amounts of soluble sugars which depends on the quality, and a low amount of starch. With these types of diets cellulolytic and saccharolytic bacteria are the most active bacteria in the rumen. Protozoa also thrive on high roughage diets. With a high roughage diet, acetate production is high. On higher concentrate diets, amylolytic bacteria thrive and the production of propionate and lactate increases. VFA are produced by specific microbial pathways and are absorbed continuously from the rumen (Owens and Goetsch, 1988). Most VFA are absorbed across the ruminal wall. Active transport is not involved, thus concentration gradients between ruminal epithelial cells, ruminal contents, and blood seem to dictate absorption. Some VFA do leave the rumen with digesta flowing to the lower gastrointestinal tract and are absorbed in the omasum (Merchen, 1988) . The rate of VFA absorption is influenced mainly by pH which is affected by the amount of free or undissociated VFA in the rumen, so concentration does indirectly affect absorption (Merchen, 1988). Absorption of VFA will 24 stabilize rumen pH. Ruminal papillae enlarge at a lower pH so as pH drops, VFA absorption may increase, however papillae surface area may be reduced. its maximum at pH 5.5. Papillae size is at pH levels lower than 5.5 usually indicate acidosis problems and papillae will be sloughed from the rumen wall causing acids to build up even more (Owens and Goetsch, 1988). With roughage diets, slow breakdown of fiber sets the pace for rumen fermentation and controls the release of easily degraded cell contents such as sugars and starches. If starches are added to roughage diets in moderation, the high fiber content causes a barrier to the rapid breakdown of starches so that the rate of VFA production and pH is lower. Under these conditions ruminal pH will remain at physiological levels (6 to 7) and most microbes thrive at this level (Owens and Goetsch, 1988). VFA concentrations are normally stable in molar ratios even though microbial populations and intake patterns change. With high roughage diets the ratios of acetate:propionate:butyrate are usually near 65:25:10. Changes from these ratios in roughage diets can be fast and unpredictable (Owens and Goetsch, 1988). 25 INDIVIDUAL VFA ABSORPTION AND METABOLISM ACETATE: Most acetate absorbed is in the blood and is carried to the liver and converted to acetyl-CoA. Acetate in this form is oxidized in the Tricarboxylic acid (TCA) cycle or used for fatty acid synthesis. Acetate is the main precursor for fat synthesis in ruminant tissues because glucose can supply only limited quantities of acetyl-CoA for fatty acid synthesis (Fahey and Berger, 1988). PROPIONATE: epithelium. Propionate is absorbed through the ruminal Two to five percent of propionate is converted to lactic acid and the rest reaches the liver where it is either oxidized to propionyl-CoA or converted to glucose. Propionyl-CoA is eventually converted to succinyl-CoA so it can enter the TCA cycle (Fahey and Berger, 1988) BUTYRATE: Butyrate is converted mostly to ketones during absorption through the ruminal epithelium. B- hydroxybutyric acid (B-HBA) accounts for more than 80% of the ketones formed. B-hydroxybutyric acid is oxidized in cardiac and skeletal muscle, and is used for fatty acid synthesis in adipose and mammary gland tissue (Fahey and Berger, 1988). 26 LIPID CONTAINING FEEDS AND VFA Lipid containing feeds fed to ruminants have been shown to change the proportions of VFA produced in the rumen (Shaw and Ensor, 1959). The researchers reported that feeding cod liver oil, oleic acid, and linoleic acid when given orally to dairy cows on normal diets decreased the ratio of acetic to propionic acid in the rumen. Total VFA was also increased with linoleic acid, the predominant fatty acid in soybeans, having the greatest affect. Brown et al. (1962) observed that changes in VFA production by adding fat to the diet were more noted on low roughage than high roughage diets. They also found an increase in valeric concentrations when fat was added. Proportions of valeric and isovaleric acids have also been increased by feeding soybean meal coated with soybean oil when compared to whole soybeans and soybean meal alone (Larson and Shultz, 1970). Perry and Macleod (1968) reported that feeding ground raw soybeans in diets with a forage to concentrate ratio of 1:1 or higher did not affect the proportions of VFA production. What was noted was a lower pH with the soybean diet for up to 6 hrs post feeding which suggested an increased VFA production and/or slower absorption. and Macleod's work, In contrast to Perry Davis and Stallcup (1967) found that total VFA production was lower with raw soybeans when compared to soybean meal and corn gluten meal. This accounted for a higher rumen pH on the raw soybean 27 treatment. It was concluded in this study that since acid production has an effect on pH, pH would be expected to remain higher on the raw soybean treatment. Other researchers have not found a difference in VFA production due to feeding full fat soybean sources compared to non lipid protein sources (Palmquist and Conrad, 1971; Stern et al., 1985; Keele et al., 1989). However, Keele et al. (1989) did report an increase in molar proportion of propionic acid and a decreasing proportion of butyric acid when whole cottonseeds were fed in comparison to extruded soybeans. They concluded that the increased intake of long chain fatty acids in the cottonseed meal could have caused rumen protozoa to decrease. Annexstad et al. This agrees to some extent with (1987) who actually found an increase in butyrate proportions when extruded soybeans were fed at high levels in dairy concentrate rations. 28 PERFORMANCE STUDIES AND SOYBEAN SUPPLEMENTS Numerous studies have reported different results on performance when full fat soybeans are utilized in rations. Many conditions may dictate whether or not these supplements are to be used. The most common rations that have utilized full fat soybeans have been dairy and feedlot rations. The overall consensus among researchers has been that raw soybeans or full fat soybeans are good protein supplement alternatives if economic conditions allow (McCormick et al., 1983; Van Dijk et al., 1983; Erickson and Barton, 1987; Mader, 1988). Mader (1988) compared soybean meal, rolled raw soybeans, and whole raw soybeans as supplements to growing steers consuming corn silage. The raw soybean treatments were fed at 2 levels, 10 and 20% of the diet. Dry matter intakes and average daily gains were lower for steers consuming the raw soybean treatments, however, feed efficiency tended to be improved. Mader concluded that the fat content of soybeans was responsible for the depressed intake but the depression of intake did not differ whether the soybeans were fed at 10 or 20% of the diet. raw soybeans did not improve animal performance. Rolling the Steers fed the whole soybeans gained faster and more efficiently suggesting a more efficient utilization of protein in the whole soybeans. McCormick et al. (1983) conducted several trials 29 comparing rolled whole soybeans against soybean meal in silage based rations to growing calves. Calf performance was not different in this trial, but in a second trial, roasting whole soybeans did improve calf gains by 14% over cottonseed meal controls. The researchers concluded that rolled raw soybeans may be substituted for conventional protein supplements on an equal protein basis. Conflicting reports exist on the effects of full fat soybeans on DMI and performance. Studies comparing extruded soybeans to raw soybeans have mainly shown no differences in DMI (Block et al., 1981; Van Dijk et al., 1983; Stern et al., 1985). When raw soybeans have been compared to soybean meal, differences in intake have been reported (Palmquist and Conrad, 1971; Erickson and Barton, 1987), and have not been reported (Perry and Macleod, 1968; Stern et al., 1985). Negative impacts on performance and intake due to feeding full fat soybeans have in large been blamed on the lipid component of full fat soybeans (Erickson and Barton, 1987). Many of the studies reported above involved feeding high levels of soybeans in rations high in concentrates such as dairy or feedlot rations. Factors affecting intake may have possibly been due to excess starch in the diets and not fat. 30 ECONOMICS The equation below can be used to determine when whole soybeans may be economical to feed in substitution of soybean meal on an equal protein basis: X = soybean meal cost/ton 38.58 X = market price of soybeans source: McCormick et al. (1983). This equation is derived by using 44% soybean meal. McCormick et al. (1983) state that 114 pounds of whole soybeans are needed to equal the amount of protein in 100 lbs of soybean meal containing 44% crude protein. The numerical value of 38.58 is derived by dividing 44 by 114 and then multiplying by 100. This value is also the approximate percentage of crude protein in whole soybeans. When the market price of whole soybeans (bushel price) falls below the figure derived by the above equation, whole soybeans may be economical to feed. This equation needs to be manipulated when protein values of soybean meal and whole soybeans vary from the above values. 31 SUMMARY The incorporation of supplemental protein into ruminant diets composed of low quality roughage is important in improving the utilization of the forage component. Soybeans are considered a high quality protein source, however, soybean protein is highly degradable in the rumen. Feeding soybeans whole, or as extruded soybeans will decrease CP degradation compared to feeding ground soybeans or soybean meal. Soybeans contain approximately 20% lipid and should be fed at a level that will not allow the entire diet to exceed 5% lipid. The highly unsaturated fatty acid composition of soybean lipid may interfere with ruminal microbial activity subsequently interfering with digestion, especially that of cellulose. Performance studies with beef cattle report that whole soybeans can be fed as protein supplements in moderate levels, exhibiting similar animal performance as soybean meal or other traditional protein supplements. The decisive factor in determining what to feed should be cost and availability of supplements. Feeding full fat soybeans has been shown to be adequate for cattle consuming moderate to high quality roughage. Since lower quality roughages are abundant, they exhibit a strong potential as inexpensive feed sources. The main question that arises is whether or not full fat soybeans can 32 be incorporated into low quality roughage diets for cattle without exhibiting deleterious effects on performance. 33 COMPARISON OF WHOLE SOYBEANS, EXTRUDED SOYBEANS OR SOYBEAN MEAL/BARLEY ON DIGESTIVE CHARACTERISTICS AND PERFORMANCE OF WEANED BEEF STEERS CONSUMING MATURE GRASS HAY. INTRODUCTION Whole soybeans (WSB) have been considered by many producers in the U.S., as an alternative source of supplemental protein. The use of WSB in substitution of soybean meal supplements for growing cattle has been shown to provide similar gains (Edwards et al., 1969), and can be economical (McCormick et al., 1983; Mader, 1988). Depression in DMI has often been associated with feeding full fat soybeans to ruminants in comparison to soybean meal (Palmquist and Conrad 1971; Erickson and Barton 1987). Most research in feeding full fat soybeans to ruminants has been conducted using high quality feeds, such as feedlot and dairy diets. Research involving the use of full fat soybeans with low quality roughage diets is lacking. Low quality roughage, such as crop residues, are abundant and can provide an economical source of feed if strategies to effectively utilize them are implemented. It is well documented by previous research that protein supplementation will increase the intake and utilization of low quality roughage (McAllan and Griffith 1987; Sniffen and Robinson 1987; DelCurto et al., 1990a). The purpose of the present study, therefore was to compare the effects of three 34 different soybean supplements on the intake, utilization, and performance of beef steers fed with a low quality grass hay. 35 MATERIALS AND METHODS Exp. 1: Digestion study. Four ruminally cannulated steers (average wt 255 kg) were assigned to the following four treatments in a 4X4 Latin Square design: 1) Control, no supplement; 2) 1.5 kg of whole soybeans (WSB); 3) 1.36 kg of extruded soybeans (ESB); and 4) 1.48 kg of a 62% soybean meal 38% rolled barley mixture (SBM+BAR). The formulation of the rations were isonitrogenous, supplying approximately .43 kganimalld4. Ground mature native cool season grass hay produced in the Willamette Valley of Western Oregon (Table 1) supplied the basal forage component. Steers were individually housed in 3mX6mpens and had access to water and a two to one mixture of trace mineralized salt' and dicalcium phosphate. Each period of the Latin square consisted of a 14 d adaptation period followed by a 7 d intake and fecal collection period. On days 15 through 21 at 0800, hay orts were weighed and sampled at 10% of the weight. Supplement was offered in separate feed bunks at this same time. After sampling orts, ground hay was offered at 120% of the previous day's as-fed forage intake. Samples of 100 g each of hay and supplements were taken daily. On d 14 at 1500, fecal collection bags were placed on the steers. On days 15 'The trace mineralized salt consisted of not less than 95% salt, .35% Zn, .3% Mn, .23% Fe, .023% Cu, .012% I, .006% Co, and .009% Se. 36 through 21 at 1500, total fecal collections were weighed and sampled at 2.5% of the daily fecal output. Ort, feed, and fecal samples were dried at 50° C for 72 h in a forced air drying oven for DM determination. All the above samples were composited by period, ground to pass a 1 mm screen and analyzed for DM and ash by standard procedures (AOAC, 1984). Feed and ort samples were analyzed for CP by the Macro Kjeldahl method (AOAC, 1984). Acid detergent fiber and NDF were analyzed for all samples (excluding ADF for fecal samples), as described by Goering and Van Soest (1970), but modified by a micro method described by Waldern (1971) . In situ rate and extent of protein degradation were determined for WSB, ESB, and SBM+BAR with methods described by Orskov (1982). One g samples of each supplement that had been ground to pass a 2 mm screen were placed in nitrogen (N) free dacron bags2 (5 cm X 10 cm). The bags had pore sizes of 53 A (± 10) and were placed in the rumen of steers on the respective treatment. Bags with sample, and empty bags used as blanks were allowed to digest for 24, 18, 12, 9, 6, and 3 h. In situ rate and extent of fiber digestion were also determined. Four g samples of the basal hay that had been ground to pass a 2 mm screen were also placed in dacron bags (10 cm X 20 cm). 2Ankom, Fairport, NY. Bags were placed in the rumen 37 of all steers and allowed to digest for 96, 72, 48, 36, 24, 18, 12, and 6 h. All bags were removed at the same time on day 22, rinsed thoroughly, and dried for 72 h at 50° C. Dry weights were recorded to determine dry matter disappearance. Residual N was determined by the Macro Kjeldahl method for bags with remaining supplement and for the blanks to account for microbial N attachment. Residual NDF was determined for hay samples by a micro method as described by Waldern (1971). Rate and lag time of digestion was calculated using log transformations and linear regression as described by Mertens and Loften, (1980). On d 22 of each period a ruminal profile was performed. Rumen fluid samples were collected at 0, 3, 6, 9, and 12 h post-supplemental feeding. Rumen fluid samples were transported to the laboratory where pH was determined using a combination electrode. Fluid was frozen at -20° C following treatment with 25% metaphosphoric acid and .1N HC1 in a 1:1 dilution for VFA and NH3 analysis respectively. Ruminal VFA concentrations were determined by gas chromatography using a fused silica capillary column3 in a gas chromatography Ruminal NH3 concentration was analyzed by quantitative enzymatic determinations using a narrow- 3Alltech Associates, Inc., Deerfield, IL. 45890 Series II gas chromatograph. Hewlett Packard Company, Analytical group, San Fernando, CA. sSigma Diagnostics. St. Louis, MO. 38 bandwidth UV spectrophotometer.° On d 23 ruminal contents were evacuated at 0 (prefeeding) and 5 h post-supplemental feeding to measure digesta kinetics. Ruminal contents were weighed, mixed, sub-sampled, and immediately replaced in the steers. Sub- sampled ruminal contents were subsequently dried at 50° C for 72 h to determine DM percentage and DM fill. Ruminal evacuation samples were ground to pass a 1 mm screen and saved for analysis of indigestible ADF (IADF) as described by Berger et al., (1979) and Ellis et al., (1984). IADF was measured as an internal marker to determine passage rate and flow rate. Statistical analysis: Data pertaining to intake, digestibility, and in situ rate and extent of digestion were analyzed as a latin square design with effects for treatment, using the general linear model procedure of Statistical analysis systems (SAS, 1985). model were steer, period, and treatment. Terms in the Digesta fill variables and fermentation characteristics were analyzed as a latin square design, split plot in time with respect to sampling times. Terms in the model were steer, period, treatment, steer x period x treatment, time, and treatment x time. Dependent variables which displayed a treatment x time interaction were analyzed within time periods, and are °Model UV 160 Shimadzu corp., Kyoto, Japan. 39 presented graphically. Differences among treatments for all variables above were noted by predetermined contrasts for 1) Control vs supplemented treatments, SBM+BAR, and Exp. 2: 2) WSB and ESB vs 3) WSB vs ESB. Performance study. Forty British X Exotic weaned steer calves (avg BW, 250 kg) were utilized in a 112 d performance trial comparing the use of different forms of soybean supplements on low quality mature grass hay (CP = 6.5%). Steers were stratified by weight and within stratum allotted randomly to two replications of four treatments. Treatments consisted of 1) Control, no supplement; 2) 1.5 kganima14d4 of whole soybeans (WSB); 3) 1.36 kganima144,d4 of extruded soybeans (ESB); and 4) 1.48 kganima14d4 of a mixture of 62% soybean meal and 38% rolled barley grain (SBM+BAR). Supplements were formulated to be isonitrogenous and supply approximately .43 kganima14d4 of CP. Approximately 71% of the CP requirement (NRC, 1984) supplied by the supplements. was Steers were fed a basal diet of ground hay which consisted primarily of native cool season mature grass hay produced in the Willamette valley of Western Oregon (table 1). A two to one mixture of trace mineralized salt and dicalcium phosphate was offered free choice for the entire trial. A 10 d adaptation period was used to allow the steers to adjust to the diets prior to the initiation of the experiment. Steers were weighed at 28 d intervals during the trial 40 to determine average daily gain and feed efficiency. Weights, for two consecutive days, were recorded and averaged at the beginning and ending of the trial. On the second weigh day at the beginning of the trial, steers were injected with 5 ml of Ivermectin7 for parasite control. Steers were housed in semi-enclosed pens and fed both supplement and hay in large wooden bunks. Each day at 1600, orts from previous feedings were pushed to the rear of the bunks and supplement was offered. Steers were allowed approximately 15 min to consume the supplement. Ground hay was subsequently weighed, recorded, and offered ad libitum post supplemental feeding. Hay orts were removed from the bunks once weekly, weighed, recorded, and sub-sampled. A 100 g sample of the ort sub-sample was then dried at 100° C for 24 h for DM determination. Basal hay and supplements were also sub- sampled at 100 g weekly. These samples were dried at 55° C for 48 h for DM determination and then composited across each period of the trial. Dry matter values of the basal hay, orts and supplement were used to determine total and forage DMI. Composited samples were ground to pass through a 1 mm screen and saved for laboratory analysis. Dry matter, ash, and CP were determined by standard procedures (AOAC, 1984). Acid detergent fiber and NDF was determined as 7MSDAGVET Division of Merck & Co., Inc., Rathway, NJ. 41 described by Goering and Van Soest (1970) but modified by a micro method as described by Waldern (1971). Statistical Analysis. All data pertaining to Intake, gain and feed efficiency were analyzed as a completely randomized design with effects for treatment, using the general linear model procedure of Statistical Analysis Systems (SAS, 1985). The experimental unit was each pen of animals. Contrast statements were pre determined for 1) Control vs supplemented treatments, 2) WSB and ESB vs SBM+BAR, and 3) WSB vs ESB. 42 RESULTS AND DISCUSSION Experiment 1: Digestion study. Supplemental protein did not affect forage DMI, however steers supplemented with WSB had a higher forage DMI than steers on ESB (P <.10). Other research has not reported differences in forage DMI when comparing WSB and ESB (Block et al., 1981; Van Dijk et al., 1983: Stern et al., 1985). supplementation (P <.10). Total DMI was increased by The increase may have been an additive effect, but voluntary intake of feed can be influenced by N in the diet (Van Soest, 1982). Apparent DM digestibility was increased by protein supplementation (P <.10). Within soybean supplements, soybean protein source had no effect on DM or NDF digestibility (P >.10). This is in agreement with previous research (McCormick et al., 1983; Stern et al., 1985). Other researchers have reported a decrease in DM digestibility when raw soybeans were compared to soybean meal supplements and have concluded that the fat content was the cause (Palmquist and Conrad, 1971; Erickson and Barton, 1987). Since NDF digestibility was not affected by treatment, it may support the theory that adequate fiber content in the diet may reduce the negative impact of fat on fiber digestibility (Erickson and Barton, 1987). In this study NDF digestibility was lower than DM digestibility, which disagrees with other studies supplementing very low quality roughage with protein 43 (DelCurto et al., 1990c). It does however support other studies using different soybean protein supplements (McCormick et al., 1983; Erickson and Barton, 1987) with higher quality roughage such as corn silage. Forage quality and fiber content is likely to be more important in this situation. The NDF content in this study was apparently not high enough to exhibit a higher digestibility than that of DM. With feeding very high fiber diets, microflora populations may be more efficient at digesting fiber than if the content was lower. A treatment X time interaction was noted for ruminal DM fill estimated by ruminal evacuations (P < .10). No differences in DM fill were observed at 0 h post supplementation (PS) but at 5 h PS, supplemented treatments had a greater DM fill by nearly 50% (P <.10). This agrees with DelCurto et al. (1990c), who also observed increases in DM fill due to protein supplementation. Full fat soybeans also had a greater DM fill when compared to SBM+BAR (P <.10). This is because forage intake was the greatest for steers consuming WSB. An increase in IADF intake was also reflected by this for steers consuming WSB. Protein supplementation did not influence IADF fill or passage rate (percentage per h); (P <.10), but WSB vs ESB had a greater IADF flow (grams per h) (P <.05). The IADF flow rate is a ratio between IADF intake and time, where IADF passage rate is the percentage of IADF fill leaving the rumen per unit of 44 time. This may explain why steers supplemented with WSB had a greater IADF flow rate but exhibited no differences in IADF passage rate. Fermentation characteristics: supplementation (P <.10). Ruminal pH was reduced by This is explained due to an increase in total VFA concentration on supplemented treatments (P <.10). Individual molar proportions of acetate were decreased by supplementation (P <.10). The ratio of acetate to propionate was not affected (P >.10) but numerically the ratio did decrease slightly due to supplementation. Isobutyrate and isovalerate proportions were increased by supplementation (P <.10). Butyrate, although not showing a treatment X time interaction, affected by supplement source. was Butyrate molar proportion was higher on SBM+BAR (P <.05) than when compared to WSB and ESB. This is in agreement with Keele et al. (1989), who reported decreased proportions of butyrate when ESB were fed to non-lactating cows. The researchers concluded that ruminal protozoa numbers may have been decreased by an increased intake of long chain fatty acids in the ESB. contrast to the above, Annexstad et al. In (1987) actually found an increase in butyrate production when high levels of ESB were fed in dairy concentrate rations. Valerate was the only VFA to exhibit a treatment X time interaction (P <.10; Fig. 1). Valerate was higher at all collection times for supplemented treatments (P <.05). 45 SBM+BAR increased valerate production when compared to WSB and ESB at 3, h post feeding (P <.05). This is in partial agreement with Larson and Schultz (1970), who found increasing proportions of isovaleric and valeric acids when soybean meal coated with soybean oil was compared against whole soybeans. This could however, negate the theory of long chain fatty acids causing a reduction in VFA production. Other studies comparing different soybean supplements have failed to report differences in ruminal VFA production (Van Dijk et al.,1983; Stern et al. 1985; Perry and Macleod, 1968). Ruminal ammonia exhibited a treatment X time interaction (P <.10; Fig 2). Protein supplementation increased ruminal ammonia levels at all sampling times (P <.05). Ammonia production peaked at 3 h post supplementation for all supplement treatments, with SBM+BAR having a higher ammonia release at 3 h than full fat soybeans (P <.05). Ammonia levels during h 6 to 12 remained similar among supplemented treatments. ESB had a more uniform release of ammonia throughout the 12 h period. WSB were also more uniform in ammonia release than was SBM+BAR. Other studies have reported slower ammonia release in ESB when compared to soybean meal (Cleale et al., 1985) and in WSB compared to soybean meal (Davis and Stallcup, 1967; Erickson and Barton, 1987). These data suggest that WSB are less degradable than soybean meal if fed in the physically 46 whole state. In this study, the physical form of the soybean supplement due to processing may have been more important in affecting ammonia production than was the lipid fraction of the supplement. Davenport et al. (1987), reported similar ammonia release values for ground raw soybeans as soybean meal. In situ dacron bag experiment: Rate (%/h) and lag time (h) of supplement DM disappearance was not different (P >.10; Table 5). Extent of supplement DM disappearance was 17.7% greater for WSB than ESB (P <.10) and tended to be higher for WSB than SBM+BAR (P =.14). WSB for this experiment were ground < 2 mm in diameter, which allowed more surface area for microbial attachment, thus enhancing DM disappearance. Rate, lag time, and extent of CP disappearance did not differ among treatments (P >.10). Numerically, SBM+BAR tended to exhibit nearly a twofold increase in rate of CP disappearance than did WSB and ESB (P =.16). This suggests that the lipid component may have aided in slowing the rate of CP disappearance. Whole soybeans tended to have a higher extent of CP disappearance than ESB (P =.13). agreement with Stern et al. This is in (1985) who reported that ground raw soybeans exhibited a higher extent of CP degradation than did processed soybeans. Processing methods of the supplements are most likely the reason for differences in this study. Extruded soybeans undergo considerable heating during processing, and this could explain why they were 47 lowest in rate and extent of CP disappearance. The high extent of CP disappearance in WSB supports research reported by Illg and Stern (1990), who reported a CP disappearance of near 90% in WSB. This may be because the WSB were ground for in situ analysis. Crude protein disappearance of whole oil seeds by in situ or in vitro methods may be an overestimate because of normal procedures used to prepare samples, such as grinding. Feeding the whole or unground seeds may slow the release of nutrients during rumen fermentation (Earleywine, 1989). When the whole seeds are ground for the respective analysis, then this factor is removed. It has been suggested that the best analysis may be to estimate a normal level of mastication and process seeds used for analysis accordingly (Earleywine, 1989). Lag time of forage DM and NDF disappearance was increased by supplementation (P <.10), while rate of NDF disappearance tended to decrease (P =.10) due to supplementation. No differences in extent of forage DM or NDF disappearance were found among any treatments suggesting that supplement source had no effect on ruminal digestibility of the forage component. This was also found true for total tract digestibility. Experiment 2: Performance study. Forage DMI was similar among treatments, however, WSB and ESB exhibited a trend to lower forage DMI compared to SBM+BAR (P =.11) (Table 6.). This agrees with previous research that reported depressed 48 DMI when full fat soybeans are fed in comparison to soybean meal supplements (Palmquist and Conrad, 1971; Erickson and Barton, 1987; Mader, 1988). Differences in forage DMI between WSB and ESB were not found which agrees with previous research (Block et al., 1981; Van Dijk et al., 1983; Stern et al., 1985). The slight decrease in forage DMI may have been due to the increased caloric density from the lipid portion of the full fat soybeans (Davenport et al., 1987). The lipid portion in the full fat soybean treatments was estimated to be less than 4% of the daily DMI. Levels of fat above 5% have been associated with reduced performance in ruminants (Shirley, 1986). Since steers consuming the control diet did not have depressed forage DMI, it may suggest that the animals were only marginally deficient in N intake (Table 7.). It was observed that Control steers consumed more forage during the first 4 weeks of the experiment than did steers on WSB or ESB treatments. During the second half of the trial, control steers began to consume less forage as the trial progressed. This suggests that deleterious effects caused by lack of protein in the control treatment took a period of time to be exerted. Steers on this experiment were apparently not in a negative protein balance during the first 4 weeks of the trial. Other research that reports no influence in intake due to protein supplementation has suggested that protein may not have been a limiting factor 49 in the diets (Rittenhouse et al., 1970; Kartchner, 1980). Protein supplementation increased average daily gain by more than twofold (P <.05) and feed efficiency by nearly twofold (P <.05; table 6.). Average daily gains were not influenced by source of soybean protein (P >.10), but steers on ESB tended to exhibit better feed efficiency over steers on WSB (P =.10). The more efficient gains by ESB may reflect a lower amount of degradable crude protein. ESB are less degradable in CP content than are WSB and soybean meal (Stern et al.,1985) and this should translate into better performance (Davenport et al., 1990). The data above support the practice of substituting full fat soybeans for soybean meal supplements when it is economical. (McCormick, 1983; Van Dijk et al., 1983; Mader 1988). It also suggests that full fat soybeans can be incorporated into growing diets for cattle consuming low quality roughage. 50 IMPLICATIONS Full fat soybeans, fed at the above levels, may be used with little if any deleterious effects on performance and digestibility. In this study, the oil component of the rations did not exceed 4% of the total daily DMI. Levels of fat above this may exert negative effects on performance and digestibility in ruminants. This study indicates that full fat soybeans can be effectively utilized in growing cattle diets consisting of low quality roughage when economic conditions allow. It also supports numerous reports of increased animal performance, forage digestibility, and utilization due to protein supplementation. FIGURE 1. INFLUENCE OF SOYBEAN PROTEIN SOURCE ON RUMINAL VALERATE CONCENTRATIONS (Exp. 1) Treatment ---WSB -HESB 'SBM+BAR 'CONTROL R 0.6.,_______,-1.---0.50 3 ...........-.."".-.--.-"-----.-.. 6 9 12 hours post-supplementation Control vs supplemented treatments differ (P <.05); all Full fat soybeans vs SBM+BAR differ (P < .05); hours. hour 3. FIGURE 2. INFLUENCE OF SOYBEAN PROTEIN SOURCE ON RUMINAL AMMONIA CONCENTRATIONS (Exp. 1) 25 Ts 20 0) Treatment E15 WSB 0 10 ESB E trz -*SBM+BAR 5 0 CONTROL 3 6 9 12 hours post-supplementation Control vs supplemented treatments differ (P <.05); all hours. Full fat soybeans vs SBM+BAR differ (P <.05); hour 3. WSB vs ESB differ (P <.05); hour 0. N 53 TABLE 1. CHEMICAL COMPOSITION OF GROUND MATURE GRASS HAY AND TREATMENT SUPPLEMENTS' (Exp. 1) Item Ground hay WSB ESB SBM+BAR DM 93.55 95.57 95.86 94.07 ASH 7.84 5.63 5.56 5.79 OM 92.17 94.38 94.44 94.21 CP 6.56 35.44 36.22 36.08 NDF 68.17 25.92 17.62 19.46 ADF 40.57 19.42 10.83 8.18 IADFb 12.97 2.12 2.21 1.88 'All values are reported on a percentage of dry matter. bIndigestible ADF. 54 TABLE 2. INFLUENCE OF SUPPLEMENTAL WHOLE SOYBEANS (WSB), EXTRUDED SOYBEANS (ESB), AND SOYBEAN MEAL-I-BARLEY (SBM+BAR) ON DM INTAKE AND APPARENT DIGESTIBILITY' (Exp. 1) Item CONTROL WSB ESB SBM+BAR SE Forageb 1.58 1.65 1.31 1.45 .106 Totalb'` 1.58 2.14 1.77 1.93 .106 DMD%` 53.96 57.87 59.16 60.57 1.28 NDFD% 50.62 52.09 47.62 49.99 1.21 DMI, %BW Total tract Digestibility aDMD = DM digestibility; NDFD = NDF digestibility. bWSB vs ESB treatments differ (P <.10). `Control vs supplemented treatments differ (P <.10). 55 TABLE 3. INFLUENCE OF SUPPLEMENTAL WHOLE SOYBEANS (WSB), EXTRUDED SOYBEANS (ESB), AND SOYBEAN MEAL+BARLEY (SBM+BAR) ON RUMINAL KINETICS' (Exp. 1) Item CONTROL WSB ESB SBM+BAR SE 0 h PS 1.41 1.61 1.40 1.31 .145 5 h PSb'c 1.82 2.42 2.08 1.94 .130 Ruminal IADF .563 .628 .502 .563 .017 1.14 1.26 1.12 1.13 .061 0 h PS 2.78 2.36 2.47 2.24 .288 5 h PS 2.16 1.94 2.02 1.98 .211 23.40 26.12 20.88 23.40 .689 Ruminal DM fill, %BW Intake, kg4 Ruminal IADF fill, kg Ruminal IADF passage, %/h Ruminal IADF flow g/hd 'Indigestible acid detergent fiber (IADF) was used to describe an indigestible fiber component of the diet. Ruminal DM fill, IADF fill, and IADF passage values were obtained from rumen evacuations 0 and 5 h post supplementation (PS). bControl vs supplemented treatments differ (P < .10). cFull fat soybeans vs SBM+BAR treatments differ (P<.10). dWSB vs ESB treatments differ (P < .05). 56 TABLE 4. INFLUENCE OF SUPPLEMENTAL WHOLE SOYBEANS(WSB),EXTRUDED SOYBEANS (ESB), AND SOYBEAN MEAL+BARLEY (SBM+BAR)ON RUMEN pH AND VFA CONCENTRATION' (Exp. 1) Item CONTROL WSB ESB SBM+BAR SE pie 6.59 6.23 6.37 6.39 .081 Total VFA mMb 96.56 108.92 109.56 108.78 3.35 Acetate M%b 71.25 70.12 68.83 67.98 .654 Propionate M% 17.67 17.63 18.98 18.25 .714 Isobutyrate M%b .533 .797 .686 .839 .055 Butyrate M%c 9.49 9.37 9.78 10.86 .256 Isovalerate M%b .488 1.23 .896 1.14 .128 A:P ratio 4.08 3.99 3.67 3.75 .182 'Data presented above did not display a treatment x time interaction (P > .10) and were averaged across time periods. bControl vs supplemented treatments differ (P < .10). `Full fat soybeans vs SBM+BAR differ (P < .05). 57 TABLE 5. IN SITU DEGRADATION OF TREATMENT SUPPLEMENTS AND INFLUENCE OF SUPPLEMENT SOURCE ON IN SITU DEGRADATION OF GROUND MATURE GRASS HAY (Exp. 1) Item CONTROL WSB ESB SBM+BAR SE 3.96 3.87 4.08 3.67 3.96 3.08 .194 .283 3.76 4.20 2.50 3.89 3.41 7.52 .884 1.52 97.85 97.09 83.12 79.44 85.93 88.28 4.22 5.97 CONTROL WSB ESB SBM+BAR SE 3.73 3.30 3.93 3.58 3.94 3.62 3.83 3.53 .064 .105 1.30 2.00 1.11 1.66 1.11 1.62 1.19 1.70 .097 .154 64.68 62.57 60.82 61.01 61.53 61.01 63.33 62.28 1.77 1.52 Supplement Disappearance Lag time, h DM CP' Rate of digestion, %/h DM CP' 24 h extent of digestion, % DMb CP' Forage Disappearance due to supplement Lag time, h DMd NDFd Rate of digestion, %/h DM NDF' 96 h extent of digestion, % DM NDF 'Full fat soybeans vs SBM+BAR exhibit a trend for differences (P >.10 <.16). bWSB vs ESB differ (P <.10). `WSB vs ESB exhibit a trend for differences (P =.1277). dControl vs supplemented treatments differ (P <.10). `Control vs supplemented treatments differ (P =.10). 58 TABLE 6. INFLUENCE OF WHOLE SOYBEANS (WSB), EXTRUDED SOYBEANS (EBB), AND SOYBEAN MEAL+BARLEY (SBM+BAR) ON DM INTAKE AND PERFORMANCE OF WEANED BEEF STEERS' (Exp. 2) Item CONTROL WSB ESB SBM+BAR SE Forage" 6.10 6.12 6.24 6.66 .42 Total"` 6.10 7.45 7.54 7.95 .42 ADGc .40 .99 1.09 1.13 .13 F/Gc.d 14.65 7.55 6.89 7.04 .22 DMI, kg. d-1 Performance 'ADG = Average daily gain; F/G = Feed/Gain ratio. "Full fat soybeans vs SBM+BAR exhibit a trend for decreased DM intake by full fat soybeans (P =.11). cControl vs supplemented treatments differ (P <.05). dWSB vs ESB differ (P =.10). 59 TABLE 7. CHEMICAL COMPOSITION OF FEEDSTUFFS2 (Exp. 2) Item Ground Hay WSB ESB SBM+BAR DM 93.08 92.86 95.12 91.03 CP 6.53 35.45 36.86 36.77 ASH 7.22 4.63 5.10 5.06 ADF 41.39 17.35 10.03 7.37 NDF 67.04 23.25 16.33 18.69 'All values are reported on a percentage of dry matter. 60 LITERATURE CITED Abdelgadir, I.E.O., J.L. Morril, J.A. Stutts, M.B. Morril, D.E. Johnson and K.C. Behnke. 1984. Effect of processing temperature on utilization of whole soybeans by calves. J. Dairy Sci. 67:2554. Annexstad, R.J., M.D. Stern, D.E. W.P. Hansen. 1987. Extruded meal as supplemental protein dairy cattle. J. Dairy Sci. Otterby, J.G. Linn and soybeans and corn gluten sources for lactating 70:814. AOAC. 1984. Official Methods of Analysis (14th Ed.). Association of Official Analytical Chemists, Washington, DC. Berger, T. Klopfenstein, and R. Britton. 1979. Effect of sodium hydroxide on efficiency of rumen digestion. J. Anim. Sci. 49:1317. Block, E., L.D. Muller, L.C. Griel jr. and D.L. Garwood. 1981. Brown mid rib-3 corn silage and heat treated extruded soybeans for early lactating dairy cows. J. Dairy Sci. 64:1813. Brown, W.H., J.W. Stull and G.H. Scott. 1962. Fatty acid composition of milk. I. Effect of roughage and dietary fat. J. Dairy Sci. 45:191. Byers, F.M. and G.T. Schelling. 1988. Lipids in ruminant nutrition. In: D.C. Church (Ed.). The Ruminant Animal. pp 298-312. Prentice Hall, Englewood Cliffs, NJ. Chalupa, W. 1975. Rumen bypass and protection of proteins and amino acids. J. Dairy Sci. 58:1198. Cheeke, P.R. and C.R. Shull. 1985. Natural Toxicants in Feeds and Poisonous Plants. pp 235-237. Avi Pub. Co. Inc., Westport, CT. Clanton, D.C. and D.R. Zimmerman. 1970. Symposium on pasture methods for maximum production in beef cattle: Protein and energy requirements for female beef cattle. J. Anim. Sci. 30:122. Clanton, D.C. 1982. Crude protein in range supplements. In: F.N. Owens (Ed.). Protein requirements for cattle: Symposium. Oklahoma State Univ. MP 109:228. 61 Cleale, R., T. Klopfenstein, J. Merril, M. Nelson and W. Stroup. 1985. Heat treated soybean products for growing beef cattle. 1985 Nebraska Beef Cattle Rep., pp 55-57. Univ. of Nebraska, Lincoln. Crenshaw, M.A. and D.M. Danielson. 1985. Raw soybeans for gestating swine. J. Anim. Sci. 60:163. Davendra, C. and D. Lewis. 1974. The interaction between dietary lipids and fibre in the sheep. Anim. Prod. 19:67. Davenport, G.M., J.A. Boling, N. Gay and L.D. Bunting. 1987. Effect of soybean lipid on growth and ruminal nitrogen metabolism in cattle fed soybean meal or ground whole soybeans. J. Anim. Sci. 65:1680. Davenport, G.M., J.A. Boling and N. Gay. 1990. Performance and plasma amino acids of growing calves fed corn silage supplemented with ground soybeans, fish meal and rumen protected lysine. J. Anim. Sci. 68:3773. Davis, G.V. and O.T. Stallcup. 1967. Effect of soybean meal, raw soybeans, corn gluten feed, and urea on the concentration of rumen fluid components at intervals after feeding. J. Dairy Sci. 50:1638. DelCurto, T., R.C. Cochran, D.L. Harmon, A.A. Beharka, K.A. Jacques, G. Towne and E.S. Vanzant. 1990a. Supplementation of dormant tallgrass-prairie forage: I. Influence of varying supplemental protein and (or) energy levels on forage utilization characteristics of beef steers in confinement. J. Anim. Sci. 68:515. DelCurto, T., R.C. Cochran, L.R. Corah, A.A. Beharka, E.S. Vanzant and D.E. Johnson. 1990b. Supplementation of dormant tallgrass-prairie forage: II. Performance of forage utilization characteristics in grazing beef cattle receiving supplements of different protein concentrations. J. Anim. Sci. 68:532. DelCurto, T., R.C. Cochran, T.G. Nagaraja, L.R. Corah, A.A. Beharka and E.S. Vanzant. 1990c. Comparison of soybean meal/sorghum grain, alfalfa hay and dehydrated alfalfa pellets as supplemental protein sources for beef cattle consuming dormant tallgrass-prairie forage. J. Anim. Sci. 68:2901. Earleywine, T.J. 1989. Raw soybeans in dairy rations. Proc. 1989 Annual and Semiannual meetings of the American Feed Industry Council. pp 41, 42. Kansas City, MO and San Antonio, TX. 62 Edwards, R.L., G.C. Skelley Jr., J.T. Gillingham, S.L. Moore and W.C. Godley. 1969. Vitamin A, corn silage and raw soybeans for finishing steers in drylot. J. Anim. Sci. 29:940. Ellis, W.C., E.M. Bailey and C.A. Taylor. 1984. A silicone esophageal cannula; Its surgical installation and use in research with grazing cattle, sheep, or goats. J. Anim. Sci. 59:204. Erickson, P.S. and B.A. Barton. 1987. Whole soybeans for market lambs. J. Anim. Sci. 64:1249. Fahey, G.C. Jr. and L.L. Berger. 1988. Carbohydrate nutrition of ruminants. In: D.C. Church (Ed.) The Ruminant Animal. pp 269-297. Prentice Hall, Englewood Cliffs, NJ. Glenn, B.P., D.G. Ely and J.A. Boling. 1977. Nitrogen metabolism in lambs fed lipid coated protein. J. Anim. Sci. 46:871. Goering, H.K. and P.J. Van Soest. 1970. Forage Fiber Analyses (apparatus, reagents, procedures, and some applications). Agric. Handbook 379. USDA, ARS, Washington, D.C. Grovum, W.L. 1988. Appetite, palatability and control of feed intake. In: D.C. Church (Ed.) The Ruminant Animal. pp 202-216. Prentice Hall, Englewood Cliffs, NJ. Gunter, S.A., M.B. Judkins, L.J. Krysl, R.K. Barton and J.T. Broesder. 1990. Ruminal and forage intake response to time of cottonseed meal supplementation. Proc. West. Sect. Am. Soc. of Anim. Sci. 41:253. Henderson, C. 1973. The effects of fatty acids on pure cultures of rumen bacteria. J. Agri. Sci. (camb.) 81:107. Hibberd, C.A. and S.K. Martin. 1990. Substitution of corn gluten meal for soybean meal in range supplements on intake and utilization of low quality native grass hay. Anim. Sci. Res. Rep. MP-129. Oklahoma Agric. Exp. Sta., Stillwater. Illg, D.J. and M.d. Stern. 1990. Full fat soybeans: Energy and protein for ruminants. Proc. of the 51st Ann. Minnesota Nutr. Conf., Sept. 18, 19 1990. pp 43-55. Bloomington. 63 Jenkins, T.C. and D.L. Palmquist. 1982. Effect of added fat and calcium soaps on rumen and total digestibility in dairy rations. J. Dairy Sci. 67:978. Johnson, R.R. 1976. Influence of carbohydrate solubility on non-protein nitrogen utilization in the ruminant. Oklahoma Agric. res. station, Stillwater. Kakade, M.L., R.D. Thompson, W.E. Engelstad, G.C. Behrens, R.D. Yoder and F.M. Crane. 1976. Failure of soybean trypsin inhibitor to exert deleterious effects in calves. J. Dairy Sci. 59:1484. Kartchner, R.J. 1980. Effects of protein and energy supplementation of cows grazing native winter range forage on intake and digestibility. J. Anim. Sci. 51:432. Keele, J.W., R.E. Roffler and K.Z. Beyers. 1989. Ruminal metabolism in nonlactating cows fed whole cottonseed or extruded soybeans. J. Anim. Sci. 67:1612. Larson, A. and L.H. Schultz. 1970. Effects of soybeans compared to soybean oil meal in the ration of dairy cows. J. Dairy Sci. 53:1233. Lee, G.L., D.W. Hennessy, P.J. Williamson, J.V. Nolan, T.J. Kempton and R.A. Leng. 1985. Response to protein meal supplements by lactating beef cattle given a lowquality pasture hay. Aust. J. of Agric. Res. 36:729. Liener, I.E. and M.L. Kakade. 1980. Protease inhibitors. In: I.E. Liener (Ed.) Toxic Constituents of Plant Foodstuffs. pp 7-71. Academic Press, New York, NY. Mader, T. 1988. Utilization of raw soybeans and whole shelled corn in corn silage based diets. 1988 Nebraska Beef Cattle Rep., pp 33, 34. Univ. of Nebraska, Lincoln. McAllan, A.B. and E.S. Griffith. 1987. The effects of different sources of nitrogen supplementation on the digestion of fibre components in the rumen of steers. Anim. Feed Sci. and Tech. 17:65. McCormick, M.E., M.E. McCullough, L.R. Sisk and E.E. Worley. 1983. Soybeans: A protein supplement for growing calves. Univ. of Georgia Coll. of Agri. Exp. Sta., Res. Rep., no. 439. 64 Merchen, N.R. 1988. Digestion, absorption, and excretion in ruminants. In: D.C. (Ed.) The Ruminant Animal. pp 172-201. Prentice Hall, Englewood Cliffs, NJ. Mertens, D.R. and J.R. Loften. 1980. The effect of starch on forage fiber digestion kinetics in vitro. J. Dairy Sci. 63:1437. Mielke, C.D. and D.J. Schingoethe. 1981. Heat-treated soybeans for lactating cows. J. Dairy Sci. 64:1579. Moore, J.H. and W.W. Christie. 1984. Digestion, absorption and transport of fats in ruminant animal. In: J. Wiseman (Ed.) Fats in Animal Nutrition. pp 123-147. Butterworths Pub. Co., London. Morrison, F.B. 1956. Feeds and Feeding. A handbook for the student and stockman. (21st Ed.). Morrison Pub. Co., Ithaca, NY. Noble, R.C. 1984. Essential fatty acids in the ruminant. In: J. Wiseman (Ed.) Fats in Animal Nutrition pp 185200. Butterworths Pub. Co., London. NRC. 1984. Nutrient Requirements of Beef Cattle (6th Ed.). National Academy Press, Washington, DC. Oirskov, E.R. 1982. 84. Protein Nutrition in Ruminants. pp 41Academic Press, New York, NY. Owens, F.N. and A.L. Goetsch. 1988. Ruminal fermentation. In: D.C. Church (Ed.) The Ruminant Animal pp 145-171. Prentice Hall, Englewood Cliffs, NJ. Palmquist, D.L. and H.R. Conrad. 1971. High levels of raw soybeans for dairy cows. J. Anim. Sci. 33(Suppl. 1):295 (Abstr.). Palmquist, D.L. and T.C. Jenkins. 1980. Review. J. Dairy Sci. 63:1. Fats in Lactation: Palmquist, D.L. and T.C. Jenkins. 1982. Calcium soaps as a fat supplement in dairy cattle feeding. Proc., XII World Congr. Dis. Cattle., pp 477-481. Perry, F.G. and G.K. Macleod. 1968. Effects of feeding raw soybeans on rumen metabolism and milk composition of dairy cows. J. Dairy Sci. 52:1233. 65 Peterson, M.K., D.C. Clanton and R. Britton. 1985. Influence of protein degradability in range supplements on abomasal nitrogen flow, nitrogen balance and nutrient digestibility. J. Anim. Sci. 60:1324. Rittenhouse, L.R., D.C. Clanton and C.L. Streeter. 1970. Intake and digestibility of winter-range forage by cattle with and without supplements. J. Anim. Sci. 31:1215. Sanson, D.W. and D.C. Clanton. 1989. Intake and digestibility of low quality meadow hay by cattle receiving various levels of whole shelled corn. J. Anim. Sci. 67:2854. SAS. 1985. SAS User's Guide: Cary, NC. Statistics. SAS Inst., Inc., Satter, L.D. and L.L. Slyter. 1974. Effect of ammonia concentration on rumen microbial protein production in vitro. Brit. J. of Nutr. 32:199. Shaw, J.C. and W.L. Ensor. 1959. Effect of feeding cod liver oil and unsaturated fatty acids on rumen volatile fatty acids and milk fat content. J. Dairy Sci. 42:1238. Shirley, R.L. 1986. Nitrogen and Energy Nutrition of Ruminants. Academic Press Inc., Orlando, FL. Sniffen, C.J. and P.H. Robinson. 1987. Symposium: Protein and fiber digestion, passage and utilization in lactating cows. J. Dairy Sci. 70:425. Snyder, H.E. and T.W. Kwon. 1987. Soybean Utilization. Nostrand Reinhold Co., Inc., New York, NY. Van Sonntag, N.O.V. 1979. Morphology and composition. In: H.E. Snyder and T.W. Kwon (Ed.) Soybean Utilization. p 34. Van Nostrand Reinhold Co., Inc. New York, NY. Stern, M.D., K.A. Santos and L.D. Satter. 1985. Protein degradation in rumen and amino acid absorption in small intestine of lactating dairy cattle fed heat-treated whole soybeans. J. Dairy Sci. 68:45. Van Dijk, H.J., G.D. O'Dell, P.R. Perry and L.W. Grimes. 1983. Extruded versus raw ground soybeans for dairy cows in early lactation. J. Dairy Sci. 66:2521. Van Soest, P.J. 1982. Nutritional Ecology of the Ruminant. O&B books Inc., Corvallis, OR. 66 Waldern, D.E. 1971. A rapid micro-digestion procedure for neutral and acid detergent fiber. Can. J. Anim. Sci. 51:67. Weston, R.H. and J.P. Hogan. 1968. Factors limiting the intake of feed by sheep. IV: The intake and digestion of mature ryegrass. Aust. J. Agric. Res. 19:567. Zinn, R.A. and F.N. Owens. 1983. in steers: Predictability. Site of protein digestion J. Anim. Sci. 56:707. APPENDIX 67 TABLE A.1 INFLUENCE OF SUPPLEMENTAL SOYBEAN SOURCE ON INTAKE AND DIGESTIBILITY. (Exp.1) STEER PER 48 83 97 138 1 1 48 83 97 138 2 2 4 2 2 2 3 48 83 97 138 3 3 3 3 4 48 83 97 138 4 1 1 3 4 4 4 TRTb 1 2 3 4 1 1 2 2 3 4 1 DM FECAL OUTPUT FDMI (lbs/d) TDMI (lbsid) (lbs/d) 9.28 7.21 9.32 5.40 12.31 10.07 12.24 5.40 5.47 4.38 4.73 2.67 55.03 56.44 61.18 49.73 49.09 43.21 52.58 47.81 9.59 10.25 9.31 4.65 9.59 13.27 12.16 7.55 4.31 5.47 4.92 2.70 54.77 58.66 59.13 62.93 52.06 49.79 47.87 48.81 9.78 11.35 10.55 0.00 12.69 11.35 13.55 0.00 5.16 5.11 5.46 0.00 59.13 54.95 59.71 0.00 48.92 48.43 49.70 0.00 10.12 12.59 12.34 10.71 12.96 15.50 12.34 13.71 4.91 6.36 5.36 5.74 61.96 58.86 56.39 58.07 54.75 49.66 54.18 51.77 DMD% TDMI = Forage DMI, TDMI = Total DMI. /Treatment 1 = WSB; 2 = ESB; 3 = SBM+BAR; 4 = CONTROL NDFD% 68 TABLE A.2 INFLUENCE OF SUPPLEMENTAL SOYBEAN SOURCE ON RUMINAL KINETICS (Exp. 1) DM FILL STEER PER TRTa 097 083 138 048 1 1 1 3 2 4 1 1 1 1 1 097 083 138 048 1 1 1 1 3 2 097 083 138 048 2 2 2 2 2 097 083 138 048 2 2 TIMEb IADF IADF FILL INTAKE IADF PASSAGE IADF FLOW (LBS) (LBS) (LBS) (%/HR) (a/hr) 1 9.06 7.30 2.67 7.43 2.28 1.42 0.58 1.72 1.24 1.00 0.68 1.25 2.27 2.94 4.91 3.04 23.51 18.99 12.90 23.68 2 2 2 2 11.68 8.98 5.47 12.55 2.43 1.76 0.90 2.43 1.24 1.00 0.68 1.25 2.13 2.38 3.14 2.15 23.51 18.99 12.90 23.68 1 1 1 3 1 4 1 9.22 9.72 4.17 9.04 2.47 2.63 1.08 2.19 1.27 1.39 0.67 1.22 2.14 2.20 2.58 2.32 24.04 26.29 12.58 23.00 2 2 2 1 3 2 4 2 13.05 15.16 8.35 11.26 2.26 2.72 1.51 2.45 1.27 1.39 0.67 1.22 2.34 2.13 1.84 2.07 24.04 26.29 12.58 23.00 097 083 138 048 3 1 1 3 3 4 1 2 1 3 3 1 11.56 12.17 6.86 9.30 2.84 2.91 2.21 2.85 1.44 1.50 0.00 1.35 2.12 2.14 0.00 1.97 27.32 28.28 0.00 25.50 097 083 138 048 3 1 3 4 3 2 2 2 2 3 3 2 16.14 14.50 12.24 12.60 3.54 3.53 2.12 2.82 1.44 1.50 0.00 1.35 1.70 1.76 0.00 1.99 27.32 28.28 0.00 25.50 097 083 138 048 4 4 4 4 4 1 3 1 1 2 1 1 11.67 10.15 11.07 10.89 3.72 3.31 2.88 3.14 1.56 1.69 1.44 1.35 1.74 2.13 2.08 1.80 29.42 32.03 27.20 25.63 097 083 138 048 4 4 4 2 2 4 4 1 2 13.70 15.19 15.96 16.76 3.86 3.60 3.39 4.31 1.56 1.69 1.44 1.35 1.68 1.96 1.77 1.31 29.42 32.03 27.20 25.63 4 1 3 2 2 2 2 'Treatment 1 = WSB; 2 = ESB; 3 = SBM+BAR; 4 = CONTROL bTiMe corresponds to evacuations at 0 h post supplementation = time 1; and 5 h post supplementation = time 2. 69 TABLE A.3 INFLUENCE OF SUPPLEMENTAL SOYBEAN SOURCE ON RUMINAL VFA CONCENTRATIONS (Exp. 1) mM concentration Steer 097 097 097 097 097 083 083 083 083 083 138 138 138 138 138 048 048 048 048 048 097 097 097 097 097 083 083 083 083 083 138 138 138 138 138 048 048 048 048 048 097 097 097 Per Trta 1 1 3 3 1 3 1 1 3 3 1 2 1 1 2 2 1 2 1 2 1 4 1 4 1 4 1 1 1 1 4 4 1 1 1 1 1 1 2 2 2 2 2 2 2 2 2 2 1 1 2 2 2 1 1 1 2 2 2 2 1 3 3 2 3 2 2 2 2 2 3 3 2 4 4 2 1 4 4 4 3 1 3 1 3 1 Time ACE 67.547 84.039 6 91.936 9 66.549 12 77.540 0 66.104 3 78.113 6 87.879 9 77.047 12 68.064 0 56.151 3 65.319 6 51.876 9 53.599 12 50.683 0 68.345 3 67.349 6 69.445 9 58.571 12 59.814 0 53.474 3 90.914 6 73.643 9 85.470 12 74.117 0 78.499 3 67.208 6 78.937 9 68.511 12 82.388 0 60.216 3 70.662 6 69.993 9 79.604 12 72.488 0 64.753 3 73.360 6 71.847 9 75.540 12 69.128 0 72.835 3 86.103 6 84.386 0 3 PRO IBU BUT IVA VAL 15.639 20.113 22.958 16.889 18.779 15.299 21.006 23.992 22.675 19.086 15.332 20.088 15.047 16.095 15.044 14.996 16.586 16.116 15.377 14.789 13.747 25.422 20.254 23.941 21.249 19.727 18.316 20.618 18.836 24.190 14.962 20.500 22.611 24.756 21.261 12.809 16.782 17.301 18.127 15.368 18.325 22.051 21.747 0.852 0.926 1.167 0.862 0.906 0.713 0.645 0.811 0.933 1.088 0.575 0.475 0.359 0.341 0.396 1.136 1.137 1.248 0.814 0.745 0.792 0.834 0.612 0.755 0.543 1.132 0.850 0.823 0.585 0.772 1.314 0.935 0.900 0.896 0.718 0.591 0.591 0.454 0.461 0.441 0.804 0.948 0.835 10.829 14.648 15.706 12.144 13.350 7.521 11.224 13.601 11.674 9.455 6.838 8.152 6.10 6.549 6.226 6.987 6.686 7.561 6.571 6.756 8.127 13.572 10.975 12.809 11.102 10.697 8.710 10.476 9.384 11.742 6.892 10.279 11.615 12.814 11.281 7.075 8.750 9.104 9.976 8.890 10.827 13.711 12.755 0.940 1.257 1.705 1.036 1.039 1.088 0.985 1.316 1.603 1.811 0.491 0.344 0.240 0.307 0.260 1.779 1.916 2.138 1.287 1.163 0.917 1.039 0.639 0.636 0.461 1.871 1.380 1.523 1.052 1.217 1.882 1.482 1.471 1.261 1.069 0.635 0.525 0.422 0.344 0.371 1.051 1.301 1.162 0.676 1.154 1.386 0.852 0.899 0.664 0.952 1.237 1.172 1.123 0.471 0.526 0.428 0.422 0.413 0.794 0.925 1.003 0.712 0.767 0.540 0.995 0.757 0.972 0.722 1.012 0.948 1.061 0.898 1.065 0.880 1.342 1.441 1.252 1.075 0.374 0.534 0.556 0.589 0.510 0.891 1.138 1.029 °Treatment 1 = WSB; 2 = ESB; 3 = SBM+BAR; 4 = CONTROL. bTVFA = Total VFA concentration. TVFAb 96.483 122.137 134.858 98.302 112.513 91.389 112.925 128.836 115.104 100.627 79.858 94.904 74.052 77.313 73.022 94.037 94.599 97.511 83.332 84.034 77.597 132.776 106.880 124.583 108.194 112.938 97.412 113.438 99.266 121.374 86.146 105.200 108.031 120.583 107.892 86.237 100.497 99.684 105.037 94.708 104.733 125.252 121.914 70 TABLE A.3 (continued) INFLUENCE OF SUPPLEMENTAL SOYBEAN SOURCE ON RUMINAL VFA CONCENTRATIONS (Exp. 1) mM concentration Steer 097 097 083 083 083 083 083 138 138 138 138 138 048 048 048 048 048 097 097 097 097 097 083 083 083 083 083 138 138 138 138 138 048 048 048 048 048 Time ACE PRO IBU BUT IVA VAL 1 9 1 12 0 81.194 86.037 72.851 70.951 85.825 65.570 74.281 60.575 77.406 77.449 69.520 68.662 62.094 72.315 67.895 66.010 66.877 66.538 67.742 79.783 81.124 79.730 76.296 79.311 88.401 79.137 77.760 70.301 87.212 85.973 86.213 86.434 71.654 86.341 81.464 76.209 82.898 20.923 22.345 15.511 17.551 19.856 14.846 17.539 17.973 24.943 26.572 22.970 24.428 14.218 18.198 17.768 17.457 19.058 14.997 17.690 19.778 19.619 19.462 18.733 23.156 25.574 22.738 22.757 15.697 21.759 20.453 19.974 21.328 15.609 20.752 19.394 17.914 19.573 0.793 0.731 0.590 0.537 0.661 0.503 0.538 0.782 0.627 0.551 0.505 0.522 0.787 0.972 0.877 0.924 0.772 0.587 0.520 0.574 0.508 0.537 0.954 0.815 0.841 0.764 0.757 0.639 0.771 0.821 0.770 0.614 0.837 0.784 0.804 0.845 0.782 12.333 12.783 8.980 9.936 12.130 9.230 10.670 7.245 12.013 13.347 11.201 11.091 8.503 11.194 10.500 10.087 11.002 9.625 10.553 12.462 12.021 11.816 10.363 14.175 16.071 13.231 13.274 8.653 12.530 12.526 11.974 13.232 8.367 11.240 10.609 9.554 10.560 1.046 0.792 0.514 0.363 0.457 0.375 0.340 1.001 0.734 0.541 0.582 0.572 1.016 1.548 1.313 1.300 0.907 0.649 0.550 0.532 0.465 0.421 1.171 1.125 1.004 0.911 0.880 0.917 1.107 1.264 1.221 0.819 1.164 1.171 0.942 1.081 0.979 0.998 0.905 0.646 0.599 0.751 0.568 0.606 0.773 1.047 1.158 0.986 0.973 0.520 1.019 0.842 0.730 0.700 0.603 0.741 0.837 0.790 0.763 0.877 1.338 1.354 1.152 1.067 0.659 0.943 1.064 0.915 0.877 0.607 0.917 0.880 0.800 0.885 Per Trt' 3 3 3 3 3 3 3 3 3 3 3 4 4 4 4 4 2 2 2 3 2 2 3 3 3 3 3 3 3 3 3 3 4 4 4 4 4 4 4 4 4 4 4 4 4 4 4 3 3 3 3 3 4 4 1 4 1 4 4 4 4 4 1 4 4 1 1 2 2 2 3 6 9 12 0 3 6 9 12 0 3 6 9 12 0 3 6 9 12 0 3 6 9 12 0 3 6 9 12 0 3 6 2 9 2 12 'Treatment 1 = WSB; 2 = ESB; 3 = SBM+BAR; 4 = CONTROL bTVFA = Total VFA concentration. TVFAb 117.287 123.593 99.092 99.937 119.680 91.110 103.974 88.349 116.770 119.618 105.764 106.248 87.138 105.246 99.195 96.508 99.316 92.999 97.796 113.966 114.527 112.729 108.394 119.920 133.245 117.933 116.495 96.866 124.322 122.101 121.067 123.304 98.238 121.205 114.093 106.403 115.677 71 TABLE A.4 INFLUENCE OF SUPPLEMENTAL SOYBEAN SOURCE ON RUMINAL pH AND AMMONIA CONCENTRATION (Exp. 1) STEER PER TRT' 048 048 048 048 048 1 1 1 1 1 1 1 1 083 083 083 083 083 1 2 1 2 1 1 2 2 2 097 097 097 097 097 1 1 3 3 1 1 3 3 00 03 06 09 1 3 12 138 138 138 138 138 1 4 1 1 1 4 4 4 4 00 03 06 09 048 048 048 048 048 2 4 2 4 4 083 083 083 083 083 1 1 1. 1 TIME 00 03 06 09 12 00 03 06 09 12 12 6.89 6.80 6.78 6.80 6.67 193.60 216.48 230.56 129.36 88.00 6.80 6.28 6.03 6.63 6.80 79.20 160.16 192.72 215.60 220.00 6.60 6.21 6.09 6.38 6.40 113.52 254.32 288.64 196.24 146.08 6.95 6.67 6.75 6.87 6.87 44.00 33.44 30.80 33.44 31.68 44.88 33.44 31.68 31.68 31.68 4 00 03 06 09 4 12 6.96 6.80 6.62 5.80 6.59 2 2 2 2 2 1 1 00 03 06 09 12 6.52 6.64 6.21 6.17 5.65 222.64 139.92 109.12 50.16 49.28 097 097 097 097 097 2 2 2 2 6.72 6.35 6.22 5.83 5.94 136.40 203.28 134.64 117.04 88.00 138 138 138 138 138 6.94 6.36 5.97 6.47 6.01 188.40 227.92 148.72 41.36 39.60 2 2 2 1 1 1 2 2 2 00 03 06 09 2 2 12 2 3 2 3 3 3 00 03 06 09 3 12 2 2 2 2 °Treatment 1 = WSB; 2 = ESB; 3 = SBM+BAR; 4 =CONTROL. 72 TABLE A.4 (continued) INFLUENCE OF SUPPLEMENTAL SOYBEAN SOURCE ON RUMINAL pH AND AMMONIA CONCENTRATION (Exp. 1) STEER 048 048 048 048 048 083 083 083 083 083 PER TRTs 3 3 3 3 3 3 3 3 3 3 3 3 4 4 4 3 3 4 4 3 097 097 097 097 097 3 138 138 138 138 138 3 3 1 1 3 3 3 1 1 1 3 TIME 6.87 6.73 6.63 6.63 6.21 99.44 242.00 163.68 138.16 89.76 00 03 06 09 6.67 6.59 6.10 6.62 6.42 27.28 26.40 34.32 28.16 27.28 6.21 5.56 5.79 5.81 5.64 125.84 189.20 116.16 130.24 103.84 6.87 6.24 6.11 6.35 6.18 41.36 98.56 64.24 49.28 22.88 6.79 6.39 6.35 6.54 6.03 130.24 196.24 154.00 168.96 160.16 6.61 6.45 5.90 6.14 6.35 106.48 210.32 116.16 70.40 78.32 6.79 6.67 6.20 6.44 6.39 56.32 54.56 30.80 29.92 34.32 6.36 6.13 6.02 6.14 5.77 113.52 183.92 179.52 184.80 121.44 12 00 03 06 09 12 3 00 03 06 09 3 2 12 048 048 048 048 048 4 2 4 4 4 2 2 2 00 03 06 09 4 2 12 083 083 083 083 083 4 3 4 3 4 4 4 3 3 00 03 06 09 3 12 097 097 097 097 097 4 4 4 4 4 4 4 4 4 00 03 06 09 4 12 138 138 138 138 138 4 4 4 4 1 1 1 1 00 03 06 09 4 1 12 °Treatment 1 NHS (ppm) 00 03 06 09 12 2 2 2 2 3 pH = WSB; 2 = ESB; 3 = SBM+BAR; 4 = CONTROL. 73 TABLE A.5 STEER PER TRT° 097 097 097 097 097 097 083 083 083 083 083 083 048 048 048 048 048 048 097 097 097 097 097 097 083 083 083 083 083 083 138 138 138 138 138 138 097 097 097 097 097 097 1 1 1 3 3 1 1 1 3 3 3 1 2 1 2 2 2 2 2 1 1 1 1 1 1 1 1 1 1 2 2 2 2 2 2 2 2 2 3 1 1 1 1 1 1 2 2 2 2 2 2 1 1 1 2 2 2 1 2 3 2 2 3 2 2 2 3 3 3 3 3 3 1 1 3 3 3 3 1 1 1 1 1 1 IN SITU DEGRADATION OF SUPPLEMENTS (Exp. 1) TIME %DM DIS %CP DIS 24 18 12 09 06 03 24 18 12 09 06 03 24 18 12 09 06 03 24 18 12 09 06 03 24 18 12 09 06 03 24 18 12 09 06 03 24 18 12 09 06 03 88.53 88.53 61.78 71.33 64.17 50.86 84.69 75.26 70.76 62.16 61.02 49.97 100.00 100.00 88.79 79.62 70.11 69.43 83.51 75.26 78.56 59.80 53.10 49.97 91.38 100.00 71.98 77.58 53.29 59.97 65.60 65.60 80.89 78.50 71.33 50.86 100.00 100.00 79.62 69.43 77.58 53.29 92.26 80.66 52.61 56.90 41.58 29.74 77.84 69.22 59.02 51.10 48.27 29.22 101.65 98.42 86.38 78.63 61.46 59.28 85.23 54.20 68.05 40.74 38.12 33.78 92.15 89.90 66.42 82.73 43.42 63.68 64.18 40.00 60.83 66.87 52.80 28.45 97.72 99.56 76.32 68.45 71.04 44.86 LAG TIME OF DM/h 4 02 LAG TIME OF CP/h 3.4 %/h RATE OF DM DIS 3.07 4.07 %/h RATE OF CP DIS 2.57 4.14 5.10 3.97 2.66 4.01 3.65 3.61 3.15 3.99 3.83 3.98 3.76 4.52 3.17 4.12 1.33 3.97 1.46 3.83 3.56 4.55 'Treatment 1 = WSB; 2 = ESB; 3 = SBM+BAR. 6.12 3.66 74 TABLE A.5 (continued) STEER PER TRT° 138 138 138 138 138 138 048 048 048 048 048 048 083 083 083 083 083 083 138 138 138 138 138 138 048 048 048 048 048 048 3 2 3 2 3 3 3 3 2 2 2 3 3 3 3 3 3 3 3 3 2 3 3 3 4 4 4 4 4 4 4 4 4 3 4 4 4 1 4 2 4 4 4 4 4 3 3 3 3 3 1 1 1 1 1 2 2 2 2 2 IN SITU DEGRADATION OF SUPPLEMENTS (Exp. 1) TIME %DM DIS %CP DIS 24 18 12 09 06 03 24 18 12 09 06 03 24 18 12 09 06 03 24 18 12 09 06 03 24 18 12 09 06 03 82.13 70.76 61.02 64.27 61.02 57.12 100.00 68.73 64.17 58.30 37.46 29.44 89.58 90.44 65.60 65.60 68.73 47.88 100.00 100.00 82.75 66.37 53.29 49.05 82.13 78.56 73.20 53.10 58.77 50.52 LAG TIME LAG TIME OF DM/h OF CP/h 80.78 4.22 3.90 43.52 48.28 %/h %/h 43.43 RATE OF RATE OF 39.02 DM DIS CP DIS 40.87 1.24 0.79 96.83 3.34 1.92 48.72 48.49 35.28 12.38 5.79 7.26 13.27 99.84 3.97 2.82 94.42 39.29 41.42 35.84 19.53 98.48 98.72 83.70 64.16 53.75 45.13 73.91 66.70 63.08 36.23 39.23 28.31 °Treatment 1 = WSB; 2 = ESB; 3 = SBM+BAR. 3.46 3.73 9.22 3.69 5.07 4.03 5.42 3.53 3.02 5.86 75 TABLE A.6 STEER PER 097 097 097 097 097 097 097 097 083 083 083 083 083 083 083 083 138 138 138 138 138 138 138 138 048 048 048 048 048 048 048 048 097 097 097 097 097 097 097 097 1 1 1 1 1 1 1 INFLUENCE OF SUPPLEMENTAL SOYBEAN SOURCE ON IN SITU DEGRADATION OF FORAGE SOURCE (Exp. 1) TBTa 3 3 3 3 3 3 1 1 3 3 2 1 2 1 2 2 2 1 1 1 1 2 1 2 2 1 4 1 1 1 4 1 4 1 4 1 1 4 4 4 1 4 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 TIME T-96 T-72 T-48 T-36 T-24 T-18 T-12 T-06 T-96 T-72 T-48 T-36 T-24 T-18 T-12 T-06 T-96 T-72 T-48 T-36 T-24 T-18 T-12 T-06 T-96 T-72 T-48 T-36 T-24 T-18 T-12 T-06 T-96 T-72 T-48 T-36 T-24 T-18 T-12 T-06 NDF% DM% (DMB) LAG TIME LAG TIME DISAP DISAP OF DM/h OF NDF/h 61.39 63.51 3.75 3.47 56.45 57.96 58.27 57.82 49.66 46.65 37.10 32.86 %/h %/h 34.86 32.87 RATE OF RATE OF 28.46 24.04 DM DIS NDF DIS 22.10 17.04 1.34 1.78 54.96 55.52 3.92 3.79 55.49 55.21 53.01 50.65 49.20 51.44 40.48 36.41 39.30 40.08 28.13 25.80 25.00 20.33 1.18 1.39 63.77 62.15 3.55 3.00 56.00 53.16 48.43 42.09 40.29 35.10 34.24 24.95 26.60 18.07 25.00 15.58 23.16 12.02 1.44 2.28 59.29 61.98 3.88 3.74 54.96 55.71 53.42 52.54 48.24 52.15 45.65 42.76 39.06 37.50 32.14 31.28 23.16 19.11 1.13 1.35 64.75 62.61 3.93 3.45 64.91 60.95 56.81 52.69 51.41 47.29 48.59 39.32 41.70 30.04 38.30 26.17 28.90 14.21 1.07 1.89 'Treatment 1 = WSB; 2 = ESB; 3 = SBM+BAR; 4 = CONTROL. 76 TABLE A.6 (continued) INFLUENCE OF SUPPLEMENTAL SOYBEAN SOURCE ON IN SITU DEGRADATION OF FORAGE SOURCE (Exp. 1) DM% STEER PER 083 083 083 083 083 083 083 083 138 138 138 138 138 138 138 138 048 048 048 048 048 048 048 048 097 097 097 097 097 097 097 097 083 083 083 083 083 083 083 083 TRTa 2 2 2 1 1 1 2 1 2 2 2 2 2 2 1 1 2 1 1 3 3 3 2 2 3 2 2 3 3 3 2 3 2 4 2 4 4 4 4 4 4 4 2 2 2 2 2 2 3 3 3 3 3 3 3 3 3 3 3 3 3 3 3 3 'Treatment 1 1 1 1 1 1 1 1 4 4 4 4 4 4 4 4 1 TIME DISAP T-96 60.50 T-72 62.45 T-48 54.11 T-36 52.60 T-24 46.02 T-18 37.62 T-12 33.16 T-06 24.42 T-96 57.87 T-72 50.92 T-48 49.97 T-36 46.02 T-24 42.07 T-18 39.43 T-12 33.16 T-06 26.27 T-96 62.21 T-72 60.74 T-48 57.87 T-36 48.59 T-24 44.70 T-18 36.10 T-12 32.52 T-06 28.02 T-96 57.25 T-72 57.58 T-48 55.51 T-36 47.17 T-24 44.27 T-18 40.29 T-12 37.57 T-06 29.43 T-96 62.00 T-72 59.29 T-48 54.93 T-36 43.00 T-24 37.82 T-18 31.07 T-12 28.13 T-06 24.00 NDF% (DMB) LAG TIME LAG TIME DISAP OF DM/h OF NDF/h 59.37 3.86 3.27 57.69 47.49 47.84 34.92 %/h %/h 27.62 RATE OF RATE OF 20.63 DM DIS DM DIS 10.29 1.27 2.22 55.16 3.97 3.55 48.90 47.76 43.68 31.40 28.75 22.13 15.61 0.88 1.63 59.45 3.86 3.39 58.83 52.81 44.75 33.87 26.71 22.28 14.11 1.18 2.01 60.50 4.05 3.84 58.88 56.11 49.88 39.08 37.65 33.27 24.93 0.92 1.22 60.52 3.69 3.44 57.24 50.63 41.35 30.31 27.14 22.29 17.98 1.41 1.77 = WSB; 2 = ESB; 3 = SBM+BAR; 4 = CONTROL. 77 TABLE A.6 (continued) INFLUENCE OF SUPPLEMENTAL SOYBEAN SOURCE ON IN SITU DEGRADATION OF FORAGE SOURCE (Exp. 1) STEER PER 138 138 138 138 138 138 138 138 048 048 048 048 048 048 048 048 097 097 097 097 097 097 097 097 083 083 083 083 083 083 083 083 138 138 138 138 138 138 138 138 TRTa 3 3 2 3 3 3 2 3 3 3 3 3 3 3 3 3 3 3 4 4 4 4 4 4 4 4 4 4 4 4 4 4 4 4 4 4 4 4 4 4 4 4 °Treatment 2 2 2 2 2 2 3 3 3 3 3 3 3 3 4 4 4 4 4 4 4 4 3 3 3 3 3 3 3 3 1 1 1 1 1 1 1 1 1 TIME T-96 T-72 T-48 T-36 T-24 T-18 T-12 T-06 T-96 T-72 T-48 T-36 T-24 T-18 T-12 T-06 T-96 T-72 T-48 T-36 T-24 T-18 T-12 T-06 T-96 T-72 T-48 T-36 T-24 T-18 T-12 T-06 T-96 T-72 T-48 T-36 T-24 T-18 T-12 T-06 NDF% DM% (DMB) LAG TIME LAG TIME DISAP DISAP OF DM/h OF NDF/h 57.58 61.90 3.97 3.73 61.07 62.25 55.95 55.74 50.13 51.69 47.17 42.99 %/h %/h 37.30 34.75 RATE OF RATE OF 33.72 29.18 DM DIS DM DIS 27.71 22.68 1.14 1.46 66.32 65.31 3.67 3.57 63.85 63.34 58.55 56.44 49.95 51.25 43.00 39.60 36.05 34.37 31.13 27.79 22.15 18.93 1.47 1.67 70.73 68.17 3.87 3.38 68.83 62.97 62.06 54.28 54.55 50.33 54.55 43.03 46.67 34.96 38.02 23.97 28.16 14.00 1.16 1.94 70.00 65.17 3.95 3.55 67.27 60.36 67.77 60.14 59.41 52.27 52.27 38.77 44.30 34.25 41.65 28.95 31.82 17.25 1.07 1.70 66.23 62.19 3.92 3.48 67.27 62.09 62.75 56.13 56.87 50.54 49.26 36.52 38.34 26.57 38.34 24.58 31.82 18.06 1.14 1.87 = WSB; 2 = ESB; 3 = SBM+BAR; 4 = CONTROL. 78 TABLE A.6 (continued) INFLUENCE OF SUPPLEMENTAL SOYBEAN SOURCE ON IN SITU DEGRADATION OF FORAGE SOURCE (Exp. 1) STEER PER 048 048 048 048 048 048 048 048 TRTa 4 4 4 4 4 4 4 4 `Treatment 2 2 2 2 2 2 2 2 1 TIME T-96 T-72 T-48 T-36 T-24 T-18 T-12 T-06 NDF% DM% (DMB) LAG TIME LAG TIME DISAP DISAP OF DM/h OF NDF/h 68.83 64.02 3.92 3.52 63.64 60.01 62.75 56.84 55.85 50.87 49.09 37.55 %/h %/h 44.24 34.74 RATE OF RATE OF 37.66 24.50 DM DIS DM DIS 31.50 17.57 1.03 1.75 = WSB; 2 = ESB; 3 = SBM+BAR; 4 = CONTROL. 79 TABLE A.7 INFLUENCE OF SUPPLEMENTAL SOYBEAN SOURCE ON FEED INTAKE (Exp. 2) WSB PEN 1 WEEK WEEK WEEK WEEK 1 2 3 4 FORAGE TOTAL DMI DMI (lbs) (lbs) 409.74 362.37 380.25 390.48 515.05 465.69 483.12 493.74 1542.84 1957.61 432.24 443.86 444.18 451.70 535.13 547.08 549.89 554.55 1771.98 2186.66 473.77 525.06 500.77 566.99 579.26 631.42 606.34 673.59 2066.60 2490.61 561.14 592.07 539.42 575.65 664.49 698.09 641.83 681.01 2268.28 2685.42 TRIAL AVERAGE PEN/LBS/D 68.30 PER/HD/D 13.66 83.22 16.64 PERIOD 1 TOTAL---> WEEK WEEK WEEK WEEK 5 6 7 8 PERIOD 2 TOTAL---> WEEK WEEK WEEK WEEK 9 10 11 12 PERIOD 3 TOTAL---> WEEK WEEK WEEK WEEK 13 14 15 16 PERIOD 4 TOTAL---> ESB PEN 2 WEEK WEEK WEEK WEEK 1 2 3 4 FORAGE DMI TOTAL (lbs) (lbs) DMI 430.51 400.07 419.15 413.29 530.42 498.18 517.20 511.25 1663.02 2057.04 442.19 441.07 471.75 481.58 540.39 539.37 572.01 578.94 1836.58 2230.72 500.44 537.27 526.10 572.97 600.32 641.82 626.66 674.53 2136.78 2543.35 589.17 592.92 556.00 586.56 687.18 703.07 653.20 686.78 2324.67 2730.23 TRIAL AVERAGE PEN/LBS/D 71.08 PER/HD/D 14.22 85.37 17.07 PERIOD 1 TOTAL---> WEEK WEEK WEEK WEEK 5 6 7 8 PERIOD 2 TOTAL---> WEEK WEEK WEEK WEEK 9 10 11 12 PERIOD 3 TOTAL---> WEEK WEEK WEEK WEEK 13 14 15 16 PERIOD 4 TOTAL---> 80 TABLE A.7 (continued) INFLUENCE OF SUPPLEMENTAL SOYBEAN SOURCE ON FEED INTAKE (Exp. 2) SBM+BAR PEN 3 WEEK WEEK WEEK WEEK 1 2 3 4 FORAGE DMI TOTAL (lbs) (lbs) DMI 439.54 421.98 449.80 427.70 540.69 520.47 548.27 513.14 1739.01 2122.57 468.75 481.51 490.70 504.38 567.41 580.64 592.48 602.71 1945.34 2343.25 547.80 588.58 554.80 623.51 649.13 691.68 656.78 726.65 2314.69 2724.25 620.99 650.36 630.28 657.02 719.51 753.74 729.01 758.81 2558.65 2961.07 TRIAL AVERAGE PEN/LBS/D 76.41 PER/HD/D 15.28 90.64 18.13 PERIOD 1 TOTAL---> WEEK WEEK WEEK WEEK 5 6 7 8 PERIOD 2 TOTAL---> WEEK WEEK WEEK WEEK 9 10 11 12 PERIOD 3 TOTAL---> WEEK WEEK WEEK WEEK 13 14 15 16 PERIOD 4 TOTAL---> CONTROL PEN 4 WEEK WEEK WEEK WEEK 1 2 3 4 FORAGE TOTAL DMI DMI (lbs) (lbs) 434.63 433.24 436.18 423.23 434.63 433.24 436.18 423.23 1727.28 1727.28 442.15 447.78 472.67 467.42 442.15 447.78 472.67 467.42 1830.02 1830.02 469.10 504.12 495.67 527.22 469.10 504.12 495.67 527.22 1996.11 1996.11 492.09 527.16 503.95 514.24 492.09 527.16 503.95 514.24 2037.44 2037.44 TRIAL AVERAGE PEN/LBS/D 67.78 PER/HD/D 13.56 67.78 13.56 PERIOD 1 TOTAL---> WEEK WEEK WEEK WEEK 5 6 7 8 PERIOD 2 TOTAL---> WEEK WEEK WEEK WEEK 9 10 11 12 PERIOD 3 TOTAL---> WEEK WEEK WEEK WEEK 13 14 15 16 PERIOD 4 TOTAL---> 81 TABLE A.7 (continued) INFLUENCE OF SUPPLEMENTAL SOYBEAN SOURCE ON FEED INTAKE (Exp. 2) ESB PEN 5 WEEK WEEK WEEK WEEK 1 2 3 4 FORAGE DMI TOTAL (lbs) (lbs) DMI 326.76 368.90 403.70 378.70 426.67 467.00 501.75 476.66 1478.06 1872.08 422.59 419.96 440.16 458.86 520.79 518.26 540.43 556.22 1741.56 2135.70 483.67 501.61 484.62 540.87 583.55 602.11 585.18 642.44 2010.77 2413.28 519.52 564.09 532.70 571.37 617.53 674.24 629.90 671.58 2187.69 2593.25 TRIAL AVERAGE PEN/LBS/D 66.23 PER/HD/D 13.25 80.48 16.10 PERIOD 1 TOTAL---> WEEK WEEK WEEK WEEK 5 6 7 8 PERIOD 2 TOTAL---> WEEK WEEK WEEK WEEK 9 10 11 12 PERIOD 3 TOTAL---> WEEK WEEK WEEK WEEK 13 14 15 16 PERIOD 4 TOTAL---> SBM+BAR PEN 6 WEEK WEEK WEEK WEEK 1 2 3 4 FORAGE TOTAL DMI DMI (lbs) (lbs) 369.94 387.17 423.23 414.14 471.09 485.66 521.71 499.58 1594.47 1978.03 445.31 446.68 469.32 489.12 543.97 545.81 571.11 587.46 1850.44 2248.35 506.54 530.23 511.10 579.43 607.87 633.33 613.08 682.58 2127.31 2536.87 570.43 610.90 552.61 540.30 668.95 714.28 651.34 642.09 2274.24 2676.66 TRIAL AVERAGE PEN/LBS/D 70.06 PER/HD/D 14.01 84.28 16.86 PERIOD 1 TOTAL---> WEEK WEEK WEEK WEEK 5 6 7 8 PERIOD 2 TOTAL---> WEEK WEEK WEEK WEEK 9 10 11 12 PERIOD 3 TOTAL---> WEEK WEEK WEEK WEEK 13 14 15 16 PERIOD 4 TOTAL---> 82 TABLE A.7 (continued) INFLUENCE OF SUPPLEMENTAL SOYBEAN SOURCE ON FEED INTAKE (Exp. 2) CONTROL PEN 7 WEEK WEEK WEEK WEEK 1 2 3 4 FORAGE DMI TOTAL (lbsl (lbsl DMI 418.47 427.39 441.21 432.48 418.47 427.39 441.21 432.48 1719.55 1719.55 447.45 451.43 460.57 474.30 447.45 451.43 460.57 474.30 1833.76 1833.75 490.05 468.71 482.03 491.64 490.05 468.71 482.03 491.64 1932.43 1932.43 463.88 481.08 497.42 497.00 463.88 481.08 497.42 497.00 1939.37 1939.37 TRIAL AVERAGE PEN/LBS/D 66.30 PER/HD/D 13.26 66.30 13.26 PERIOD 1 TOTAL---> WEEK WEEK WEEK WEEK 5 6 7 8 PERIOD 2 TOTAL---> WEEK WEEK WEEK WEEK 9 10 11 12 PERIOD 3 TOTAL---> WEEK WEEK WEEK WEEK 13 14 15 16 PERIOD 4 TOTAL---> WSB PEN 8 WEEK WEEK WEEK WEEK 1 2 3 4 FORAGE DMI TOTAL DMI (lbs) (lbs) 336.51 356.26 390.38 368.86 417.66 459.58 493.24 472.12 1452.01 1842.61 417.57 416.94 429.85 446.47 520.45 520.16 535.57 549.32 1710.83 2125.51 408.98 495.88 482.43 561.04 514.47 600.96 588.00 667.64 1948.35 2371.07 558.19 599.75 551.59 576.14 661.54 705.77 654.01 681.50 2285.68 2702.82 TRIAL AVERAGE PEN/LBS/D 66.04 PER/HD/D 13.21 80.73 16.15 PERIOD 1 TOTAL---> WEEK WEEK WEEK WEEK 5 6 7 8 PERIOD 2 TOTAL---> WEEK WEEK WEEK WEEK 9 10 11 12 PERIOD 3 TOTAL---> WEEK WEEK WEEK WEEK 13 14 15 16 PERIOD 4 TOTAL---> 83 TABLE A.8 INFLUENCE OF SUPPLEMENTAL SOYBEAN SOURCE ON PERFORMANCE" (Exp. 2) TREATMENT PEN 1 WSB WEIGH DAYS OF TRIAL AND WEIGHT OF STEERS DAYS----> STEERS 73 5 104 123 121 PEN WT AV WT 0 1 28 56 84 111 112 654 684 608 582 554 672 688 624 580 556 718 740 668 618 581 801 816 719 672 640 875 915 833 742 722 933 935 872 799 769 944 956 896 818 778 3082.00 3120.00 620.20 3325.00 665.00 3648.00 729.60 4087 .00 817 .40 4308.00 870.00 4392 > > 224.00 224.00 8.00 8.00 1.60 323.00 547.00 11.54 9.77 2.31 PEN GAIN FOR PERIOD CUMULATIVE PEN GAIN ADG FOR PERIOD CUMULATIVE ADG ADG PER ANIMAL 439 986 15 11 .00 .00 .68 .74 3 .14 263.00 1249.00 9.39 11.15 1.88 PEN'S DM FEED CONSUMPTION FOR PERIOD > 1957.61 2186.65 2490.61 2685.42 PEN'S CUMULATIVE DM FEED CONSUMPTION > 1957.61 4144.26 6634.87 9320.29 AVG DM FEED CONSUMPTION FOR PERIOD > 391.5219 437.33 498.122 537.084 AVG CUMULATIVE DM FEED CONSUMPTION > 391.5219 828.8519 1326.974 1864.058 FEED/GAIN/PERIOD CUMULATIVE FEED/GAIN 8.74 8.74 6.77 7.58 'Units for all numerical values are given in lbs. 5.67 6.73 10.21 7.46 84 TABLE A.8 (continued) INFLUENCE OF SUPPLEMENTAL SOYBEAN SOURCE ON PERFORMANCE' (Exp. 2) PEN 2 TREATMENT WEIGH DAYS OF TRIAL AND WEIGHT OF STEERS DAYS----> STEERS 55 70 39 107 142 PEN WT AV WT ESB 0 1 28 56 84 111 112 698 678 624 580 586 698 688 632 584 590 752 755 687 626 638 827 848 747 697 722 926 930 840 789 801 979 991 898 830 859 981 998 892 850 870 3166.00 3192.00 635.80 PEN GAIN FOR PERIOD CUMULATIVE PEN GAIN ADG FOR PERIOD CUMULATIVE ADG ADG PER ANIMAL > > > > > 3458.00 691.60 279.00 279.00 9.96 9.96 1.99 3841.00 768.20 383.00 662.00 13.68 11.82 2.74 4286.00 4557.00 4591.00 857.20 914.80 445.00 1107.00 15.89 13.18 3.18 288.00 1395.00 10.29 12.46 2.06 PEN'S DM FEED CONSUMPTION FOR PERIOD > 2057.04 2230.71 2543.33 2730.23 PEN'S CUMULATIVE DM FEED CONSUMPTION > 2057.043 4287.753 6831.083 9561.313 AVG DM FEED CONSUMPTION FOR PERIOD > 411.4086 446.142 508.666 546.046 AVG CUMULATIVE DM FEED CONSUMPTION > 411.4086 857.5506 1366.217 1912.263 FEED/GAIN/PERIOD CUMULATIVE FEED/GAIN > > 7.37 7.37 5.82 6.48 'Units for all numerical values are given in lbs. 5.72 6.17 9.48 6.85 85 TABLE A.8 (continued) INFLUENCE OF SUPPLEMENTAL SOYBEAN SOURCE ON PERFORMANCE* (Exp. 2) PEN 3 TREATMENT SBM+BAR WEIGH DAYS OF TRIAL AND WEIGHT OF STEERS DAYS----> STEERS 137 12 27 35 163 PEN WT AV WT 0 1 28 56 84 111 112 806 636 628 566 538 790 640 632 568 548 904 728 713 637 609 986 800 780 693 661 1092 868 887 774 741 1171 940 950 829 806 1169 945 950 833 820 3174.00 3178.00 635.20 PEN GAIN FOR PERIOD CUMULATIVE PEN GAIN ADG FOR PERIOD CUMULATIVE ADG ADG PER ANIMAL 3591.00 718.20 > > > > > 415.00 415.00 14.82 14.82 2.96 3920.00 784.00 329.00 744.00 11.75 13.29 2.35 4362.00 4696.00 4717.00 872.40 941.30 442.00 1186.00 15.79 14.12 3.16 344.50 1530.50 12.30 13.67 2.46 PEN'S DM FEED CONSUMPTION FOR PERIOD > 2122.57 2343.24 2724.24 2961.07 PEN'S CUMULATIVE DM FEED CONSUMPTION > 2122.573 4465.813 7190.053 10151.12 AVG DM FEED CONSUMPTION FOR PERIOD > 424.5146 468.648 544.848 592.214 AVG CUMULATIVE DM FEED CONSUMPTION > 424.5146 893.1626 1438.011 2030.225 FEED/GAIN/PERIOD CUMULATIVE FEED/GAIN > > 5.11 5.11 7.12 6.00 *Units for all numerical values are given in lbs. 6.16 6.06 8.60 6.63 86 TABLE A.8 (continued) INFLUENCE OF SUPPLEMENTAL SOYBEAN SOURCE ON PERFORMANCE' (Exp. 2) PEN 4 TREATMENT CONTROL WEIGH DAYS OF TRIAL AND WEIGHT OF STEERS DAYS----> STEERS 105 3 91 139 47 PEN WT AV WT 0 1 28 56 84 111 112 666 612 594 572 556 662 602 596 570 560 694 644 635 600 592 704 668 670 617 608 742 692 676 648 644 756 696 704 677 654 768 708 703 691 659 3000.00 2990.00 599.00 PEN GAIN FOR PERIOD CUMULATIVE PEN GAIN ADG FOR PERIOD CUMULATIVE ADG ADG PER ANIMAL 3165.00 633.00 > > > > > 170.00 170.00 6.07 6.07 1.21 3267.00 653.40 102.00 272.00 3.64 4.86 0.73 3402.00 3487.00 3529.00 680.40 701.60 135.00 407.00 4.82 4.85 0.96 106.00 513.00 3.79 4.58 0.76 PEN'S DM FEED CONSUMPTION FOR PERIOD > 1727.28 1830.02 1996.11 2037.44 PEN'S CUMULATIVE DM FEED CONSUMPTION > 1727.281 3557.301 5553.411 7590.851 AVG DM FEED CONSUMPTION FOR PERIOD > 345.4562 366.004 399.222 407.488 AVG CUMULATIVE DM FEED CONSUMPTION > 345.4562 711.4602 1110.682 1518.17 FEED/GAIN/PERIOD CUMULATIVE FEED/GAIN > > 10.16 10.16 17.94 13.08 'Units for all numerical values are given in lbs. 14.79 13.64 19.22 14.80 87 TABLE A.8 (continued) INFLUENCE OF SUPPLEMENTAL SOYBEAN SOURCE ON PERFORMANCE' (Exp. 2) PEN 5 TREATMENT WEIGH DAYS OF TRIAL AND WEIGHT OF STEERS DAYS----> STEERS 95 64 135 102 157 PEN WT AV WT ESB 0 1 28 56 84 111 112 660 626 590 586 544 678 650 612 608 570 729 722 647 653 624 811 801 712 702 682 890 892 948 959 832 827 811 946 957 828 834 779 3006.00 3118.00 612.40 PEN GAIN FOR PERIOD > CUMULATIVE PEN GAIN > ADG FOR PERIOD > CUMULATIVE ADG > ADG PER ANIMAL PEN'S DM FEED CONSUMPTION FOR PERIOD > PEN'S CUMULATIVE DM FEED CONSUMPTION > AVG DM FEED CONSUMPTION FOR PERIOD > AVG CUMULATIVE DM FEED CONSUMPTION > FEED/GAIN/PERIOD CUMULATIVE FEED/GAIN 3375.00 675.00 3708.00 741.60 782 794 745 4103.00 820.60 4377.00 4344.00 872.10 313.00 313.00 11.18 11.18 2.24 333.00 646.00 11.89 11.54 2.38 395.00 1041.00 14.11 12.39 2.82 257.50 1298.50 9.20 11.59 1.84 1872.08 2135.70 2413.28 2593.25 1872.08 4007.78 6421.06 9014.31 374.416 427.14 482.656 518.65 374.416 5.98 5.98 801.556 1284.212 1802.862 6.41 6.20 'Units for all numerical values are given in lbs. 6.11 6.17 10.07 6.94 88 TABLE A.8 (continued) INFLUENCE OF SUPPLEMENTAL SOYBEAN SOURCE ON PERFORMANCEA (Exp. 2) PEN 6 TREATMENT WEIGH DAYS OF TRIAL AND WEIGHT OF STEERS DAYS----> STEERS 66 34 120 94 130 PEN WT AV WT SBM+BAR 0 1 28 56 84 111 112 654 616 592 604 562 672 628 608 615 572 732 700 677 675 643 799 746 729 736 684 857 825 839 830 774 928 808 893 880 862 930 798 854 866 840 3 028.00 3095.00 612.30 PEN GAIN FOR PERIOD CUMULATIVE PEN GAIN ADG FOR PERIOD CUMULATIVE ADG ADG PER ANIMAL 3427.00 685.40 > > > > > 365.50 365.50 13.05 13.05 2.61 3694.00 738.80 267.00 632.50 9.54 11.29 1.91 4125.00 825.00 431.00 1063.50 15.39 12.66 3.08 PEN'S DM FEED CONSUMPTION FOR PERIOD > 1978.03 2248.35 2536.86 PEN'S CUMULATIVE DM FEED CONSUMPTION > 1978.03 4226.38 6763.24 AVG DM FEED CONSUMPTION FOR PERIOD > 395.6059 449.67 507.372 AVG CUMULATIVE DM FEED CONSUMPTION > 395.6059 845.2759 1352.648 FEED/GAIN/PERIOD CUMULATIVE FEED/GAIN > > 5.41 5.41 8.42 6.68 5.89 6.36 'Units for all numerical values are given in lbs. 4371.00 4288.00 865.90 204.50 1268.00 7.30 11.32 1.46 2676.66 9439.9 535.332 1887.98 13.09 7.44 89 TABLE A.8 (continued) INFLUENCE OF SUPPLEMENTAL SOYBEAN SOURCE ON PERFORMANCE° (Exp. 2) PEN 7 TREATMENT CONTROL WEIGH DAYS OF TRIAL AND WEIGHT OF STEERS DAYS----> STEERS 72 11 109 116 92 PEN WT AV WT 0 1 28 56 84 111 112 720 636 628 586 546 700 630 615 570 532 738 664 656 621 565 758 680 669 636 585 782 688 698 663 597 812 715 722 686 621 818 703 738 687 618 3116.00 3047.00 616.30 PEN GAIN FOR PERIOD CUMULATIVE PEN GAIN ADG FOR PERIOD CUMULATIVE ADG ADG PER ANIMAL 3244.00 648.80 > > > > > 162.50 162.50 5.80 5.80 1.16 3328.00 665.60 84.00 246.50 3.00 4.40 0.60 3428.00 685.60 100.00 346.50 3.57 4.13 0.71 3556.00 3564.00 712.00 132.00 478.50 4.71 4.27 0.94 PEN'S DM FEED CONSUMPTION FOR PERIOD > 1719.55 1833.75 1932.43 1939.38 PEN'S CUMULATIVE DM FEED CONSUMPTION > 1719.55 3553.3 5485.729 7425.109 AVG DM FEED CONSUMPTION FOR PERIOD > 343.9099 366.75 386.486 387.876 AVG CUMULATIVE DM FEED CONSUMPTION > 343.9099 710.6599 1097.146 1485.022 FEED/GAIN/PERIOD CUMULATIVE FEED/GAIN > > 10.58 10.58 21.83 14.42 19.32 15.83 'Units for all numerical values are given in lbs. 14.69 15.52 90 TABLE A.8 (continued) INFLUENCE OF SUPPLEMENTAL SOYBEAN SOURCE ON PERFORMANCE* (Exp. 2) TREATMENT PEN 8 WSB WEIGH DAYS OF TRIAL AND WEIGHT OF STEERS DAYS----> STEERS 54 86 50 58 125 PEN WT AV WT 0 1 28 56 84 111 112 648 638 612 590 576 652 654 625 588 578 712 688 658 658 618 775 734 704 724 683 846 826 778 798 758 869 842 833 882 830 880 861 838 890 805 3064.00 3097.00 616.10 PEN GAIN FOR PERIOD CUMULATIVE PEN GAIN ADG FOR PERIOD CUMULATIVE ADG ADG PER ANIMAL 3334.00 666.80 3620.00 724.00 4006.00 801.20 4256.00 4274.00 853.00 > > > > > 253.50 253.50 9.05 9.05 1.81 286.00 539.50 10.21 9.63 2.04 386.00 925.50 13.79 11.02 2.76 259.00 1184.50 9.25 10.58 1.85 PEN'S DM FEED CONSUMPTION FOR PERIOD > PEN'S CUMULATIVE DM FEED CONSUMPTION > 1842.61 2125.50 2370.43 2703.82 1842.61 3968.11 6338.54 9042.36 'Units for all numerical values are given in lbs. 91 TABLE A.9 FEED COST, AND COST/UNIT OF GAIN (Exp. 2) Soybean Meal - $246.00/T Rolled Barley - $130.00/T Soybean Meal/Barley - $201.92/T..@ 1.82 tons fed $367.49 Whole Soybeans $288.00/T..@ 1.85 tons fed $532.80 Extruded Soybeans --- $355.00/T..@ 1.66 tons fed $589.30 Ground Hay $45.00/T..@ 30.95 tons fed...$1392.75 Total Feed Cost $2881.54 TREATMENT ADG Cost of gain SBM+BAR HAY TOTAL $367.49 $369.23 $736.72 2.49 lbs 1.13 kg $.26/lb $.58/kg WSB HAY TOTAL $532.80 $339.29 $872.09 2.18 lbs .99 kg $.36/lb $.79/kg ESB HAY TOTAL $589.30 $345.95 $935.25 2.40 lbs 1.09 kg $.35/lb $.77/kg CONTROL HAY $338.18 .88 lbs .40 kg $.34/lb $.75/kg