−LaNbO Interfacial Charge Transfer and Chemical Bonding in a Ni
advertisement

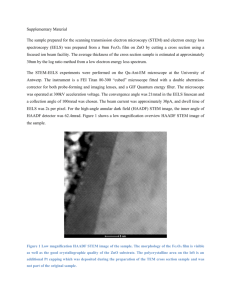
Article pubs.acs.org/cm Interfacial Charge Transfer and Chemical Bonding in a Ni−LaNbO4 Cermet for Proton-Conducting Solid-Oxide Fuel Cell Anodes Despoina-Maria Kepaptsoglou,*,†,∥ Kianoosh Hadidi,† Ole-Martin Løvvik,†,‡ Anna Magraso,§ Truls Norby,§ Anette E. Gunnæs,† Arne Olsen,† and Quentin M. Ramasse*,∥ † Department of Physics, University of Oslo, FERMiO, Gaustadalleen 21, NO-0349 Oslo, Norway SINTEF Materials and Chemistry, Forskningsveien 1, P.O. BOX. 124 Blindern, NO-0314 Oslo, Norway § Department of Chemistry, University of Oslo, FERMiO, Gaustadalleen 21, NO-0349 Oslo, Norway ∥ SuperSTEM Laboratory, STFC Daresbury Campus, Keckwick Lane, WA4 4AD Daresbury, U.K. ‡ S Supporting Information * ABSTRACT: In this work, we present an atomic scale study of the structural and chemical characteristics of interfaces between Ni and LaNbO4 grains in Ni−LaNbO4 cermets, a model composite material for anodes in proton-conducting solid-oxide fuel cells (SOFC). Electron energy loss spectroscopy (EELS) performed in an aberration-corrected scanning transmission electron microscope reveals the absence of reaction or interdiffusion layers at the interface. Changes in the valence state of Ni as well as in the electronic structure of La, reflected by changes in the EELS fine features at the interface, are shown to be related to charge transfer across the interface. The experimental results are in excellent agreement with ab initio calculations based on density functional theory, which predict that direct chemical bonds are formed between the metal and the ceramic at this abrupt interface, resulting in a redistribution of electronic charge across the interface. KEYWORDS: scanning transmission electron microscopy, electron energy loss spectroscopy, density functional theory, charge transfer, proton-conducting solid oxide fuel cells, electrodes, metal/oxide interfaces, LaNbO4 1. INTRODUCTION The demand for efficient and clean energy technologies has led to an increased interest in the direct conversion of gaseous fuels into electricity using fuel cells. High-temperature solid-oxide fuel cells (SOFCs) can be divided into two subcategories, oxygen- and proton-conducting SOFCs, depending on the type of conductivity of the electrolyte material of the cell (oxidic or protonic, respectively).1 Proton-conducting solid-oxide fuel cells (PC-SOFCs) employ acceptor-doped oxides with a high density of oxygen vacancies, where protons dissolve as hydroxide defects in the oxide at the expense of the vacancies.2 The oxide then becomes proton-conducting as the protons are able to jump between their oxide ion hosts. In PC-SOFCs, the electrical current is produced by the electrochemical oxidation of H2 fuel to protons in the anode, which typically consists of porous composite materials,3 such as cermets (ceramic−metal composites). It is thought that the H2 is dissociatively adsorbed from the fuel gas phase onto the metal or ceramic surfaces at or near confined spatial sites, called three-phase boundaries, where electrolyte, gas, and electrode are in contact.3−5 The porosity of cermet anodes provides for this purpose plenty of three-phaseboundary contacts between the percolating phases of the ceramic proton conductor, the metallic electron conductor and the gas phase. Although the effects of geometry and kinetics of fuel cell electrodes are widely studied,3,6−10 the role of the local © 2012 American Chemical Society electronic structure of metal/oxide interfaces and three-phase boundaries in determining the cermet anode functionality is not yet fully understood. In particular, the interface between a metal and an oxide is often characterized by a redistribution of electronic charges across the interface. The spatial extent of this phenomenon depends on the specific system and can arise from various causes such as chemical bonding, formation of secondary phases, or differences in the material work function on either side of the interface. The description and understanding of this charge transfer (which can be positive or negative), while essential for the design and further optimization of PC-SOFCs, is rather complex: different physical and chemical mechanisms can be at play. To a first approximation, the contact can be described electronically in terms of a Fermi level alignment between the oxide and the metal.11 This alignment results in the formation of a space charge layer at the oxide surface and depends on the metal work function and the band gap of the oxide. Locally, the charge redistribution is governed by the presence of discrete energy states in the oxide band gap, which originate from delocalized metal wave functions. These energy Received: July 23, 2012 Revised: October 1, 2012 Published: October 1, 2012 4152 dx.doi.org/10.1021/cm302317f | Chem. Mater. 2012, 24, 4152−4159 Chemistry of Materials Article states are referred to as metal-induced gap states (MIGS)12 and normally extend only a few atomic layers across the interfacial region. Further parameters to be taken into consideration are chemical bonding as well as local structural changes (such as lattice distortions), as all will result in the creation of local electronic interfacial states that could in turn affect the transfer of charges. In this work, we make use of the subangstrom spatial resolution of analytical aberration-corrected scanning transmission electron microscopy (STEM) in order to probe the structure and electronic configuration of a metal/oxide interface at the atomic scale. The material selected for this study is a cermet composite combining Ni- and Ca-doped (0.5% at.) LaNbO4 (hereafter referred to as LNO for simplicity), a potential electrolyte in PC-SOFCs.2,13−15 LNO exhibits relatively high and pure proton conductivity, which, in combination with its excellent chemical stability and chemical compatibility with Ni,16−18 make it a good case study material for the investigation of the electronic structure of interfaces in PC-SOFC anodes. We combine state-of-the-art STEM imaging and electron energy loss spectroscopy (EELS) with ab initio calculations of the electronic and atomistic structure of a Ni/ LNO interface model, in an effort to shed light on the interfacial bonding and charge transfer mechanisms at such technologically important interfaces. was subtracted using a 2-step baseline function, the slope of which was determined by a linear fit across a 50 eV window at the continuum after the L2 Ni white line. The 2-step function was then formed using the continuum slope and steps with a 2:1 height ratio according to the ratio of initial states that should result in a 2:1 ratio transition from the 2p3/2 and 2p1/2 to the continuum.24 The onsets of the steps were set at the maxima of the L3 and L2 peaks, respectively. The ratio of the Ni white lines was then determined by the ratio of the integrated intensity of the baseline-subtracted signal, using Lorenz functions to fit the Ni L3 and L2 edges. The same fitting procedure was applied to spectra of pure Ni as well as those with overlapping Ni and La edges as a validation of the procedure. The edge overlap was separated by fitting the Ni L3 and La M4 edges using Lorentz functions. This background subtraction procedure was followed for all acquired spectrum images for which the overlap in the spectrum images did not exceed 1−1.5 nm (as this corresponds to the estimated delocalization tails of the La M4,5 signal in our experimental conditions25) for individual spectra from the linescans, as well as by averaging 2 to 3 adjacent spectra from each linescan to improve signal-to-noise in the resulting processed data. The experimental O K EEL spectra were compared with spectra calculated with the FEFF9 code that uses the real-space full multiple-scattering theory within the self-consistent muffin-tin potential approximation26 to simulate the near-edge region (ELNES). The spectra were simulated using the initial state approximation (no core-hole) for clusters including 150−200 atoms. The atomic structure and density of states calculations were carried out using spin-polarized density functional theory (DFT)27,28 through the projected augmented wave method (PAW),29,30 as implemented in the Vienna ab initio simulation package (VASP). The generalized gradient approximation within the Perdew−Burke−Ernzerhof scheme (PBE-GGA)31,32 was utilized to account for the exchange-correlation corrections. Convergence tests confirmed that the numerical uncertainty in the calculated total energy for the bulk unit cell of undoped monoclinic LaNbO4 (LNOm) was less than l meV, when using a 6 × 4 × 6 Monkhorst−Pack k-point mesh and a cutoff energy of 850 eV. An undoped LaNbO4 model was used here for simplicity. The addition of a small amount of Ca to LaNbO4 has been shown to improve the conduction properties of the material, and at moderate doping levels, Ca was found to segregate at the LNO domain boundaries.2 However, no Ca was detected through EELS in the anode material investigated for this study, likely due to the very small doping levels involved. The electronic structure of the pure compound can thus be considered a very good approximation to that of the experimentally studied Ca-doped LNO. To reach the same accuracy in the case of the Ni bulk unit cell, a Γ-centered 8 × 8 × 8 k-point mesh and a cutoff energy of 370 eV were sufficient. The ionic geometries in bulk Ni and LNOm were relaxed until the residual forces were less than 0.05 eV/Å. The ionic relaxation of the interface structure was performed using only the Γ-point in k space and a convergence criterion of 0.1 eV/Å for the remaining forces. For the final electronic structure calculations of the total energy of the supercell, we used a cutoff energy of 850 eV and a 3 × 3 × 2 k-point grid. Experimental covalent radii of 1.69, 1.37, 0.73, and 1.21 Å for La, Nb, O, and Ni, respectively, were used to project the local density of states (LDOS). Soft PAW potentials were utilized,31,32 treating only the outermost shells as valence electrons. The valence electronic levels were 5s25p66s25d1 for La, 4p65s24d3 for Nb, 2s22p4 for O, and 4s23d8 for Ni. In order to model the Ni/LNOm interface structure, the LNOm(101) and Ni(100) surfaces were merged, reflecting the present experimental observations of sharp interfaces devoid of any intergranular film. The model structure included 48 atoms in 4 layers of LNOm and 75 Ni atoms distributed in 5 layers. The number of layers for LNO was chosen so that the resulting LNOm slab would be large enough to describe fully the (101) LNO surface. The reasoning behind this choice is described in more detail in ref 4. The Ni lattice was adjusted to the bulk relaxed lattice vectors of LNOm based on the coherent method.33 The Ni primitive translation vector was thus stretched along the longer lattice vector of the (101) LNOm surface at the interfacial plane and compressed along the shorter direction. This 2. EXPERIMENTAL AND COMPUTATIONAL DETAILS The anode cermet was prepared in pellet form by cold-pressing of NiO and LNO powders in a 1:1 ratio and by sintering the resulting pellets at a temperature of 1250 °C. The NiO−LNO pellets were subsequently reduced to a Ni−LNO cermet by heat treatment at 700 °C in wet H2 atmosphere. Further details about the anode preparation and performance are discussed elsewhere by Magraso et al.14,16 Specimens for electron microscopy were prepared from the reduced Ni−LNO pellets; 3 mm disks were drilled out of the center of the pellets using an ultrasonic drill. The disks prepared by mechanical polishing and ion beam thinning at 4 keV using a Gatan PIPS ion mill. High-resolution high-angle annular dark field (HAADF) STEM images and EELS were acquired from three instruments; TEAM 0.5,19 an optimized double-corrected FEI Titan3 microscope equipped with a Gatan Image Filter Tridiem, operated at a primary beam energy of 300 keV; a Cs-corrected VG HB501 dedicated STEM instrument20 operated at 100 keV equipped with a Gatan Enfina spectrometer; and, finally, a Nion UltraSTEMTM100 dedicated STEM operated at 100 keV and also equipped with a Gatan Enfina spectrometer. The inner and outer radii of the HAADF detectors in the conditions used for the experiments were calibrated at 62−280 mrad for TEAM 0.5, 70−210 mrad for the VG HB501, 85−195 mrad for the UltraSTEM, with probe convergence semiangles of 29, 20, and 31 mrad, respectively. For the core-loss EELS, the collection semiangles were 32, 18, and 34 mrad, respectively. In order to determine the ratio of the L3/L2 EELS edges of Ni at the interface between Ni and LaNbO4 grains, the overlapping La and Ni edges must be separated. Except where noted otherwise, the raw coreloss data were denoised using principal component analysis as implemented in the HREM Research MSA plug-in for Digital Micrograph.21 The spectra recorded on the VG HB501 instrument were initially corrected for multiple inelastic scattering by the Fourier ratio method,22 using low-loss EEL spectra recorded in identical experimental conditions: the shorter inelastic mean free path at the lower beam energy of this instrument made this additional step necessary for accurate data interpretation. The background intensity of the spectrum images was subtracted using a power-law baseline, and the intensity of the spectrum image was normalized to the intensity of the continuum after the Ni L2 edge. The methodology described by Pearson et al.23,24 was employed for the determination of the Ni L3/L2 ratio. The remaining background intensity under the Ni white lines 4153 dx.doi.org/10.1021/cm302317f | Chem. Mater. 2012, 24, 4152−4159 Chemistry of Materials Article Figure 1. (a) HAADF STEM images, acquired in the TEAM 0.5 instrument, of a Ni/LNO interface; (b) close-up HAADF image of the same interface showing the rotation of the LNO surface from (−101)m to (101)m across the twinned domains. Figure 2. (a) HAADF STEM image of the Ni/LNOm interface and the path of the EELS linescan; (b) raw EELS spectrum image of the La M4,5 and Ni L2,3 core loss edges, showing the La M5 and Ni L3 overlap; (c) Ni and La white line ratio values plotted as a function of position, together with the intensity of the HAADF image along the linescan. The presented experimental data were acquired in the TEAM 0.5 instrument. leads to lattice mismatches of −6.2% (compression) and 6%, respectively, along these two lattice directions. The Ni lattice constant was kept unchanged perpendicularly to the interfacial plane, only adjusted by the monoclinic angle of LNOm in this direction. The interfacial energy was calculated as 0.045 J/m2; such a low value of the interfacial energy indicates that the supercell model is relatively stable and thus physically relevant. More details about DFT modeling work on the Ni−LaNbO4 system are provided by Hadidi et al.4 the t* subscript: at* = ct* = 5.40 Å, bt* = 11.66 Å, and [010]m∥[010]t*. The transformation from the tetragonal to the monoclinic structure upon cooling past 520 °C is accompanied by the formation of a large density of twin-like domains parallel to the (2 0 5.10)m/(5.10 2 0)m planes.34 Their formation has a visible impact on the orientation of the LNOm surfaces and interfaces; Figure 1a,b shows HAADF STEM images of a Ni/ LNOm interface. In such Z-contrast images, the intensity of each atomic column can be directly related to the nth power (n = 1.5−2) of its average atomic number Z and hence be used as a guide for chemical identification.35 While the La−Nb bright (heavy) columns are clearly resolved, the O positions could not be observed in the HAADF images due to insufficient contrast. All examined interfaces are faceted. The faceting is concave and convex within the same grain in a zigzag manner in order to accommodate the grain distortion due to the rotation between adjacent domains. From the HAADF image (Figure 1b), it is clear that the surface of the LNOm grain rotates sequentially from one type of surface to another across each boundary (in 3. RESULTS AND DISCUSSION At room temperature, LNO has a monoclinic Fergusonite type crystal structure (space group C2/c, am = 5.56 Å, bm = 11.529 Å, cm = 5.206 Å, αm = γm = 90°, and βm = 94.09°). Above 520 °C, it undergoes a structural transformation to the tetragonal Scheelite-type structure (space group I41/α, at = 5.40 Å, ct = 11.66 Å). For simplicity, crystallographic planes and directions are henceforth denoted by m and t subscripts for the monoclinic and tetragonal LNO phases, respectively. Furthermore, the tetragonal structure is hereafter described using the axis of the monoclinic structure and will be denoted using 4154 dx.doi.org/10.1021/cm302317f | Chem. Mater. 2012, 24, 4152−4159 Chemistry of Materials Article Figure 3. (a) HAADF STEM image of the Ni/LNOm interface showing the EELS line scan trace; (b) raw O K core-loss spectra corresponding to the linescan in panel a; (c) experimental EELS O K edge from the Ni/LNOm interface region and simulated O K spectra for several La, Ni, and O containing compounds, carried out using the FEFF9 code. The presented data were acquired in the TEAM 0.5 instrument. reported in the literature for metallic Ni.37−39 Approaching the interface, the L3/L2 ratio increased up to 4 ± 0.3, a value that agrees with those reported for NiO,37,39 suggesting a valence change at the interface and the presence of Ni in a higher valence state (although not necessarily a +2 state) in the vicinity of the LNOm grain. The same increasing trend was observed in all processed spectrum images recorded on all three microscopes, in grains of different orientations, with absolute values showing an overall deviation of 0.3. Moreover, a slight shift of ∼0.5 eV toward lower energies can be observed in the Ni L2,3 edge from the bulk of the Ni grain to the interface (inset in Figure 2c). This shift is indicative of a change in the Fermi energy of Ni: it is systematically observed (although smaller than the energy spread of the instrument used to acquire the data in Figure 2, 0.8 eV), including in data acquired on the VG HB501 instrument (not shown) whose energy resolution is 0.35 eV. These observations thus add strength to the conclusion that a change of valence state of Ni is taking place at the interface.22 Variations in the Ni white line ratio could be explained by the presence of an interfacial reaction layer such as NiOx, which would indeed result in a local valence change. However, no intergranular layer was directly visible in the HAADF images, although as mentioned earlier, due to the polycrystalline nature of the sample, few of the investigated interfaces were perfectly abrupt. Furthermore, the fine features of the O K core-loss EELS edge are known to be sensitive to the local bonding environment. The presence of an oxide phase would therefore be reflected in noticeable EELS signal variations. Figure 3a,b shows an EELS linescan for the O K edge across a typical Ni/ LNO interface and clearly demonstrates that no oxygen is detected on the Ni side of the interface. (See also Figure S1 of Supporting Information.) Background-subtracted spectra from the linescan (Figure 3b) reveal a drop in the total O intensity, but no fine structure change as the probe approaches the interface. In some other data sets (not shown) featuring a slight Ni/LNOm grain overlap, a weak O K edge appears within the overlapping region, but again without any significant change of the edge fine structure. In order to rule out the presence of an intergranular film, FEFF9 simulations of the O K edge were carried out using the code for LaNbO4 and a variety of possible secondary phases, such as La2O3 and La2NiO4 (Figure 3c). The calculations were performed for different crystallographic orientations, showing Figure 1b from (101)m to (101)m and so on), following the rotation of the domains. These surfaces correspond to the (101)t* surface of the high temperature Scheelite phase and will bend in an accordion manner during the inevitable thermal cycling from preparation conditions via room temperature to the eventual operating conditions of the fuel cell. The rotation of the surfaces can thus be expected to introduce further strain and defects at the metal/oxide interface. Although no significant chemical bonding differences are expected at the metal/oxide interface between pristine tetragonal and monoclinic phases,5 elastic strain is expected to influence ionic mobility across the heterointerfaces.36 The electronic structure of the Ni/LNOm interfaces was assessed experimentally by STEM EELS. The white line intensity ratio of transition metals is known to be sensitive to their valence state.21 Therefore, the determination of the L3/L2 intensity ratio for Ni can provide valuable information about its oxidation state at the interface. Core-loss spectra were recorded along lines across Ni/LNOm interfaces; many such data sets were acquired with the LNOm grains in different crystallographic orientations (here showing [010]) in order to exclude orientation and channelling effects, while post mortem images were recorded after the EELS acquisition in order to exclude beam-induced damage to the sample. As the only Nb EELS edge (M4,5) within the usable energy range for these experiments is mostly devoid of any fine features whose changes could indicate electronic reconfiguration, the rest of this discussion will focus on the La, Ni, and O components of the metal/oxide interface. Some as-recorded La M4,5 and Ni L2,3 EEL spectrum images (an example of a linescan is presented in Figure 2a,b, and inset in c), showed a limited overlap of the La M4 and Ni L3 intensity around the interface. As no reaction layer could be directly observed from the HAADF images, the core-loss edge intensity overlap is attributed to grain overlap. The polycrystalline nature of the cermet sample and the random orientation of the grains made the detection and imaging of a perfectly abrupt interface challenging. Some degree of overlap between the La M4 and Ni L3 edges is also expected due to the large EELS tails for the La M4,5 edge signal.25 Figure 2c shows the plot of the Ni L3/L2 and La M5/M4 ratios corresponding to the linescan in Figure 2a, as well as the simultaneously acquired HAADF intensity along the linescan. The L3/L2 ratio deep within the Ni grain was determined to be 3.3 ± 0.2, in good agreement with values 4155 dx.doi.org/10.1021/cm302317f | Chem. Mater. 2012, 24, 4152−4159 Chemistry of Materials Article Figure 4. Ball and stick models showing the simulated interface between the (101) LNOm surface and (100) Ni surface (a) unrelaxed and (b) relaxed. conflicting interpretations.47 However, DFT calculations can be used in order to rationalize the electronic structure variations observed experimentally. The experimental HAADF images thus motivated the creation of an atomistic LNOm(101)/ Ni(100) interface model by merging directly the LNOm(101) and Ni(100) surfaces (Figure 4a,b). Although the supercell model was complex, including 48 and 75 atoms in 4 and 5 layers of LNOm and Ni, respectively, the final relaxed structure was found to possess an interfacial energy of only 0.045 J/m2. Such a low value of the interfacial energy, coupled with the minimal distortions of the Ni lattice, demonstrate the relative stability of this supercell and confirm the physical plausibility of the model. Table 1 presents the calculated interatomic no significant difference with orientation; the simulated spectra plotted in Figure 3c are therefore averaged spectra calculated at the so-called magic angle, with no additional broadening applied. The simulated spectra in Figure 3c are plotted together with an experimental O K spectra from the Ni/LNOm interface (second from bottom curve in Figure 3b), bulk LNOm from the same experiment and NiO from a standard specimen. None of the simulated edges for the secondary oxide compounds exhibit the broad overall shape of the experimental O K edge, which in turn fits rather well the simulated fine structure for the O K in LNOm. Futhermore, the experimental O K edges at the interface are distinctly different from those acquired from the NiO specimen: the latter presents a rather large intensity hump at ∼543 eV (see also ref 40), which is clearly not present in our experimental results. From the above, it becomes clear that the apparent oxidation change of Ni is unlikely to be a consequence of the presence of a NiOx interfacial layer: another mechanism must be at play. Another interesting observation arising from the EELS study is that the white line ratio of La shows a decreasing trend toward the metal/oxide interface, from a value of 1.0 well inside the LNOm grain. In rare earth elements, the M4,5 lines correspond to transitions from the 3d orbitals to unoccupied 4f states (3d104fn → 3d94fn+1 transitions), which are highly localized.41 In some cases such as Ce and Pr, this localization is known to generate changes in the white line intensity ratio depending on the occupancy of the 4f state.42−44 By contrast, for La, which in general is found in a +3 oxidation state, no variation of the La white line ratio has ever been reported, to our knowledge, even though La2+ compounds are known to exist.45 In particular, Mikheeva et al. suggest that no significant change should occur in the 4f band during La oxidation and that occupancy changes should only be expected in the 5d states, therefore not affecting the M4,5 white line intensity ratio in EELS.46 Nevertheless, this La white line ratio change was consistently observed at all interfaces including those with no significant grain overlap, and it must therefore reflect a change in the electronic structure of La, although perhaps not a pure valence change. EELS simulations for the La edges (and more generally for rare earth elements) are notoriously difficult to match accurately to experimental spectra and can lead to Table 1. Interatomic Distances (in Å) between Ni and LNO Elements within the First Coordination Shell, for the Relaxed Interface Structure, Compared to Experimental Values for Bulk Compounds atoms relaxed experimental Ni−La 2.85, 2.94, 2.98 2.92 in all La−Ni compositions Ni−Nb 2.7−2.98 2.69−2.79 Ni6Nb6O Ni−O 1.86−1.98 2.08−2.95 La(NiO3), NiO, NiO2 distances of the modeled LNOm(101)/Ni(100) interface, compared to relevant experimental bond lengths for known compounds.48 In the interfacial model, the calculated interatomic distances between Ni and all constituting elements in LNO were similar to, or smaller than, these experimental interatomic distances. This is a strong indication that chemical bonds are established at the interface between Ni−O, as frequently observed in metal oxide heterointerfaces,49,50 but rather interestingly also between La−Ni. This interpretation is confirmed by the calculated density of states (DOS) for the model interface. Figure 5 presents the LDOS projected onto the La and O atoms at the interface layer as well as that of their nearest Ni neighbors. Only the valence orbitals reflecting chemical reactions have been plotted. The valence orbitals of Ni clearly overlap with the valence orbitals of La (Figure 5b,e) and O (Figure 5a,d). These overlaps can be considered as indirect evidence of chemical bonding between 4156 dx.doi.org/10.1021/cm302317f | Chem. Mater. 2012, 24, 4152−4159 Chemistry of Materials Article Figure 5. Local density of states projected on the atoms at the interfacial LNO and Ni layers (panels, a−e). Each panel presents a specific angular momentum orbital of an interfacial La (solid black) or O (dashed red) atom along with its nearest neighbor Ni (solid magenta) atom. Panels c and f compare the La electronic structure at the interface and in the bulk. The La d and f orbitals are shown for the interfacial layer (La-1; black), the second LNO layer from the interface plane (La-2; gray), and for La in bulk LNO (La-b; dashed green). Figure 6. Contour plot of the electron localization function. A value of 1 means very high localization (low kinetic energy of the electrons), while 0 means no localization. The plots are drawn within planes containing intersecting neighbor Ni and La atoms (left) or neighbor Ni and O atoms (right). The changes in the white line ratio of La are arguably more difficult to relate to the DFT results. Nevertheless, it is clear from the calculated LDOS (Figure 5c,f) that the d and f orbitals of La, both of which have unoccupied electronic states and therefore contribute to the formation of the EELS signal, undergo a considerable change when going from bulk LNOm to the interface structure. For instance, comparing the La d orbitals in the bulk and at the interface reveals that the LNOm band gap disappears at the interface. This phenomenon is Ni−La and Ni−O. It was recently shown that overlap population (or crystal orbital overlap population) diagrams can be used very effectively to qualitatively interpret X-ray absorption near-edge spectroscopy (XANES) or EELS spectra.51 The calculated overlap between the Ni and the La or O atoms LDOS at the LNOm at the interface is thus likely to be the cause of the change in valence of Ni, as experimentally reflected by the increase of the EELS white line ratio of Ni. 4157 dx.doi.org/10.1021/cm302317f | Chem. Mater. 2012, 24, 4152−4159 Chemistry of Materials Article LNOm and is expected to be confined to one atomic layer at the interfacial plane. Experimentally, the change in Ni valence and the La electronic reconfiguration can be considered as direct experimental indicators of this charge exchange. However, because of the complexity of the electronic configuration at the Ni/LNOm interface that cannot be fully described by a fully ionic model, a direct quantitative correlation of the measured white line ratio with the actual charge transfer is difficult to perform. Experimentally, the charge transfer appears to extend over several atomic layers (a distance of about 2 nm; Figure 2c). This extended charge transfer area can likely be attributed to the variation of the relative orientation of the experimental interface with respect to the electron beam throughout the thickness of the TEM specimen. The interface is not perfectly abrupt resulting in partial overlaps of the metal and oxide along the beam direction, at least in some parts. The spatial localization of the valence measurement might also be affected by electron channelling or other delocalization effects of the EELS signal and will in turn result in observing the charge transfer further away from the interface. This study shows that, given a stable coexistent pair of electrolyte and electrode, it is possible to obtain directly a thorough structural, compositional, and electrical characterization of electrode interfaces at an atomic level using advanced analytical microscopy. Although this study does not specifically account for the presence of protons, when combined with atomistic and electron structure modeling, this approach opens up unprecedented possibilities for understanding and interpreting the electrochemical characteristics and performance of SOFC and PC-SOFC electrodes since the electronic structure is decisive for the local solubility and diffusivity of protons across the interface (see relevant work on hydrogen energetics in ref 5). We expect that such studies will eventually be able to predict activation barriers for rate-limiting steps in electrochemical redox reactions, for instance, to be compared with polarization and impedance spectroscopy data from experimental investigations of the electrodes and devices under operating conditions. expected at a metal/oxide interface: the metal and the oxide electronic structures undergo Fermi level alignment12 resulting in the formation of MIGS,5,13 which, in this case, are projected at the La d orbital. Moreover, in the conduction band, we observe a 2.0 eV shift toward lower energies in the f orbital of the interfacial La compared to that in the bulk (Figure 5f). These changes in the LDOS reflect a strong rearrangement in the electronic configuration of La close to the interface. Hence, it is possible to assume that, in a complete electronic picture of the La atoms at the interface, the rearrangement of orbitals, due to the interfacial bonding, alters altogether the availability of the final f states, and this could, in turn, be the cause for the drop in La white line ratio observed in the EELS data. We have thus clearly established the presence of direct chemical bonds between the Ni and LNOm parts of the cermet anode. In order to clarify the nature of the bonds, we have calculated the electron localization function (ELF) at the interface, which gives a direct spatial view of the bond location.52 Contour plots of the ELF have been displayed in Figure 6. The presence of electronic charge density between the La and the Ni, as well as between the Ni and O atoms, is clear evidence that a direct chemical bond is indeed established between these elements at the interface. The high degree of localization in the vicinity of the La and O ions rather than around their Ni ion counterparts results from the presence of delocalized valence electrons in the Ni d orbital. The ELF results thus confirm that charge transfer is taking place from Ni to LNO atom-wise, with direct chemical Ni−La and ionic Ni− O bonds being responsible for the interface dipole. Hence, Bader analysis was employed for the numerical estimation of charge transfer between the atomic species at the interface.32 The sum of Bader charges for each layer relative to the bulk (reference) state of Ni and LNOm is plotted in Figure 7, as a function of position across the interface, the layers being numbered separately for Ni and LNOm sequentially away from the interface. This charge analysis reveals that a partial charge transfer of 1.6 e for the entire simulated model (corresponding roughly to 0.1 e per Ni atom) does take place from Ni to 4. CONCLUSIONS In this work, the detailed atomic distribution and the electronic structure at the interface between Ni and LaNbO4 has been investigated, with particular focus on the origins of charge transfer across the interface. This was achieved through a joint experimental and modeling study, combining atomic resolution scanning transmission electron microscopy and electron energy loss spectroscopy investigations with density functional theory calculations. The experimental results revealed a drastic change of the electronic structure when moving from the bulk to the interface region, quantified by a significant change of the white line ratios of Ni and La, respectively. Despite the consistent increase in the Ni oxidation state, the presence of a Ni oxide layer was excluded. Thus, the interface was shown to be chemically abrupt, leading to the assumption that bonding occurs between Ni and the O of the LaNbO4 phase. The experimental results were compared to DFT calculations of a defect-free model of the Ni/LNO interface. They demonstrated direct chemical bonding and a significant exchange of electrons from Ni to La and O at the interface layer. This charge transfer was quantified by theoretical calculations in very good agreement with the experimental findings. The drastic changes of the La f states at the Ni/LaNbO4 interface found in the calculated LDOS are thought to be the cause of the change in Figure 7. Calculated electronic charge transfer for the four layers of LNO (red squares) and five layers of Ni (blue discs) in the interface super cell. A Bader analysis was used to quantify the charge at different atoms. A positive charge transfer means a gain of negative charge compared to bulk atomic states. The layers are numbered sequentially from the first interfacial layer to the next one along each component (see also Figure 4). The charge differences between interfacial neighbor layers have been indicated: lines are drawn as guide to the eye only. 4158 dx.doi.org/10.1021/cm302317f | Chem. Mater. 2012, 24, 4152−4159 Chemistry of Materials Article (20) Krivanek, O. L.; Dellby, N.; Lupini, A. R. Ultramicroscopy 1999, 781. (21) Watanabe, M.; Ackland, D. W.; Burrows, A.; Kiely, C. J.; Williams, D. B.; Krivanek, O. L.; Dellby, N.; Murfitt, M. F.; Szilagyi, Z. Microsc. Microanal. 2006, 12, 515. (22) Egerton, R. F. Electron Energy-Loss Spectroscopy in the Electron Microscope; Plenum Press: New York, 1996. (23) Pearson, D. H.; Fultz, B.; Ahn, C. C. Appl. Phys. Lett. 1988, 53, 1405. (24) Pearson, D. H.; Ahn, C. C.; Fultz, B. Phys. Rev. B 1993, 47, 8471. (25) Shah, A.; Ramasse, Q.; Wen, J.; Bhattacharya, A.; Zuo, J. M. Micron 2011, 42, 539. (26) Rehr, J.; Ankudinov, A.; Ravel, B.; Jorissen, K. FEFF User’s Guide, version 9.03, 2009. (27) Hohenberg, P.; Kohn, W. Phys. Rev. B 1964, 136, 864. (28) Kohn, W.; Sham, L. J. Phys. Rev. A 1965, 140, 1133. (29) Kresse, G.; Joubert, D. Phys. Rev. B 1999, 59, 1758. (30) Blöchl, P. E. Phys. Rev. B 1994, 50, 17953. (31) Kresse, G.; Furthmüller, J. Phys. Rev. B 1996, 54, 11169. (32) Bader, R. F. W. Atoms in Molecules: A Quantum Theory; Oxford University Press: Oxford, U.K., 1990. (33) Schnitker, J.; Srolovitz, J. D. Modell. Simul. Mater. Sci. Eng. 1998, 6, 153. (34) Prytz, Ø.; Taftø, J. Acta Mater. 2005, 53, 297. (35) Pennycook, S. J.; Nellist, P. D. Scanning Transmission Electron Microscopy; Springer: New York, 2011. (36) Fabbri, E.; Pergolesi, D.; Traversa, E. Sci. Technol. Adv. Mater. 2010, 11, 054503. (37) Leapman, R.; Grunes, L. Appl. Phys. Lett. 1980, 45, 397. (38) Leapman, R.; Grunes, L.; Fejes, P. Phys. Rev. B 1982, 26, 614. (39) Sparrow, T.; Williams, B.; Rao, C.; Thomas, J. Chem. Phys. Lett. 1984, 108, 547. (40) Detemple, E.; Ramasse, Q. M.; Sigle, W.; Cristiani, G.; Habermeier, H.-U.; Benckiser, E.; Boris, A. V.; Frano, A.; Wochner, P.; Wu, M.; Keimer, B.; Van Aken, P. A. Appl. Phys. Lett. 2011, 99, 211903. (41) Moser, H. R.; Delley, B.; Schneider, W. D.; Baer, Y. Phys. Rev. B 1984, 29, 2947. (42) Manoubi, T.; Colliex, C.; Rez, P. J. Electron Spectrosc. Relat. Phenom. 1990, 60, 1. (43) Arai, S.; Muto, S.; Sasaki, T.; Tatsumi, K.; Ukyo, Y.; Kuroda, K.; Saka, H. Solid State Commun. 2005, 135, 664. (44) Fortner, J.; Buck, E. Appl. Phys. Lett. 1996, 68, 3817. (45) Hitchcock, P. B.; Lappert, M. F.; Maron, L.; Protchenko, A. V. Angew. Chem. 2008, 47, 1488. (46) Mikheeva, M. N.; Nazin, V. G. Phys. Solid State 2006, 48, 1219. (47) Tanaka, I.; Mizoguchi, T. J. Phys.: Condens. Matter 2009, 21, 104201. (48) Inorganic Crystal Structure Database (ICSD). http://icsd.fizkarlsruhe.de/. (49) Bocher, L.; Gloter, A.; Crassous, A.; Garcia, V.; March, K.; Zobelli, A.; Valencia, S.; Enouz-Vedrenne, S.; Moya, X.; Marthur, N. D.; Deranlot, C.; Fusil, S.; Bouzehouane, K.; Bibes, M.; Barthélémy, A.; Colliex, C.; Stéphan, O. Nano Lett. 2012, 12, 376. (50) Tusche, C.; Meyerheim, H. L.; Jedrecy, N.; Renaud, G.; Kirschner, J. Phys. Rev. B 2006, 74, 195422. (51) Hoffmann, R. Angew. Chem. 1987, 26, 846. (52) Becke, A. D.; Edgecombe, K. E. J. Chem. Phys. 1990, 92, 5397. the La white ratio experimentally observed. These results show for the first time how atomic-scale analytical electron microscopy measurements coupled with ab initio calculations can be used to characterize directly the nature of the chemical bonds forming across a metal/oxide interface in a complex cermet material. Such understanding is essential for further design and optimization of solid-oxide fuel cells. ■ ASSOCIATED CONTENT S Supporting Information * HAADF survey image and signal map; normalized O K edge spectra; HAADF STEM images. This material is available free of charge via the Internet at http://pubs.acs.org. ■ AUTHOR INFORMATION Corresponding Author *Tel: +44 (0) 1925 864 902. E-mail: dmkepap@superstem.org (D.-M.K.); qmramasse@superstem.org (Q.M.R.). Notes The authors declare no competing financial interest. ■ ACKNOWLEDGMENTS This work is financed by the Research Council of Norway under the NANOMAT program (project 182065/S10). Part of this work was performed at National Center for Electron Microscopy, Lawrence Berkeley National Laboratory, which is supported by the Office of Science, Office of Basic Energy Sciences of the U.S. Department of Energy under Contract No. DE-AC02-05CH11231. The SuperSTEM Laboratory is funded by the U.K. Engineering and Physical Sciences Research Council (EPSRC). ■ REFERENCES (1) Mingh, N. J. Am. Ceram. Soc. 1993, 76, 563. (2) Haugsrud, R.; Norby, T. Nat. Mater. 2006, 5, 193. (3) Mogensen, M.; Skaarup, S. Solid State Ionics 1996, 86−88, 1151. (4) Hadidi, K.; Hancke, R.; Norby, T.; Gunnæs, A. E.; Løvvik, O. M. Int. J. Hydrogen. Energy 2012, 37, 6674. (5) Hadidi, K; Norby, T.; Løvvik, O. M.; Gunnæs, A. E. Int. J. Hydrogen. Energy. 2012, 37, 8033. (6) Weppner, W. Ionics 2001, 7, 404. (7) Chu, W.; Thangadurai, V.; Weppner, W. Ionics 2006, 12, 1. (8) Knauth, P. J. Electroceram. 2000, 5, 111. (9) Stancovski, V.; Stidhar, S.; Pal, U. B. J. Electroceram. 199, 3, 279. (10) Kek, D.; Bonanos, N. Vaccum 2001, 61, 453. (11) Fu, Q.; Wagner, T. Surf. Sci. Rep. 2007, 62, 431. (12) Tersoff, J. Phys. Rev. Lett. 1984, 52, 465. (13) Fjeld, H.; Kepaptsoglou, D.; Haugsrud, R.; Norby, T. Solid State Ionics 2010, 181, 104. (14) Magraso, A.; Fontaine, M.; Larring, Y.; Bredesen, R.; Syvertsen, G. E.; Lein, H. L.; Grande, T.; Huse, M.; Strandbakke, R.; Haugsrud, R.; Norby, T. Fuel Cells 2011, 11, 17. (15) Lin, B.; Wang, S.; Liu, X.; Meng, G. J. Alloys Compd. 2011, 478, 355. (16) Magraso, A.; Haugsrud, R.; Norby, T. J. Am. Ceram. Soc. 2010, 93, 2650. (17) Magraso, A.; Xuriguera, H.; Varela, M.; Sunding, M.; Strandbakke, J.; Haugsrud, R.; Norby, T. J. Am. Ceram. Soc. 2010, 93, 1874. (18) Sunding, M. F.; Kepaptsoglou, D. M.; Diplas, S.; Norby, T.; Gunnæs, A. E. Surf. Interface Anal. 2010, 42, 568. (19) Kisielowski, C.; Freitag, B. M.; Bischoff, B.; van Lin, H.; Lazar, S.; Knippels, G.; Tiemeijer, P.; van der Stam, M.; von Harrach, S.; Stekelenburg, M.; et al. Microsc. Microanal. 2008, 14, 08090. 4159 dx.doi.org/10.1021/cm302317f | Chem. Mater. 2012, 24, 4152−4159