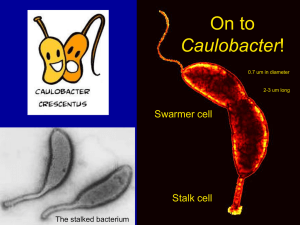
I 201 SEPO SciP: A novel inhibitor of CtrA transcriptional...
advertisement

SciP: A novel inhibitor of CtrA transcriptional activity in Caulobacter crescentus N MASSACHUSE I SEPO by i 201 Kasia G. Gora LIBRAR'ES B.S. Biology California Institute of Technology, 2006 ARCHNVES SUBMITTED TO THE DEPARTMENT OF BIOLOGY IN PARTIAL FULLFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY IN BIOLOGY AT THE MASSACHUSETTS INSTITUTE OF TECHNOLOGY August 8, 2011 © Kasia G. Gora. All rights reserved. The author hereby grants MIT permission to reproduce and distribute publicly paper and electronic copies of this thesis document in whole or in part. Signature of author: Department of Biology August 8, 2011 z I/ A/7 Certified by: Michael T. Laub Associate Professor of Biology Thesis supervisor 1~~) Accepted by: Robert T. Sauer Professor of Biology Co-chair, Biology Graduate Committee vI UT Room 14-0551 77 Masc husetts Avenue MITLibraries Document Services Cambridge, MA 02139 Ph: 617.253. 2800 Email: docs@rit.edu http:ilibraries.m . eduldocs DISCLAIMER OF QUALITY Due to the condition of the original material, there are unavoidable flaws in this reproduction. We have made every effort possible to provide you with the best copy available. If you are dissatisfied with this product and find it unusable, please contact Document Services as soon as possible. Thank you. There are some duplicate numbered pages in this SciP: A novel inhibitor of CtrA transcriptional activity in Cauobacter crescentus by Kasia Gora Submitted to the Department of Biology on August 8, 2011 in partial fulfillment of the requirements of the degree of Doctor of Philosophy in Biology at the Massachusetts Institute of Technology Abstract Cells must sense changes in their environment and respond appropriately in order to survive. A common survival strategy is for cells to translate an environmental signal into the activity of a transcription factor they effects a change in gene expression. In this way, cells can express a gene just-in-time for its biological function. In addition, cells can coordinate the activity of many transcription factors to construct genetic regulatory networks that integrate many inputs to control complex cellular behaviors. In this thesis, I use the model system Caulobactercrescentus to examine the regulation of CtrA, the essential transcription factor at the core of the Caulobactercell cycle regulatory network. CtrA regulates the activity of approximately 100 cell cycle genes many of which are critical for cell cycle progression. CtrA activity is regulated at the level of abundance, post-transcriptional modification, and here I show that CtrA is also regulated by a novel protein-protein interaction. I identify SciP, a GI specific inhibitor of CtrA transcriptional activity and show that SciP forms a complex with CtrA at CtrA dependent promoters. The SciP/CtrA interaction likely prevents CtrA from recruiting RNA polymerase thereby blocking the activation of transcription. In addition, I show that SciP is restricted to G1 by regulated proteolysis. I identify the Lon as the SciP protease and show that Lon is required for SciP proteolysis in vivo and that E. coli Lon degrades SciP in vitro. Finally I engineer a stable allele of sciP and show SciP proteolysis is critical for proper cell cycle progression. Thesis Advisor: Michael T. Laub Title: Associate Professor of Biology Acknowledgements I would like to thank Mike Laub for taking me on as his graduate student and giving me the opportunity to work in his lab. Mike has been a wonderful mentor. He is wicked rigorous and wicked smart. Thank you for investing so much time in developing my project and sharpening my communication skills. I hope that I have risen to meet your high expectations. I would also like to acknowledge Barrett Perchuk for his invaluable scientific and moral support. Without his technical expertise I could not have been so productive. Barrett is also my dear friend. Barrett, you made every day in lab a pleasure, even that bad ones. Thank you for your unwavering friendship and support. It has been a privilege to work with all the Laub lab members past and present. I would especially like to acknowledge Christos Tsokos, Erin Chen, and Andy Yuan for helping me with key experiments and Jeff Skerker for his contributions to building the infrastructure of the lab. I would like to thank Emily Capra, Andy Fan, Erin Chen, Josh Modell, and Celeste Peterson for all their invaluable help editing this manuscript. Emily and Andy Fan went above and beyond to help me edit the introduction. Emily is my two-component fairy godmother and Andy is the yeast master. In addition Emma Lubin, Kristina Jonas, Chris Aakre, Anna Podgornaia and Diane Baer have all contributed to making the Laub lab a wonderful place to work. It has been a true pleasure to work with our honorary lab member Orr Ashenberg and his fellow Keating lab member Aaron Renkie. I would like to thank Matt Wohlever for his enthusiastic work performing in vitro degradation. I would also like to thank the members of my thesis committee Angelika Amon and Alan Grossman for years of thoughtful comments, helpful advice, and advocacy. Thank you Eric Alm for being my outside examiner and a generally cool dude. In addition, Frank Solomon has also been an incredible mentor in science, career, and life. Thank you Tania Baker, Bob Sauer, Amy Keating, and Steve Bell (and Radar) for taking the time out of your busy schedules to chat. Finally, a special thank you to Tony Sinskey for putting in a good word and helping me find my first job. I would like to thank all my friends who have supported me over the years. Thank you Jennifer Patton, Alex McCauley, Marya Shiekh, Paul Wiggins, Hila Hashemi and the entire CMT crew, Michael Betancourt, Matt Walker, Richard Eager, Cindy Chen, Eric Spear, Mary Lee, Lori Ling, Bernardo Pando, Drew Nager, Mihir Mehta, Emilia Bora, Mihai Bora, John Allman, Jared Leadbetter, Eric Matson, Alden Waters, Kaveh Milaninia, Paola Rubesco, Jane & Marty, Zenzie Brooks, and especially Matthieu Couturier and our fluff-nugget Darwin. Thank you to the cheerful staff of Starbucks for fueling my Ph.D. Most of all I'd like to thank my family for their unconditional love and support that have made possible this amazing opportunity. Mom, Dad, and Michael, thank you for holding my hand through the last few months, I could not have finished without you. Thank you Oscar, Poosha, and Peanut for all your love and cuteness. Table of Contents Ab stract ................................................................................................................................ 2 Acknowledgements....................................................................................................... 3 Chapter 1: Introduction................................................................................................... 9 Regulation of transcription ................................................................................ 10 Transcriptionand the yeast cell cycle ................................................... 15 Two-component systems........................................................................ 18 Regulating two-component systems .................................................... 23 Elaboration of two-component systems in the Caulobactercell cycle..............26 Caulobacteras a cell cycle model ........................................................ 27 CtrA, master regulatorof the Caulobactercell cycle............................ 30 Regulation of CtrA ................................................................................ 35 Identifying missing regulatorsof CtrA ................................................ 39 References ........................................................................................................ Chapter 2: A cell-type-specific protein-protein interaction modulates transcriptional activity of a master regulator in Caulobactercrescentus ............................................ 41 46 Abstract ................................................................................................................. 47 Introduction ...................................................................................................... 47 1 Resu lts....................................................................................................................5 Identification of sciPas a candidate regulatorof CtrA and the cell cycle51 SciP is present only in GJ swarmer cells............................................... 54 sciP is requiredfor proper swarmer cell development and cell cycle progression........................................................................................... 55 Depletion of SciP affects CtrA -dependentgene expression...................58 Overexpressing sciP disrupts the cell cycle and drives the downregulationof CtrA -dependent genes ..................................................... 59 SciP does not affect CtrA degradationorphosphorylation...................63 SciP binds directly to CtrA .................................................................. 65 SciP binds CtrA without disruptingDNA-binding................................ 67 Discussion ....................................................................................................... 70 Regulation of SciP................................................................................. 72 M echanism of inhibition CtrA ................................................................... 73 Regulating response regulators............................................................ 74 Concludingremarks .............................................................................. 75 Experimental procedures .................................................................................. 76 Acknowledgements............................................................................................ 87 References..............................................................................................................88 Chapter 3: The Caulobactercell cycle regulator SciP is proteolyzed at the G1 -to-S transition by Lon ................................................................................................................ 91 Introduction............................................................................................................92 2 R esu lts....................................................................................................................9 SciP is regulatedprimarily at a post-transcriptionallevel ................... 92 SciP is an unstable protein, but is not degradedby ClpXP, ClpAP, or HslUV .................................................................................................... 93 SciP is degradedby Lon ....................................................................... 96 Addition of a C-terminal tag interferes with SciPproteolysis...............97 Proteolysis of SciP is necessaryfor proper cell cycle progression........... 99 Discussion............................................................................................................102 Post-translationalRegulation of SciP ..................................................... 102 Regulated Proteolysis by Lon .................................................................. 102 ConcludingRemarks................................................................................104 Experim enal Procedure........................................................................................105 Acknowledgem ents..............................................................................................109 References............................................................................................................110 Chapter 4: Conclusions and future work ......................................................................... 112 Characterizing the interaction between CtrA and SciP........................................114 Does SciP contactDNA and if so, how? .................................................. 114 Why does SciP requirefree DNA for complex formation?...................... 117 What is the molecular surface that mediates the interactionbetween CtrA 118 andSciP ................................................................................................... Regulated proteolysis and the Caulobactercell cycle.........................................119 Outstanding questions in cell cycle regulated transcription in Caulobacter....... 121 List of Figures and Tables Chapter 1: Introduction Figure 1.1 Three modes of activation of gene expression by transcription factors 1 Figure 1.2 Activation of eukaryotic transcription............................................. 13 Figure 1.3 Regulation of metazoan transcription factor E2F.............................14 Figure 1.4 S. cerevisiaecell cycle..................................................................... 16 Figure 1.5 S. cerevisiae cell cycle transcription network ................................. 17 Figure 1.6 Two-component architecture.......................................................... 22 Figure 1.7 Connector proteins in two-component systems...............................25 Figure 1.8 Caulobactercell cycle..................................................................... 28 Figure 1.9 Flagellar assembly............................................................................ 29 Figure 1.10 CtrA activity and regulation through the cell cycle.......................30 Figure 1.11 Dual function of CtrA in regulation of the origin and cell-cycle regulated transcription ....................................................................................... 32 Figure 1.12 PhoB activation by phosphorylation ............................................ 34 Figure 1.13 Activation of transcription by CtrA...............................................35 Figure 1.14 Regulation of CtrA transcription ................................................... 36 Figure 1.15 Differential activation of CtrA at the swarmer and stalked pole........37 Figure 1.16 CtrA proteolysis by ClpXP ........................................................... 38 Chapter 2: A cell-type-specific protein-protein interaction modulates transcriptional activity of a master regulator in Caulobactercrescentus Figure 2.1 Identification of sciP as a key regulator of cell cycle progression.......52 Figure 2.2 Multiple sequence alignment of SciP orthologs...............................53 Figure 2.3 Flow cytometry analysis of cells from Figure 2.1E ......................... 55 Figure 2.4 SciP depletion phenotype ................................................................ 57 Figure 2.5 Effects of sciP overexpression and depletion on CtrA-dependent gene . 61 expression ........................................................................................................ Figure 2.6 Phenotypic consequences of sciP overexpression............................62 Figure 2.7 Morphology and chromosomal content offtsZ depletion strain...........65 Figure 2.8 SciP and CtrA interact directly in a yeast two-hybrid assay ............ 66 Figure 2.9 Model of SciP structure ................................................................... 67 Figure 2.10 Electrophoretic mobility shift assays with CtrA and SciP ............. 69 Figure 2.11 Regulation of CtrA-dependent gene expression during cell cycle p rogression ............................................................................................................. 71 T able 2.1 Strains list ......................................................................................... 85 Table 2.2 Prim ers list ......................................................................................... 86 Chapter 3: The Caulobactercell cycle regulator SciP is proteolyzed at the GI -to-S transition by Lon Figure 3.1 Post-transcriptional regulation of SciP............................................ 93 Figure 3.2 SciP stability in various protease mutant strains .............................. 95 Figure 3.3 In vitro proteolysis of SciP by E. coli Lon ..................................... 97 Figure 3.4 Stabilization of SciP through an C-terminal M2 tag ........................ 98 Figure 3.5 Phenotypic consequences of stable SciP ............................................ 100 T able 2.1 Strains list ............................................................................................ 108 T able 2.2 Prim ers list ........................................................................................... 108 Chapter 4: Conclusions and future work Figure 4.1. Dispensability of the putative SciP motif in vitro and in vivo...........116 Figure 4.2. Modeling the three-dimensional structure of SciP ............................ 118 Figure 4.3. Electrophoretic mobility assays with mutant CtrA and SciP ............ 119 Chapter 1 Introduction Introduction Regulation of transcription in cells Signal transduction is fundamental to the lives of all cells. In order to sense environmental stimuli or to internally regulate cellular events, a cell must properly translate an internal or external signal into an effect. One of the most common effects, or outputs, of signaling pathways is a change in activity of a transcription factor and, consequently, a change in gene expression. In this way, cells adaptively regulate the expression of genes just-in-time for their biological function. How do transcription factors activate gene expression? In bacteria, genes are transcribed by a five subunit RNA polymerase complex (a2pp'o) in association with a sigma factor [1]. The sigma factor typically recognizes DNA sequences near the -35 and -10 positions relative to the transcriptional start site allowing polymerase to bind to the double stranded DNA and form a closed-complex [1, 2]. Next the complex undergoes a conformational change, melting the DNA strands to form an open-complex that can initiate transcription [2]. Transcription factors can activate transcription in at least three general ways: regulated recruitment of RNA polymerase, activation of RNA polymerase, and direct promoter activation [2]. A classic example of the regulated recruitment of RNA polymerase by a transcription factor comes from studies of the lac operon of E. coli. The lac operon consists of several genes involved in lactose metabolism including lacZ, a gene encoding p-galactosidase, an enzyme that cleaves lactose [2]. Although E. coli can metabolize lactose, glucose is the preferred energy source and the lac operon has evolved to highly express lactose metabolism genes when glucose is unavailable and lactose is present [3]. The lac operon is normally repressed by the binding of the lac repressor, Lac, to the -10 region of the lac promoter thereby occluding RNA polymerase and preventing transcription (Fig. 1.1A) [4]. Allolactose, a lactose metabolite, can bind LacI, thereby relieving repression of the lac operon. In the absence of glucose, the transcription factor CAP (catabolite activator protein) is binds the signaling molecule cAMP activating CAP to bind the lac promoter and recruit RNA polymerase that is bound to 070 through a direct interaction with the a subunit of RNA polymerase (Fig. 1. lA) [5]. In this way E. coli can induce lactose metabolism genes just when they are needed. allolactose .3t ~~~~0~ lacZ W44IacZ .35 inerT Figure 1.1. Three modes of activation of gene expression by transcription factors. (A) Regulated recruitment of RNAP by CAP. (B) NtrC activation of RNAP. (C) MerT activation of the mer operon promoter. Adapted from [2]. In contrast to the lac operon, the gin operon of E. coli requires polymerase activation rather than recruitment [2]. The gin operon encodes three genes important for nitrogen metabolism including ginA, a gene that encodes glutamine synthetase, an enzyme involved in glutamine synthesis [6]. The amino acid glutamine is an important component of proteins and precursor for the synthesis of other nitrogen containing molecules by the cell [6]. Expression of the gin operon is activated by NtrC, a transcription factor that binds more than 100 nucleotides upstream of the promoter [6]. NtrC binds DNA in response to nitrogen starvation and the NtrC/DNA complex loops around to make contact with the closed complex of RNA polymerase bound to the c54 leading to isomerization from the closed to open complex (Fig. 1.1.B) [6]. In this way NtrC activates the gin operon to express glutamine synthetase and make glutamine when the cell is starved of nitrogen. As a final example, the mer operon requires activation of the promoter [2]. In many Gram-negative bacteria, the transposon encoded mer operon (merTPAD) confers resistance to the toxic heavy metal mercury [7]. Expression of the mer operon is regulated by the transcription factor MerR [7]. In the absence of mercury, MerR forms an inactive closed-complex with RNA polymerase at the mer operon promoter, but when mercury is present, it binds MerR, causing a conformational change in the protein that allows it to distort the DNA and thereby activate transcription (Fig 1.1.C) [8]. This mechanism allows MerR to turn on mer operon only in the presence of mercury. In many ways, transcriptional activation in eukaryotes is an elaboration of prokaryotic mechanisms. Unlike prokaryotes, eukaryotes have several different RNA polymerase enzymes but the majority of genes are transcribed by Poll which is structurally similar to prokaryotic RNA polymerase [5]. Eukaryotic Poll promoters are often characterized by a TATA box, an AT-rich region 50-70 base pairs upstream of the transcriptional start site which plays an analogous role to the prokaryotic -10 and -35 boxes [5]. Unlike bacteria, yeast require many global transcription factors including TBP and TFIIB to activate transcription [5]. TBP (TATA binding protein) plays the critical role of recognizing and binding the TATA box and in association with a host of associated factors forms the TFIID complex. In a gross simplification, TFIID recruits the holoenzyme consisting of PolIl and many other factors through TFIIB, a factor that acts as a protein bridge between TBP and the holoenzyme complex (Fig. 1.2) [9]. In yeast, transcription factors generally have two separable domains, a DNA binding domain and an activating domain that together can activate transcription through regulated recruitment of PolII [5]. The activating domain interacts with the holoenzyme and components of TFIID while the DNA binding domain binds between 100 and 250 base pairs upstream of the transcriptional start site (Fig. 1.2) [5]. TATA Figure 1.2. Activation of eukaryotic transcription. The TFlD complex recognizes the TATA box and binds the RNA polymerase holoenzyme through TFIlB. Eukaryotic transcription factors engage in regulated recruitment of the holoenzyme by interacting with DNA through a DNA binding domain (BD) and the holoenzyme through the activating domain (AD). Adapted from [5]. Transcription factors play a key role in regulating gene expression so it is no surprise that their activity is strictly regulated. For instance the metazoan transcription factor E2F drives the expression of hundreds of genes in late G1, many of which are critical for proper progression of S phase [10]. E2F activity is regulated in at least three ways: abundance, protein-protein interactions, and post-transcriptional regulation (Fig. 1.3). First E2F abundance is regulated at the transcriptional level with mRNA accumulating in GI [11]. Secondly, in GO, E2F is prevented from binding DNA and activating transcription by a protein-protein interaction with the Rb protein [10]. During G1, Rb becomes phosphorylated and releases E2F initiating the late G1 transcriptional program [10]. Finally in S phase, E2F itself becomes phosphorylated which reduces its ability to bind promoters and ensures that the E2F regulon is only expressed during a brief window in late GI [12]. GO GI Figure 1.3. Regulation of metazoan transcription factor E2F. In GO, E2F is inactivated by a protein-protein interaction with Rb and is released by Rb phosphorylation in G1 at the same time E2F mRNA accumulates. E2F is phosphorylated and inactivated in S phase. Transcription and the yeast cell cycle Our previous examples have focused on the regulation of individual genes or operons by individual transcription factors. However, many transcription factors can interact to form genetic regulatory networks that control complex processes. For instance, a complex transcription factor network controls cell cycle gene expression in yeast ensuring that these genes are expressed at their time of function [13]. This genetic regulatory network interacts with a biochemical oscillator to drive progression of the cell cycle. Because the biochemical oscillator was characterized before the genetic regulatory network, I will begin with a brief overview of its key players and then discuss the role of regulated transcription in the cell cycle. In eukaryotes, cyclin-dependent kinases (Cdks) are at the core of the biochemical cell cycle oscillator. While metazoans have several Cdks, the budding yeast Saccharomyces cerevisiae has only one, Cdc28, which phosphorylates different substrates based on its association with 9 different cyclins: Cln3 interacts with Cdc28 in GI, Clnl and Cln2 at the G1-to-S transition, Clb5 and Clb6 in S phase, and Clbl, Clb2, Clb3, and Clb4 in M phase (Fig 1.4) [14, 15]. For instance in S phase, the Cdc28/Clb5 complex phosphorylates key proteins that activate origins of replication to permit the initiation of DNA synthesis [16]. During M phase, another complex, Cdc28/Clb2, phosphorylates Pdsl, protecting it from degradation [17]. Pdsl is an inhibitor of Espl, a chromosome seperase protein. Therefore Cdc28/Clb2 activity prevents premature chromosome seperation by stabilizing Pds 1 [18]. These are just a few of the many examples of key cell cycle processes that are regulated by Cdc28 activity. Clbi,2 3,4 G2 Cdc28 cn Clb5,6 So Cini,2 S assoiatin wih vDNArosinoteisTe replication elld ylrtae r clrcdeensherlvn cycinsareindcae ngretpanel [1,1] bud fo Start Figure 1.4. S. cerevisiae cell cycle. Key events of the yeast cell cycle are regulated by Cdc28 in association with various cyclin proteins. The cell cycle stages are color-coded and the relevant cyclins are indicated in grey panels [14, 15]. In addition to the biochemical oscillator, a group of transcription factors ensures that cell cycle genes are expressed at the appropriate time. The key players include Mbp 1, Swi4, Swi6, Mcml, Fkhl, Fkh2, Nddl, Swi5, and Ace2 (Fig. 1.5) [13]. In late GI the SBF complex (Swil and Swi6) and the MBF complex (Mbpl and Swi6) drive the expression of 200 genes, the so-called Cln cluster [19]. SBF controls the expression of genes involved in budding while MBF regulates genes involved in DNA replication and repair [19]. Next, in G2 phase, Fkhl and Fkh2 activate transcription of the Clb2 cluster, -35 that are involved in mitosis [20]. Activation of Fkh2 requires the phosphorylated transactivator Nddl [21]. In M phase, Mcml drives the expression of the MCM cluster of genes involved in DNA synthesis [20]. Finally in late M phase and G1, Swi5 and Ace2 control expression of the Sici cluster [22, 23]. These transcription factors form a regulatory network in which transcription factors expressed in one stage of the cell cycle activate those expressed in the next stage [13]. For instance, in late GI, SBF and MBF both regulate expression of Nddl, the key activator of G2 expression by Fkh2 [13]. Then in G2, Fkh2 and Nddl regulate the expression of Swi5 and Ace2 which act in M phase [13]. Finally, M phase Mbpl activates the expression of Swi4 that acts in late GI closing the regulatory loop (Figl.5) [13]. Swi5 Ace2 Mcm1 Fkh2 Nddi Swl6 G2 S Swl6 Sw14 Mbp1 start Figure 1.5. S. cerevisiae cell cycle transcription network. Key transcription factors are indicated in grey ellipses at their time of activity. General interactions between phase specific clusters are indicated by arrows. Adapted from [13]. The biochemical oscillator and transcription network driving cell cycle progression in yeast have many points of interaction. For instance Swi5 and Ace2 regulate the Cln3 promoter [13]. In turn both Swi4 and Swi6 are targets of Cdc28/Cln3 in vitro and Swi6 has been shown to be phosphorylated in a Cdc28/Cln3 dependent manner in vivo [22, 24- 17 26]. In addition, SBF is inhibited by association with the protein Whi5 until Whi5 becomes phosphorylated by Cdc28/Cln3 and releases SBF to activate transcription [27]. SBF and MBF also bind the Clnl,2 and Clb5,6 promoters [13]. Furthermore the transactivator Nddl is a substrate of Cdc28/Clb2 creating a positive feedback loop that ensures Clb2 expression occurs only late in S phase [28]. Cdc28 activity clearly influences cell cycle gene expression; however, recent work shows that the majority of cell cycle regulated transcription remains periodic in a strain lacking all S and M phase cyclins suggesting that the transcription factor network is autonomous of the biochemical oscillator [29]. Two-component systems A common way of modulating gene expression in bacteria involves two-component signal transduction systems. These signaling pathways allow cells to sense and respond to their internal or external environment. At their core, two-component systems consist of a histidine kinase and a response regulator. In response to a signal, the kinase typically autophosphorylates a conserved histidine residue and then specifically transfers the phosphoryl group to a conserved aspartate residue on the response regulator. Many response regulators contain DNA binding domains, and thus phosphorylation can lead to downstream transcription by activating DNA binding ability [30]. In addition to phosphorylating response regulators, many histidine kinases are bifunctional and can act as phosphatases, dephosphorylating their cognate regulator when not activated to autophosphorylate [30]. Histidine kinases consist of a modular kinase core coupled to a sensor domain. Sensor domains vary greatly but a common topology is a periplasmic sensor consisting of two transmembrane helices [31]. Other kinases are soluble and contain PAS or GAF domains that allow them to bind and sense small molecules [31]. In contrast to the sensor domain, the catalytic core contains a highly conserved C-terminal catalytic and ATP binding (CA) domain and a slightly more variable dimerization and histidine phosphotransfer (DHp) domain [31]. The CA domain catalyzes the transfer of the phosphoryl group from ATP to a conserved histidine residue located in the DHp domain [31]. As the name suggests, the DHp domain also mediates dimerization, which is essential for autophosphorylation, although the autophosphorylation reaction can occur either in cis or trans [30]. Unlike many eukaryotic kinases, histidine kinase dimerization is not induced by the signal; histidine kinases usually exist as dimers even when not active as kinases [31]. Histidine kinases are modular and functional chimeras can be created by swapping sensor domains and kinase cores of different proteins [32]. Response regulators are also modular and consist of a receiver domain and an effector domain [31]. Receiver domains are conserved and contain the active site that receives the phosphoryl group from the kinase on a conserved aspartate residue. Effector domains are more variable but most encode DNA binding domains [31]. In these transcription factor response regulators, phosphorylation of the receiver domain can promote protein-protein interactions, like dimerization, that activate the response regulator to bind DNA and change gene expression [30]. One of the best-studied two-component systems is the EnvZ-OmpR system that controls osmoregulation in E. coli (Fig. 1.6A). In response to osmotic shock, the histidine kinase EnvZ is activated and phosphorylates its cognate response regulator OmpR. Phosphorylation of OmpR induces dimerization allowing OmpR to bind DNA and act as a transcription factor. OmpR regulates the expression of two porin genes, ompF and ompC, whose differential expression allows the cell to balance ions between the cytoplasm and periplasmic space and thus protect the inner membrane from excessive turgor pressure [31, 33]. An elaboration of the canonical two-component pathway is the phosphorelay. In a phosphorelay, the histidine kinase transfers a phosphoryl group to a response regulator, which in turn transfers the phosphoryl group to a histidine phosphotransferase (HPt), which transfers it to another response regulator [31]. The addition of extra steps in a phosphorelay has been speculated to allow for more complex regulation of the output. A classic example of a phosphorelay controls the initiation of sporulation in B. subtilis (Fig 1.6B). In response to physiological cues, KinA and several other kinases phosphorylate an aspartate residue on the response regulator SpoOF. SpoOF then phosphorylates a histidine residue on the phosphotransferase SpoOB. In turn, SpoOB phosphorylates the terminal response regulator SpoQA which increases its affinity for DNA and hence its ability to activate target genes [34]. SpoOA directly regulates the expression of over 100 genes involved in initiating the sporulation genetic regulatory network [35]. The intermolecular connectivity between components of a phosphorelay can vary. In the sporulation example, each component comprises its own protein. However different components are often combined within a single protein. Generally it is the kinase that is fused to the first receiver domain to form a so-called hybrid kinase [30]; the histidine phosphotransferase can also be fused to a hybrid kinase. For instance the ArcB-ArcA system of E. coli contains the same number of elements as the B. subtilis sporulation phosphorelay, but the first three are fused into a single hybrid kinase, ArcB, with the terminal regulator, ArcA, a separate, soluble protein (Fig. 1.6C). In response to anoxia, ArcB autophosphorylates on the conserved histidine in the catalytic core and relays phosphate to an aspartate on a receiver like domain, then to a histidine on a HPt like domain, and finally to an aspartate on the response regulator ArcA. ArcA in turn represses genes involved in aerobic respiration which would be energetically wasteful under anaerobic conditions [36]. A. E. coi Osmoregulation P mechanical stress froms osmotic shock? P P regulation of porin genes ompF, ompC OmpR EnvZ B. B. subtilis Sporulation P His P P KinA SpoOF SpoOB * P ~ SpoOA Induction of the sporulation genetic regualtory network KinB-D B. E. coil Anoxic Response redox state of eclector carriers or membrane potential? 6 ArcB j P P P represison of aerobic metabolism genes " ArcA Figure 1.6. Two-component architecture. (A)The EnvZ/OmpR two-component system from E. coli. Phosphate flow from the kinase core (yellow bullet) to the receiver domain (blue box) is indicated by the arrow. The input and output domains are represented by the gray and purple arrows respectively. (B) The SpoQA phosphorelay from B. subtilis schematized as in (A) with the addition of a receiver domain (teal box) and an HPt (orange ellipse). (C) The ArcB hybrid kinase from E. coli. Adapted from [30, 37]. Given the utility and flexibility of two-component systems, it comes as no surprise that most bacterial genomes encode dozens to hundreds kinase-regulator pairs that allow cells to respond to diverse environmental signals [32]. Unlike eukaryotic kinases that often phosphorylate many target proteins, histidine kinases are generally specific for their cognate response regulators and preferentially phosphorylate them over other possible targets [32]. How then do cells ensure the specificity of so many paralogous signaling proteins? The answer lies in molecular recognition between the kinase and regulator rather than contextual strategies such as scaffolds. Histidine kinases typically have a strong kinetic preference for their cognate regulator over all other regulators in a cell [38]. Recent studies have identified a small set of residues in the DHp domain of E. coli EnvZ that confer specificity for its cognate regulator OmpR. Mutating three residues in the wild-type E. coli EnvZ to those found in RstB was sufficient to change the specificity of EnvZ from OmpR to RstA, the cognate regulator of RstB [39]. Subsequent work demonstrated that mutating just four residues in E. coli OmpR to the RstA sequence was sufficient to change specificity for EnvZ to RstB [40]. Thus, histidine kinases and response regulators can ensure the specificity of partner recognition through a few key specificity residues. Regulating two-component systems Two-component signaling pathways transduce information by converting various input signals into the output of a response regulator's phosphorylation level. As described in the previous section, activation of a histidine kinase by its ligand or signal results in autophosphorylation and then phosphotransfer to its cognate regulator. However, additional factors can influence the flow of phosphate and therefore the output of the system. For example, protein phosphatases exist that dephosphorylate some response regulators. Other factors can modify the output of a response regulator without affecting their phosphorylation status, often by binding directly to a response regulator and preventing its interaction with DNA. Collectively, factors that modulate phosphate flow through a two component system are called connectors because they can provide links between different regulatory systems [41]. Connectors can act at any step of the phosphorelay; below I will discuss a few key examples for connectors whose mechanism of action has been elucidated. Some connectors act at the first step of the phosphorelay by regulating the autophosphorylation of a histidine kinase. One such example is the B. subtilis protein Sda, which blocks autophosphorylation of KinA (Fig. 1.7A) [42]. Sda is thought to bind directly to KinA and prevent the CA domain from passing the phosphoryl group to the conserved histidine in the DHp domain. [43]. The sda gene is activated by the replication initiation/transcription factor DnaA; Sda protein is highly expressed when DnaA activity is high as may occur when during the initiation of replication at the beginning of each cell cycle [44]. Therefore expression of Sda prevents downstream phosphorylation of SpoOA, preventing the initiation of the sporulation in cells that have not finished replicating their DNA [44]. Other connectors act as phosphatases and directly dephosphorylate response regulators. Several examples of phosphatases exist in the B. subtilis sporulation phosphorelay. For instance members of the Rap family of phosphatases (RapA, RapB, RapE, and RapH) all directly dephosphorylate the response regulator SpoOF (Fig. 1.7A) [45-47]. Dephosphorylation of SpoOF results in back transfer of phosphate from SpoOA to SpoOB back onto SpoOF, effectively preventing sporulation by dephosphorylating SpoOA [45]. The expression of some Rap phosphatase genes is induced during competence, an alternative differentiation pathway where the bacteria can take up foreign DNA, and others are induced during vegetative growth [45-47]. Thus the Rap phosphatases allow B. subtilis to prevent inappropriate sporulation when the cells are growing vegetatively or initiating competence. The SpoOE family of phosphatases, including SpoOE, YisI, and YnzD, directly dephosphorylate SpoOA and are also differentially regulated by alternative differentiation and growth pathways (Fig 1.7A) [48, 49]. RapA/BIHIE SpoOE/YisIlYnzD Sdax p ilm SpoOF SpoOB SpoOA KinB-D SixA V! P P ArcB ArcA A \w RapH ComA Spx R \Y !I spx ComA Figure 1.7. Connector proteins in two-component systems. (A) Inhibition of KinA autophosphorylation by Sda (red pentagon). Dephosphorylation of the receiver domains of SpoOF and SpoQA by the Rap and SpoOE family of phosphatases (green crescent) respectively in B. subtilis. (B) Dephosphorylation of the HPt of ArcB by SixA in E. coli. (C) Inhibition of ComA DNA binding by RapH in B. subtilis. (D) Inhibition of transcriptional activation by ComA by the RNA binding protein Spx (red half circle) in B. subtilis [30, 37]. Another target for phosphatases may be histidine kinases and phosphotransferases although to date there has only been one report of a phosphatase for these types of molecules. The E. coli protein SixA was suggested to dephosphorylate the histidine phosphotransferase domain of the hybrid kinase ArcB, although the in vivo relevance of SixA remains unclear (Fig. 1.7B) [50]. In addition to affecting the flow of phosphate in two-component systems, connectors can also modulate the output of response regulators in other ways. In the case of response regulators that are transcription factors, connectors can inhibit DNA-binding without affecting phosphorylation. For example, although RapH acts as a phosphatase for SpoOF, it has a different mechanism for regulating the ComP-ComA two-component system which controls genetic competence in response to cell density signals [51]. RapH directly binds ComA to prevent it from binding to target promoters (Fig. 1.7C) [46]. While RapH acts directly on ComA, another B. subtilis protein, Spx, blocks the activity of ComA by interfering with its ability to recruit RNA polymerase (Fig. 1.7D). Spx binds to the c-subunit of RNA polymerase, preventing the formation of a ComA-RNAPpromoter complex [52]. While these examples are by no means exhaustive, they give a flavor of the diverse mechanisms that have evolved to modulate the activity of two-component systems. Elaboration of two-component systems in the Caulobacter cell cycle In the examples above I have focused on two-component signaling pathways that regulate environmental and stress responses. However, these signaling proteins also play a critical role in regulating the cell cycle and the development of Caulobactercrescentus, the focus of my thesis work. Caulobacter as a cell cycle model Caulobactercrescentus is a fresh water oligotrophic alpha-proteobacterium. Members of the Caulobacter genus were first described at the turn of the 20 'b century and were characterized by their long stalks and tendency to form clumps of cells called rosettes [53]. The lab strain Caulobacter crescentus (CB15) was not isolated until 1960 [52]. However, because of its unique cell cycle, Caulobactercrescentus quickly emerged as a system for studying differentiation and the bacterial cell cycle [53]. The Caulobacter cell cycle is characterized by an asymmetric cell division and cellular differentiation. Each cell division yields a distinct mother cell (stalked cell) and daughter cell (swarmer cell) (Fig. 1.8) [54]. The stalked cell is sessile and marked by a polar stalk while the swarmer is motile and marked by a single polar flagellum and several pili [54]. Stalked cells are competent to immediately initiate DNA replication while swarmer cells are in a G 1-like phase and must differentiate into stalked cells to initiate DNA replication and proceed through the cell cycle. Caulobacter has proven to be an attractive model system for understanding the bacterial cell cycle and cellular differentiation as each cell cycle transition is coordinated with polar morphogenetic events [54]. The G1-to-S transition is marked by the loss of the flagellum and pili and the development of a stalk at the same pole, while the end of S phase is marked by the synthesis of a new flagellum at the nascent swarmer pole opposite the stalked pole [54]. swarmer cell late predvisional N_= - stalked cell S chromosome segregation DNA replicati n flagellar synthesis pili synthesis early predivisional Figure 1.8. Caulobacter cell cycle. Asymmetric cell division yields one swarmer cell and one stalked cell. Stalked cells immediately initiate DNA replication while swarmer cells differentiate into stalked cells to proceed through the cell cycle. Key events are indicated in text while visible polar morphogenetic events are highlighted in red. Cell cycle phases are color-coded. Much of the early work on Caulobacter focused on dissecting these visible polar morphogenetic events. Flagellar morphogenesis was especially amenable to genetic analysis through the isolation of non-motile mutants [55]. Through detailed epistatic analysis of these mutants, a specific hierarchy of flagellar assembly was established (Fig. 1.9). This hierarchy consists of four classes of genes that are expressed in an order that corresponds to the order of flagellar assembly [55]. Expression of the next level in the hierarchy requires proper expression of the previous level [55]. The flagellum is assembled from the inside out with class II genes encoding for proteins that make up the 28 internal membrane structures including the MS ring, switch, and a secretion apparatus that allows for the export of the extra-cytoplasmic components [55]. The class III genes encode proteins that constitute the periplasm spanning basal body as well as the extracellular hook while class IV genes encode the proteins of the flagellar filament [55]. The class I designation was reserved for the initiator(s) of flagellar biosynthesis, which was ultimately identified as the response regulator CtrA [56]. class IV I fljJ fijM fljK fljL fjN fijO filament flgF* flgBC flgi hook class Ill flgK hook operon basal body .. fihA class 11 class I fliF* jffiQ* fLM SfliOP ctrA Figure 1.9. Flagellar assembly. Flagellar assembly requires the expression of four classes of genes. The flagella is synthesized from the inside out with the class 11,111, and IV genes coding for structural components of the basal body, hook, and filament respectively. CtrA is the only class I gene and initiates the hierarchy. Asterisks indicate operons. Adapted from [55, 57]. In addition to regulating flagellar biogenesis, CtrA is an important regulator of the cell cycle and is essential for viability [55, 56]. Initially, CtrA was found to bind a motif in class II promoters that had previously been identified in the promoter of the gene for an essential methyltransferase, ccrM, and in the origin of replication [56]. These observations gave the first clues to the importance of CtrA for the regulation of many different developmental processes. CtrA, master of the Caulobacter cell cycle Subsequent work established CtrA as a master regulator of Caulobacter cell cycle because it inhibits the initiation of DNA replication and regulates the transcription of a large set of cell cycle genes (Fig. 1.10). Like most response regulators, CtrA is activated by phosphorylation [56] and regulated changes in CtrA phosphorylation (CtrA-P) drive progression of the cell cycle. CtrA-P is abundant in G1 swarmer cells, where it inhibits the initiation of DNA replication by binding the origin [58, 59]. The cell then clears CtrA at the G1-to-S transition through a combination of dephosphorylation and degradation, thereby releasing the origin and permitting initiation in stalked cells [58]. After being cleared from the cell, CtrA is subsequently transcribed and phosphorylated in predivisional cells, where it directly activates the expression of approximately 100 genes involved in cell cycle progression and morphogenesis [60]. Thus, CtrA controls the asymmetric morphogenesis of Caulobacter as well as the asymmetry in replicative capacities of the two daughter cells. CtrA-P GI G2 S CtrA-actIvated Origin repression CtA rotoyiA one ex rslon tAphshrlto Figure 1.10. CtrA activity and regulation through the cell cycle. The presence of CtrA-P is indicated in blue. Cell cycle specific activities of CtrA are indicated in white boxes and regulation of CtrA is indicated in grey boxes. In Caulobacter both CtrA and the widely conserved replication initiation factor DnaA regulate the initiation of DNA replication [61]. DnaA binds at least four sites in the origin of replication and promotes origin melting (Fig 1.1 1A) [59]. Although the mechanisms are not yet clear, changes in DnaA activity set the periodicity of the Caulobacter cell cycle [62]. DnaA must be activated to initiate each round of replication then deactivated to prevent over-replication. On top of the regulation by DnaA, CtrA blocks DNA replication specifically in swarmer cells through the occupancy of five binding sites in the origin of replication (Fig. 1.11 A) [59, 62]. Sites D and E are immediately adjacent to two of the conserved DnaA boxes suggesting that CtrA binding may occlude DnaA (Fig) [59]. In addition, sites A and B overlap and repress the hemE promoter, expression from which is also thought to play a role in origin melting and initiation (Fig 1.11 A) [63]. Mutating three of the CtrA binding sites (sites B,C, and D) in the origin of replication leads to premature initiation, i.e. shorter periods between replication events, and thus chromosome accumulation emphasizing the importance of CtrA silencing of the origin [62]. Progression of the Caulobactercell cycle also depends heavily on regulated transcription [64]. Developmental genes are expressed just-in-time for their function. For instance, the flagellar gene hierarchy is expressed in predivisional cells just as the flagellum is assembled [60]. Expression of 533 genes in the Caulobacter genome is cell cycle regulated and expression of nearly a quarter of these genes depends on CtrA [64]. Chromatin-immunoprecipitation and microarray experiments have shown that CtrA directly regulates approximately 100 of these genes (Fig. 1.11B)[64]. CtrA activates expression of the majority of its regulon in predivisional cells. About a dozen genes are A. C D E RB Orgin of Replication B. * ,1 CtrA reaulon CC# gene CC396 CC2700 CC70 CC2704 CC1211 CC3474 CC3445cigT CC3476 CSNW p protein function L-lactata 2-monooxygenaea reguato n potllc hyote protein cIe protein CCV02 olgupr hpthicl rlt a i RNA potynsrease gator in tlwpto prote h fin r ra ily hprota gaWrm e = Ionrepresdor CC363 cfA cg dl g tcranscrnptional regulator CC0N6Omne@( Sadenoeytelornneeyntiietase transcriptionalregulator,LaGIaml C231CC2317 mrphl melluylacepllng c-eoti prote= CC2641 ft@Z cell divielonloj ldeet p p rotein tein flalin modca CCO23 R CCO234 NmO fliin modification protein CCO378on D metsylnratalaa ypoeptng ohearotaxte protein mthy C-430 mh - chemyottcu protein CC043i cP CCp432 doe heaido mPro regulator CCO0 heA cheanotaxlrinaea cco43 chew chemotaxiaadapterprotein chaR chemortexie protein mathyitrterae. CC0436 prote endncaio k n CC0436 citel chemfotede giutematemtityetr ra blenheirgua T CC1490 regulator 00043 cheYl chemnotenirapne CCOM3 citD chemnotaxieprtn 00042 citeU chemnotabtproee CC0440 cheYW chetnteae eapne regulator che;;mxiepoti CC041 citED itglr motor switch protein UG CONS0 000907 fbS fla rti flagellar mo tch protei 0C0906 MN C0009 11W responsaeregulator COMti fgS flagagar baaiody rodprotein =aa-bdrod protein Wig fiegetr CC0954 hoctaabody compleoprotein CC09MIRAE flagatier .1 -0.6 CC14t7 fliG Ilageflin oiiaio rti fiagelier btoayntiieeiaregulator CCI458 fnb CC1469OWP feglrprt o rti Ngtrbeibd COl ffpP o rti CC2OS4florG faelrbabd tledcl ifeetain euao CC2462 ple cycle roepone regulator dIffaretiatonloli CC2463 rvK 002641 ftA calldivisionprotein cell division protein CC2542 feQ CC2NIl mnor peptidoglycan aynthesis enzyme CC2692 flaW call dintelan prolte CC228 itfa holdfeetsyntheis protein holdaatyetheetepWat CC2629 itAll CC2366 nouSrol log(rdo) 0 0.6 1 ~polaPlled CC2NO CC2961 00253 rpoN CC=22 CC0429 CCO7U1 C00794 130 Ctiti0 CC1102 hy poM.tical- prtein hyphtical protein RN.pO1ymwrevesigma-fi factor hypotheticalProtein hypothetia protein lagi faein conerad hypothetical protein 011"ieiclprlt 00136 cop CC2324 ATP-depontM proltee hyid hslotn kIdas CC2943 cp5 C02544 cpeD CC2ll44 cpaD CC21141cpall CC2947 cptA CUMS4 ptA CC3219 CC3205 rcdA COl03 aciP plleeaaebly protin plus assemblyprotein plluaassemblyprotein pus asamubly protei pllueaseemblyprotein pilot subunit protein hybrid hisetc kinae loial prtoti hyp CtrA tnrnacriltinal Inhibiter prolytoc subunit Figure 1.11. Dual function of CtrA in regulation of the origin and cell-cycle regulated transcription. (A). Organization of regulatory elements in the origin of replication. CtrA binding sites are indicated in blue and DnaA sites are indicated in green. The developmentally regulated hemE promoter is indicated by a pink arrow [59]. (B) Expression of the CtrA regulon in two CtrA loss of function strains ctrAt' and cckAt. Genes repressed by CtrA are in yellow and those activated by CtrA are in blue [65]. directly repressed by CtrA and only expressed at the G1-S transition when CtrA is cleared from cells (Fig 1.11 B). The CtrA regulon consists of genes involved in diverse functions like polar morphogenesis, DNA methylation, cell wall remodeling, proteolysis, and other two-component proteins and transcription factors [60]. CtrA regulates transcription by binding a specific DNA sequence found in target promoters, and phosphorylation likely activates CtrA by increasing its affinity for DNA [66]. Although the specific mechanism of activation of CtrA is unknown, activation by phosphorylation of related response regulators has been studied in atomic detail [37]. In general, phosphorylation of the receiver domain, a doubly wound a/p fold, results a significant displacement of the a4-p5-a5 surface (Fig. 1.12A) [37]. Phosphorylation of the conserved aspartate changes the orientation of two key switch residues (Ser/Thr on P4 and Phe/Tyr on p5) from facing away from (exposed) to toward (buried) the active site, thus transmitting phosphorylation status to changes in protein structure [37]. In the related protein PhoB to E. coli, structural changes induced by phosphorylation lead to homodimerization of the a4-p5-a5 surfaces, likely bringing together the DNA binding domains of each monomer in a conformation that allows them to bind to respective halfsites on the DNA (Fig. 1.12B) [67]. Although the details of RR activation probably vary for each specific response regulator, CtrA is likely to be activated by a similar phosphorylation induced dimerization mechanism. a4 al B. . active dimer DNA a2 Figure 1.12. PhoB activation by phosphorylation. (A) Crystal structures of the phosphorylated vs. unphosphorylated PhoB receiver domain. Green indicates the unphosphorylated structure while purple is phosphorylated. Key switch residues are indicated in orange and red. Image from [37]. (B) Phosphorylation of PhoB results in homodimerization positioning the DNA binding domains to interact with DNA [67]. How does CtrA binding to DNA regulate transcription? The short answer is that CtrA bound to target promoters can recruit RNA polymerase to that promoter and thus activate transcription [68]. The majority of genes activated by CtrA contain the CtrA binding motif (TTAA-N7-TTAA) near the -35 position of the promoter and binding of a CtrA dimer is thought to recruit RNA polymerase (Fig. 1.13A) [68]. In contrast, genes that are repressed by CtrA typically have a CtrA binding motif overlapping the -10 position of the promoter (Fig. 1.13B) [69]. In these cases CtrA binding probably occludes RNA polymerase from binding the promoter thereby repressing transcription. Finally, some genes contain multiple CtrA binding sites. For instance, pilA has a typical -35 site plus three additional upstream CtrA sites, the regulatory consequences of which are unknown (Fig. 1.14C) [70]. B. P1 -35 0 -35 -10 ctrA C. HA Figure 1.13. Activation of transcription by CtrA. (A) CtrA (blue barbell) may activate transcription by binding the -35 box of target promoters and recruiting RNA polymerase. (B) CtrA inhibits transcription by binding the -10 box and occluding RNA polymerase. (C) Multiple CtrA binding sites regulate genes such as pilA. Regulation of CtrA In accordance with the importance of CtrA for cell cycle progression and viability, Caulobacter has evolved several redundant mechanisms for regulating the activity of CtrA. These include the regulated transcription of ctrA and cell-type specific phosphorylation and proteolysis. Like the expression of the CtrA regulon, the expression of the ctrA gene is cell cycle regulated. ctrA is first transcribed in stalked cells shortly after the initiation of DNA replication with expression peaking in late predivisionals (Fig. 1.14A). The ctrA gene has two distinct promoters, an early P1 and a late P2 promoter, and expression is subject to both negative and positive autoregulation (Fig. 1.14A) [69]. P1 contains a CtrA binding site in the -10 box and is repressed by CtrA while P2 has a CtrA binding site in the -35 box and is activated by CtrA (Fig. 1.14B) [69]. Expression from P1 turns on by an unknown mechanism following the initiation of S phase when CtrA is cleared from the cell, leading to an initial burst of CtrA protein [69]. The newly synthesized CtrA then feeds back to inhibit transcription from P1 but activate expression from the stronger P2 promoter, leading to the rapid accumulation of CtrA [59]. Thus the negative and positive feedback loops ensure CtrA is produced to high levels shortly after being cleared during the GI-S transition [71]. A ctrA P1 P2 relative time of expression P1 -10 P2 -35 T T Repressed Activated Figure 1.14. Regulation of CtrA transcription. (A). Expression of ctrA as a function of the cell cycle including the contribution of the P1 and P2 promoters. [69] (B). Negative and positive feedback at the ctrA promoter. Each CtrA monomer is represented by a blue barbell. CtrA is also activated by phosphorylation via a phosphorelay similar to that used in B. subtilis sporulation [72]. The hybrid histidine kinase CckA first autophosphorylates and then passes a phosphoryl group to an attached receiver domain, which can transfer it to the histidine phosphotransferase ChpT which then directly phosphorylates CtrA (Fig. 1.15) [73]. CckA can also act as a phosphatase and pull phosphate off CtrA by reversing the phosphorelay [74]. CckA is activated as a kinase by DivL at the swarmer pole and becomes a phosphatase through localization at the stalked pole where DivL is either absent or directly inhibited by the phosphorylated, single domain response regulator DivK (Fig. 1.15) [74]. DivK is phosphorylated at the stalked pole by its cognate kinase DivJ and dephosphorylated at the swarmer pole by the bifunctional kinase PleC (Fig. 1.15) [75]. Thus the pole specific activity of DivK dictates CckA activity and ensures CtrA is phosphorylated in nascent swarmer cells and dephosphorylated in the stalked compartment through the establishment of a gradient of CtrA-P [74, 76, 77]. P P P P1 swarmer pole stalked pole Figure 1.15. Differential activation of CtrA at the swarmer and stalked pole. (A) The hybrid HK CckA exhibits kinase activity at the swarmer pole activates CtrA through the ChpT phosphorelay. (B) Swarmer pole factors promote CckA phosphatase activity resulting in dephosphorylation of CtrA at the stalked pole [76]. In addition to transcriptional control and phosphorylation, CtrA activity is also regulated by proteolysis. CtrA proteolysis is regulated by the same phosphorelay that phosphorylates CtrA (Fig. 1.16) [73]. In addition to CtrA, ChpT also phosphorylates the single domain response regulator CpdR [73]. Unphosphorylated CpdR is required for regulated proteolysis of CtrA by the ClpXP protease during the G1-S transition [78]. In swarmer cells when CckA is active as a kinase, CpdR is phosphorylated and thus unable to mediate CtrA proteolysis. When CckA kinase activity is down-regulated by phosphorylated DivK during the G1-to-S transition CpdR becomes dephosphorylated, enabling it to somehow stimulate CtrA proteolysis. CtrA is targeted for proteolysis by a bipartite degradation signal, present in the first 56 amino acids and in the last 15 amino acids [79]. In addition, the protein RcdA is thought to contribute to proteolysis by helping bring CtrA to ClpXP although RcdA does not affect CtrA proteolysis by ClpXP in vitro [80, 81]. stalked pole oe 00 0'CpdR CckA p1 P CtrA p ChpT p P CpdR CtrA Figure 1.16. CtrA proteolysis by CipXP. Dephosphorylation of CpdR leads to localization of CipXP to the swarmer pole and CtrA proteolysis. Identifying missing regulators of CtrA. Despite our detailed understanding of the regulation of CtrA by transcription, phosphorylation and degradation, several observations suggest that CtrA may be subject to additional modes of control. For instance, constitutive expression of a non-degradable allele of ctrA is not lethal while expression of a non-degradable, phosphomimetic allele is suggesting that dephosphorylation is necessary and sufficient to deactivate CtrA at the G1-S transition. Although CckA phosphatase activity at the swarmer pole normally dephosphorylates CtrA, CckA phosphatase activity is also not essential [74]. The mutant CckA(V366P) lacks phosphatase activity in vitro but can replace wild-type CckA without severe consequences to cell cycle progression [74]. This raises the possibility of an unidentified CtrA phosphatase. Given the precedence of multiple phosphatases in the B. subtilis sporulation pathway, it is possible that CtrA may be dephosphorylated directly or indirectly by one or more phosphatases. Another missing piece of the puzzle involves the absence of CtrA transcriptional activity in swarmer cells. Although CtrA is phosphorylated and abundant in both swarmer and predivisional cells, the majority of CtrA activated genes are expressed only in predivisional cells [60]. This raises the possibility that CtrA lacks a co-activator in swarmer cells or that there exists a swarmer-specific inhibitor of CtrA activity. In the following chapters I will document our discovery and characterization of a CtrAspecific transcriptional inhibitor called SciP. In chapter 2, I will describe the computational screen we used to identify SciP as well our characterization of its expression during the cell cycle. I will also present a series of experiments that lead to a model in which SciP interacts directly with CtrA at CtrA dependent promoters and blocks the recruitment of RNA polymerase. In chapter 3, I will describe the post-transcriptional regulation of SciP itself. Specifically I will demonstrate that SciP is proteolyzed by the Lon protease and that a failure to degrade SciP during the G1 -S transition has significant deleterious effects on cell cycle progression in Caulobacter. References 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. 12. 13. 14. 15. 16. 17. 18. 19. Gruber, T.M. and C.A. Gross, Multiple sigma subunits and the partitioningof bacterialtranscriptionspace. Annual Review of Microbiology, 2003. 57: p. 441466. Ptashne, M. and A. Gann, Genes & Signals2002, Cold Spring Harbor, New York: Cold Spring Harbor Laboratory Press. Moses, V. and C. Prevost, Cataboliterepressionof beta-galactosidasesynthesis in Escheriachiacoli. Biochemical Journal, 1966. 100(2): p. 336-&. Dickson, R.C., et al., Genetic-regulation- Lac control region. Science, 1975. 187(4171): p. 27-35. Ptashne, M. and A. Gann, Transcriptionalactivation by recruitment. Nature, 1997. 386(6625): p. 569-577. Magasanik, B., The regulationof nitrogen-utilizationin enteric bacteria.Journal of Cellular Biochemistry, 1993. 51(1): p. 34-40. Summers, A.O. and S. Silver, Microbialtransformationsof metals. Annual Review of Microbiology, 1978. 32: p. 637-672. Frantz, B. and T.V. Ohalloran, DNA distortionaccompanies transcriptional activation by the metal-responsive gene-regulatoryprotein MerR. Biochemistry, 1990. 29(20): p. 4747-475 1. Zawel, L. and D. Reinberg, Common themes in assembly andfunction of eukaryotic transcriptioncomplexes. Annual Review of Biochemistry, 1995. 64: p. 533-561. Dyson, N., The regulation ofE2F by pRB-family proteins. Genes & Development, 1998. 12(15): p. 2245-2262. Kaelin, W.G., et al., Expression cloning of a cDNA-encoding a retinoblastomabindingprotein with E2F-likeproperties.Cell, 1992. 70(2): p. 351-364. Xu, M., et al., Cyclin-A Cdk2 binds directly to E2F-1 and inhibits the dna-binding activity of E2F-J/DP-Jbyphosphorylation.Molecular and Cellular Biology, 1994. 14(12): p. 8420-843 1. Simon, I., et al., Serial regulation of transcriptionalregulators in the yeast cell cycle. Cell, 2001. 106(6): p. 697-708. Futcher, B., Cyclins and the wiring of the yeast cell cycle. Yeast, 1996. 12(16): p. 1635-1646. Alberts, B., et al., MolecularBiology of the Cell. 4 ed2002, New York: Garland Science. Sclafani, R.A. and T.M. Holzen, Cell cycle regulationofDNA replication.Annual Review of Genetics, 2007. 41: p. 237-280. Holt, L.J., A.N. Krutchinsky, and D.O. Morgan, Positivefeedbacksharpens the anaphaseswitch. Nature, 2008. 454(7202): p. 353-357. Enserink, J.M. and R.D. Kolodner, An overview of Cdkl-controlledtargetsand processes. Cell Division, 2010. 5. Iyer, V.R., et al., Genomic binding sites of the yeast cell-cycle transcription factors SBF and MBF. Nature, 2001. 409(6819): p. 533-538. 20. 21. 22. 23. 24. 25. 26. 27. 28. 29. 30. 31. 32. 33. 34. 35. 36. 37. 38. Spellman, P.T., et al., Comprehensive identificationof cell cycle-regulatedgenes of the yeast Saccharomyces cerevisiae by microarrayhybridization.Molecular biology of the cell, 1998. 9(12): p. 3273-97. Koranda, M., et al., Forkhead-like transcriptionfactors recruit Nddl to the chromatin of G2/M-specific promoters. Nature, 2000. 406(679 1): p. 94-98. Wittenberg, C. and S.I. Reed, Cell cycle-dependent transcriptionin yeast: promoters, transcriptionfactors, and transcriptomes.Oncogene, 2005. 24(17): p. 2746-2755. Zhu, G.F., et al., Two yeastforkheadgenes regulate the cell cycle and pseudohyphal growth. Nature, 2000.406(6791): p. 90-94. Amon, A., et al., Mechanisms that help the yeast-cell cycle clock tick - G2 cyclins transcriptionallyactivate G2 cyclins and repress GJ cyclins. Cell, 1993. 74(6): p. 993-1007. Sidorova, J.M., G.E. Mikesell, and L.L. Breeden, Cell-cycle regulated phosphorylationof Swi6 controls its nuclear-localization.Molecular Biology of the Cell, 1995. 6(12): p. 1641-1658. Geymonat, M., et al., Clb6/Cdc28 and Cdc14 regulatephosphorylationstatus andcellular localizationofSwi6. Molecular and Cellular Biology, 2004. 24(6): p. 2277-2285. Costanzo, M., et al., CDK activity antagonizes Whi5, an inhibitor of GJ/S transcriptionin yeast. Cell, 2004. 117(7): p. 899-913. Ubersax, J.A., et al., Targets of the cyclin-dependent kinase Cdkl. Nature, 2003. 425(6960): p. 859-864. Orlando, D.A., et al., Global control of cell-cycle transcriptionby coupled CDK andnetwork oscillators.Nature, 2008. 453(7197): p. 944-U78. Stock, A.M., V.L. Robinson, and P.N. Goudreau, Two-component signal transduction.Annual Review of Biochemistry, 2000. 69: p. 183-215. Gao, R. and A.M. Stock, BiologicalInsightsfrom Structures of Two-Component Proteins.Annual Review of Microbiology, 2009. 63: p. 133-154. Laub, M.T. and M. Goulian, Specificity in two-component signal transduction pathways. Annual Review of Genetics, 2007. 41(18076326): p. 121-45. Egger, L.A., H. Park, and M. Inouye, Signal transductionvia the histidyl-aspartyl phosphorelay.Genes to cells : devoted to molecular & cellular mechanisms, 1997. 2(3): p. 167-84. Hoch, J.A., Regulation of the phosphorelayand the initiationof sporulationin Bacillus subtilis. Annual Review of Microbiology, 1993. 47(8257105): p. 441-65. Piggot, P.J. and D.W. Hilbert, SporulationofBacillus subtilis. Current opinion in microbiology, 2004. 7: p. 579-86. Bauer, C.E., S. Elsen, and T.H. Bird, Mechanismsfor redox control ofgene expression. Annual Review of Microbiology, 1999. 53: p. 495-523. Gao, R. and A. Stock, Biological insightsfrom structures of two-component proteins. Annual review of microbiology, 2009. 63: p. 133-54. Skerker, J.M., et al., Two-Component Signal TransductionPathways Regulating Growth and Cell Cycle Progressionin a Bacterium: A System-Level Analysis. PLoS Biology, 2005. 3(10): p. e334. 39. 40. 41. 42. 43. 44. 45. 46. 47. 48. 49. 50. 51. 52. 53. 54. Skerker, J.M., et al., Rewiring the specificity of two-component signal transduction systems. Cell, 2008. 133(6): p. 1043-54. Capra, E.J., et al., Systematic Dissection and Trajectory-ScanningMutagenesis of the MolecularInterface That EnsuresSpecificity of Two-Component Signaling Pathways. Plos Genetics, 2010. 6(11). Mitrophanov, A.Y. and E.A. Groisman, Signal integrationin bacterialtwocomponent regulatorysystems. Genes & Development, 2008. 22(19): p. 2601-11. Burkholder, W.F., I. Kurtser, and A.D. Grossman, Replication initiationproteins regulate a developmental checkpoint in Bacillus subtilis. Cell, 2001. 104(2): p. 269-279. Rowland, S.L., et al., Structure and mechanism of action of'Sda, an inhibitorof the histidine kinases that regulate initiationof sporulation in Bacillus subtilis. Molecular Cell, 2004. 13(5): p. 689-701. Veening, J.W., H. Murray, and J. Errington, A mechanismfor cell cycle regulationof sporulationinitiation in Bacillus subtilis. Genes & Development, 2009. 23(16): p. 1959-1970. Perego, M., et al., Multiple protein aspartatephosphatasesprovide a mechanism for the integrationof diverse signals in the control of development in bacillussubtilis. Cell, 1994. 79(6): p. 1047-1055. Smits, W.K., et al., Temporal separationof distinct differentiationpathways by a dual specificity Rap-Phrsystem in Bacillus subtilis. Molecular Microbiology, 2007. 65(1): p. 103-120. Jiang, M., R. Grau, and M. Perego, Differentialprocessingofpropeptide inhibitorsof Rap phosphatasesin Bacillus subtilis. Journal of Bacteriology, 2000. 182(2): p. 303-310. Ohlsen, K.L., J.K. Grimsley, and J.A. Hoch, Deactivationof the sporulation transcriptionfactor SpoOA by the SpoOE proteinphosphatase.Proceedings of the National Academy of Sciences of the United States of America, 1994. 91(5): p. 1756-60. Perego, M., A new family of aspartylphosphatephosphatases targetingthe sporulation transcriptionfactorSpoOA of Bacillus subtilis. Molecular microbiology, 2001. 42(1): p. 133-43. Matsubara, M. and T. Mizuno, The SixA phospho-histidinephosphatase modulates the ArcB phosphorelaysignal transductionin Escherichiacoli. Febs Letters, 2000. 470(2): p. 118-124. Grossman, A.D., Genetic networks controlling the initiation of sporulationand the development of genetic competence in Bacillus subtilis. Annual Review of Genetics, 1995. 29: p. 477-508. Nakano, S., et al., A regulatoryprotein that interferes with activator-stimulated transcriptionin bacteria.Proceedings of the National Academy of Sciences of the United States of America, 2003. 100(7): p. 4233-4238. Poindexter, J.S., Biologicalproperties + classificationof the Caulobactergroup. Bacteriological Reviews, 1964. 28(3): p. 231-&. Shapiro, L., Differentiationin the Caulobactercell-cycle. Annual Review of Microbiology, 1976. 30: p. 377-407. 55. 56. 57. 58. 59. 60. 61. 62. 63. 64. 65. 66. 67. 68. 69. 70. Gober, J.W. and J.C. England, ProkaryoticDevelopment2000, Washington D.C.: ASM Press. Quon, K.C., G.T. Marczynski, and L. Shapiro, Cell cycle control by an essential bacterialtwo-component signal transductionprotein.Cell, 1996. 84(1): p. 83-93. Skerker, J.M. and M.T. Laub, Cell-cycle progressionand the generationof asymmetry in Caulobactercrescentus. Nature Reviews Microbiology, 2004. 2(4): p. 325-37. Domian, I.J., K.C. Quon, and L. Shapiro, Cell type-specificphosphorylationand proteolysis of a transcriptionalregulatorcontrols the GJ-to-S transitionin a bacterialcell cycle. Cell, 1997. 90(3): p. 415-24. Quon, K.C., et al., Negative controlof bacterialDNA replication by a cell cycle regulatoryprotein that binds at the chromosome origin. Proceedings of the National Academy of Sciences of the United States of America, 1998. 95(1): p. 120-5. Laub, M.T., et al., Genes directly controlledby CtrA, a master regulatorof the Caulobactercell cycle. Proceedings of the National Academy of Sciences of the United States of America, 2002. 99(7): p. 4632-7. Gorbatyuk, B. and G.T. Marczynski, Physiologicalconsequences of blocked Caulobactercrescentus dnaA expression, an essential DNA replicationgene. Molecular microbiology, 2001. 40(2): p. 485-97. Jonas, K., Y.E. Chen, and Michael T. Laub, Modularity of the Bacterial Cell Cycle EnablesIndependent Spatialand Temporal Control of DNA Replication. Current Biology, 2011. Marczynski, G.T., K. Lentine, and L. Shapiro, A developmentally regulated chromosomal origin of replicationuses essential transcriptionelements. Genes & development, 1995. 9(5499): p. 1543-57. Laub, M.T., et al., Global analysis of the genetic network controllinga bacterial cell cycle. Science (New York, NY), 2000. 290(5499): p. 2144-8. Gora, K.G., et al., A cell-type-specific protein-proteininteractionmodulates transcriptionalactivity of a master regulatorin Caulobactercrescentus. Molecular Cell, 2010. 39(3): p. 455-67. Reisenauer, A., K. Quon, and L. Shapiro, The CtrA response regulatormediates temporal control of gene expression during the Caulobactercell cycle. Journal of bacteriology, 1999. 181(3): p. 2430-9. Bachhawat, P., et al., Mechanism of activationfortranscriptionfactor PhoB suggested by different modes of dimerization in the inactive and active states. Structure, 2005. 13(9): p. 1353-1363. Ouimet, M.C. and G.T. Marczynski, Analysis of a cell-cycle promoter bound by a response regulator.Journal of Molecular Biology, 2000. 302(4): p. 761-775. Domian, I.J., A. Reisenauer, and L. Shapiro, Feedbackcontrol of a master bacterialcell-cycle regulator.Proceedings of the National Academy of Sciences of the United States of America, 1999. 96(12): p. 6648-53. Skerker, J.M. and L. Shapiro, Identification and cell cycle control of a novel pilus system in Caulobactercrescentus. The EMBO journal, 2000. 19(13): p. 3223-34. 71. 72. 73. 74. 75. 76. 77. 78. 79. 80. 81. Reisenauer, A. and L. Shapiro, DNA methylation affects the cell cycle transcriptionof the CtrA global regulatorin Caulobacter.The EMBO journal, 2002. 21(18): p. 4969-77. Jacobs, C., et al., Cell cycle-dependent polarlocalization of an essential bacterial histidinekinase that controls DNA replicationand cell division. Cell, 1999. 97(1): p. 111-20. Biondi, E.G., et al., Regulation of the bacterialcell cycle by an integratedgenetic circuit.Nature, 2006. 444(7121): p. 899-904. Chen, Y.E., et al., Dynamics of Two PhosphorelaysControllingCell Cycle Progressionin Caulobactercrescentus. Journal of Bacteriology, 2009. 191(24): p. 7417-7429. Wheeler, R.T. and L. Shapiro, Differential localizationof two histidine kinases controllingbacterialcell differentiation.Molecular cell, 1999. 4(5): p. 683-94. Tsokos, C.G., B.S. Perchuk, and M.T. Laub, A Dynamic Complex ofSignaling Proteins Uses PolarLocalization to Regulate Cell-FateAsymmetry in Caulobactercrescentus. Developmental Cell, 2011. 20(3): p. 329-341. Chen, Y.E., et al., Spatialgradientofproteinphosphorylation underlies replicativeasymmetry in a bacterium. Proceedings of the National Academy of Sciences of the United States of America, 2011. 108(3): p. 1052-1057. Iniesta, A.A., et al., A phospho-signalingpathway controls the localizationand activity of a proteasecomplex criticalfbr bacterialcell cycle progression. Proceedings of the National Academy of Sciences of the United States of America, 2006. 103(29): p. 10935-40. Ryan, K.R., E.M. Judd, and L. Shapiro, The CtrA response regulatoressentialfor Caulobactercrescentus cell-cycle progressionrequires a bipartitedegradation signalfor temporally controlledproteolysis. Journal of molecular biology, 2002. 324(3): p. 443-55. McGrath, P.T., et al., A dynamically localizedprotease complex and a polar specificity factor control a cell cycle master regulator.Cell, 2006. 124(3): p. 535547. Chien, P., et al., Direct and adaptor-mediatedsubstraterecognitionby an essential AAA plus protease.Proceedings of the National Academy of Sciences of the United States of America, 2007. 104(16): p. 6590-6595. Chapter 2. A cell-type-specific protein-protein interaction modulates transcriptional activity of a master regulator in Caulobactercrescentus This work was published in Molecular Cell, 2010. 39(3): p. 455-67 by Kasia G Gora, Christos G Tsokos, Y Erin Chen, Balaji S Srinivasan, Barrett S Perchuk, Michael T Laub B.S.S. designed and coded the computational screen. K.G and M.T.L analyzed data and wrote the manuscript. K.G. performed all the experiments with the expection of in vivo phosphorylation performed by C.G.T, microarrays perfomed by B.S.P, and in vitro phosphorylation peformed by Y.E.C. Abstract Progression through the Caulobactercell cycle is driven by the master regulator CtrA, an essential two-component signal transduction protein that regulates the expression of nearly 100 genes. CtrA is abundant throughout the cell cycle except immediately prior to DNA replication. However, the expression of CtrA-activated genes is generally restricted to S-phase. Here, we identify the conserved protein SciP and show that it accumulates during GI where it inhibits CtrA from activating target genes. The depletion of SciP from G 1 cells leads to the inappropriate induction of CtrA-activated genes and, consequently, a disruption of the cell cycle. Conversely, the ectopic synthesis of SciP is sufficient to inhibit CtrA-dependent gene expression, which also disrupts the cell cycle. We demonstrate that SciP binds to CtrA without affecting stability or phosphorylation; instead SciP likely prevents CtrA from interacting with RNA polymerase. CtrA activity is thus tightly regulated by a novel protein-protein interaction which is critical to cell cycle progression. Introduction All dividing cells must ensure the correct timing and order of DNA replication, chromosome segregation, and cell division. These cell cycle events are carefully orchestrated to ensure genome stability and cellular survival. Their execution and, hence, proper cell cycle progression requires complex transcriptional programs, with batteries of genes turned on and off according to their times of action during the cell cycle [1]. These gene expression programs are controlled by master regulators whose activities are tightly regulated. For example, in metazoans the transcription factor E2F regulates the expression of hundreds of genes during late G1, many of which are critical to the ensuing S phase [2]. To prevent the premature, inappropriate expression of E2F targets in early G 1, the retinoblastoma protein Rb binds directly to E2F and prevents it from stimulating gene expression, but without disrupting DNA binding [3]. The regulated inactivation of Rb by phosphorylation in late G1 thus unleashes E2F activity and propels cells into S phase. In the yeast S. cerevisiae, the transcription factors SBF and MBF drive the expression of nearly 200 genes in late G1 [4]. Analogous to Rb, the yeast protein Whi5 binds directly to SBF and MBF to block transcription during early G1; the subsequent hyperphosphorylation of Whi5 relieves this inhibition and permits SBF and MBF to drive gene expression crucial to cell cycle progression [5]. The regulation of gene expression during the bacterial cell cycle remains poorly understood. The Gram-negative bacterium Caulobacter crescentus is a tractable model for elucidating the mechanisms underlying the prokaryotic cell cycle [6, 7]. The Caulobacter cell cycle begins with a Gl-phased swarmer cell that is motile and cannot initiate DNA replication until it differentiates into a stalked cell. Concomitant with the swarmer-to-stalked cell transition, the cell enters S phase during which it replicates its chromosome and partitions the new chromosomes to opposite poles of the predivisional cell. Each cell division is asymmetric, producing two different daughter cells - a G1 swarmer cell and a stalked cell that can immediately enter S phase. DNA replication in Caulobacteroccurs in a once-and-only-once manner, resulting in distinct GI, S, and G2 phases. Additionally, Caulobactercells can be easily synchronized, facilitating temporal analysis of the cell cycle. In Caulobacter,cell cycle progression requires the periodic activation and inactivation of the master regulator CtrA, which functions both as a transcription factor for nearly 100 genes and as a direct repressor of DNA replication initiation [8-10]. CtrA is a response regulator and hence part of a two-component signaling pathway, the dominant form of signal transduction in bacteria [11, 12]. These pathways typically initiate with a sensor histidine kinase that autophosphorylates and then transfers the phosphoryl group to a cognate response regulator. Phosphorylation of the response regulator activates an output domain that can drive changes in cellular behavior. Phosphorylation of CtrA enhances its ability to bind DNA [13, 14] and, consequently, to modulate transcription and silence the origin of replication. CtrA activity is controlled by a combination of regulated phosphorylation, proteolysis, and transcription [8, 15-19]. Phosphorylated CtrA is abundant in swarmer/G1 cells where it silences the origin of replication [10], likely by occluding the replication initation factor DnaA. At the G1-S transition, CtrA is dephosphorylated and degraded, freeing the origin and enabling DNA replication to commence. As cells proceed through S phase, ctrA is transcribed, and the newly synthesized CtrA is stabilized against proteolysis and activated by phosphorylation. CtrA then activates the expression of more than 60 target genes, many of which are important for late stages of the cell cycle and cell division [20]. Following septation, CtrA is maintained in the phosphorylated state in daughter swarmer cells but proteolytically cleared from daughter stalked cells to permit another round of DNA replication. These mechanisms ensure that CtrA is abundant and phosphorylated in both swarmer and predivisional cells. Consistent with this pattern of activity, CtrA-repressed genes are expressed only in stalked cells after CtrA has been degraded. However, most of the genes that are directly activated by CtrA are transcribed only in predivisional cells [20]. A handful of CtrA-activated genes have mRNAs that are also abundant in swarmer cells, but this pattern may result from transcription in predivisional cells followed by stabilization of the mRNA in swarmer cells [21, 22]. These previous findings thus imply the existence of either a predivisional-specific transcriptional cofactor for CtrA or a swarmer cell-specific inhibitor of CtrA-dependent gene expression. Neither such factor has been identified to date. Here, we identify SciP, or small CtrA inhibitory protein, and demonstrate that it binds directly to CtrA and inhibits its ability to activate transcription without disrupting DNA binding or the repression of certain target genes. SciP is restricted to G1 swarmer cells and thus plays a role in establishing the differential fates of daughter cells. SciP is critical for proper cell cycle progression and we show that SciP levels must be tightly regulated, as the ectopic production of SciP in predivisional cells is sufficient to block CtrAdependent gene expression and, consequently, proper cell cycling. SciP is highly conserved and present in nearly every organism containing a CtrA ortholog emphasizing the key role this molecule plays in cell cycle progression in a wide range of bacteria. SciP represents a new class of molecules that modulate the output of two-component signaling pathways without affecting phosphorylation, indicating that these critical signaling pathways are subject to even more complex regulation than previously appreciated. Results Identification of sciP as a candidate regulator of CtrA and the cell cycle To identify putative regulators of CtrA transcriptional activity we used a bioinformatic screen for genes that are coexpressed, coinherited, or colocated in bacterial genomes with ctrA [23]. We previously used a similar approach to identify the direct CtrA phosphodonor, ChpT [15]. This screen identified CC0903, which we named sciP for small CtrA inhibitory protein. The sciP gene is annotated to encode a 93 amino acid protein of no known function. Orthologs of sciP are often found immediately adjacent to or within a few genes of ctrA in a-proteobacteria such as Rhodobacter sphaeroides, Sinorhizobium meliloti, Bartonellabacilliformis, Brucella melitensis, and Mesorhizobium loti (Fig. 2. 1A). Orthologs of sciP were found in virtually all a-proteobacteria indicating strona coconservation with ctrA with most orthologs nearly 70% identical and 85% similar to the C. crescentus SciP (Fig. 2.2). chpT Caulobacter croscentus -4 05ctrA scip I-a----L -M/ scIP ctrA 1 Rhodobacter sphaeroldes sciP ctrA =--L--- Bartonella bacillformis chpT ctrA sciP SInothizoblum mellotip 1 chpT Brucellameltensls -ciP Mesorhizoblum lot/ crA SCIP chpT AsciP 2 0 ' -0- AsciP 2 4 4 2 Ow: 0 6 8 10 12 14 time (h) 1 2 3 12 3 # of chromosomes E ~~** 01 0 . 10 20 S 30 40 50 .02 G2 60 70 - 80 90 100 SW ST CtrA 40 SciP "" M length (sm) Figure 2.1. Identification of sciP as a key regulator of cell cycle progression. (A) Genomic organization of ctrA, chpT, and sciP orthologs in C. crescentus and other a-proteobacteria. (B) Growth curve of AsciP and wild type grown in PYE. (C) Cellular morphology (top) and flow cytometry analysis (bottom) of AsciP compared to wild type. Samples were treated with rifampin and 50,000 cells from each population were analyzed. (D) Quantification of the distribution of cell lengths in C. (E) Cell cycle abundance of SciP. Synchronized wild-type swarmer cells were released into PYE and allowed to proceed through one cell cycle with samples collected every 10 min for Western blot analysis with CtrA or SciP anti-sera. After 100 min, the culture was again synchronized to separate swarmer and stalked daughter cells (lanes labeled SW and ST). The cell cycle diagram shown on top indicates relative stage of the cell cycle for each time point. C.crescentus B.0elitensis M.loti F.pelagi H.Magnetotacticum B.japonicum R .pa lusteris I.autotrophicus ELQQQiTNSRGE lMO VM --=ib vmE IEKQ il*B-T P &S A 3 - -- EgRWVIPRRKAL.VVAAVRGG LSL, EAc RYLVLILS TV I PR KAFVVAA\VRGG( I SNLEEAC R% 1T EF1 11TRR: ;V IRRHKAIV VA AV RG I .EEI C .1 ' .IIS1 1TRRiN ;VI1RRPKAELVVAAVRGGLSLEEAC 'L1j I11_WQ NTR 1 TTRPRWVFR KAE FV VAAVRPG 1; 1 TYN R VI RTH KA 6;V L Rh melilat leguainosarum S.aggregata B.quintana B.hennelae B.bacilliforais P.lavamentivorans M.aaris R. sphaeroides G, I X R 1 T V A V VAV\ 1S 1 lRRVI R 1RK, 1VA PFEEAC ) 1i1,SE1 WQ0S 1 I -IYTL T VEELELS RKAE VVAAVR-;G PR RKAF VUA L' L A.:j 1,A, vR(U U L 1. PRLE KE----- T R x :1711VMA G I EA' S I TtV'I l- I I Y TJ R ILT fY f TVEF I DRY ILTE L E I W ;1,AG1,PFI 1GI ,,A 1 rP(10'1: 0Il :Il RH P , GL AG6LRT HG fl iL I Si IQQYR's _1TTR Df HGL; GLR i'TR HGILA ' ; 1L S WQ' 1; EE ,IIswv E NW0 RY SEQIMS---j TLKRVDG- S TV" EL.IISW1-1QI R YTL11TVE A S 1.Q IR "P' RE;V C LLEA I-UEA 1:1 i'YTI H AL R; PP.K V'*.' 1;1 BK T KinraTg--i LXESQ---U LEEA SLV A NGG_1 I R A. tue f-acen. B . i R. R V P UVAAVRIZG~i. D. LGRPR1QYR ) 'L G RI t'YR 1 .A I R11 )jYRI SID R AG P 1: O Rf HGL,. HI iGl-AG!.K I Q, YR 1 1. TH "I Q YR lFd TY Y R T IO ,RH R' RLAG.LTTi0YR- Figure 2.2. Multiple sequence alignment of SciP orthologs. SciP orthologs from a sampling of alpha-proteobacterial genomes were aligned and conservation visualized using BOXSHADE. Residues shaded in black and grey match or are similar to, respectively, the consensus at that position. To test whether sciP is important for cell cycle progression, we deleted the chromosomal copy of the gene from the wild type (CB15N) and replaced it with a tetracyclineresistance cassette to produce the strain AsciP. In the C. crescentus genome, sciP is located between two operons that encode flagellar proteins, but is transcribed independently [24], so the deletion of sciP is unlikely to affect nearby genes. Although the AsciP strain is viable, it exhibited significant growth and cell cycle defects with a doubling time of -120 minutes in rich PYE medium compared to -80 minutes for the wild type (Fig. 2. 1B). Cells from a mid-log phase culture of AsciP were filamentous with lengths ranging from slightly elongated to extremely filamentous (Fig. 2.lC). The filamentous cells were either smooth and unpinched or had a single constriction which is likely the site of active cell division. Flow cytometry analysis of the AsciP strain revealed a modest, but reproducible accumulation of cells with 3N chromosomal content compared to the wild type (Fig. 2.1C). These growth, morphological, and chromosomal content defects were rescued by providing a wild-type copy of sciP in trans at the chromosomal xylX promoter (see below). These results indicate that SciP is required for proper cell cycle progression in Caulobacter. SciP is present only in GJ swarmer cells To determine when SciP functions during the cell cycle, we measured its abundance during the cell cycle using a polyclonal antibody specific to SciP. Wild-type swarmer cells were then harvested and allowed to proceed synchronously through the cell cycle with samples isolated every 10 minutes for immunoblot analysis (Fig. 2.lD). We found that SciP was abundant in swarmer cells but rapidly disappeared at the Gi-S transition and did not accumulate again until very late in the cell cycle, coincident with the first appearance of new swarmer cells (Fig. 2.3). This temporal pattern suggests that SciP may be restricted primarily to the Gi/swarmer stage of the cell cycle. To test this conclusion, we used density centrifugation to separate new swarmer cells from new stalked cells immediately following cell division. Immunoblots of samples from each population revealed that SciP was present only in the swarmer fraction (Fig. 2.1E, last two lanes). Together, these data indicate that SciP is present primarily, and perhaps exclusively, in Gi-phased swarmer cells. We note, however, that sciP mRNA was previously found to be cell cycle-regulated, reaching peak levels in predivisional cells [25]. The delay in SciP accumulation following its transcription suggests sciP expression may be posttranscriptionally regulated. time (min.) 0 10 20 30 40 150 0 60 70 80 90 100 IN 2N Figure 2.3. Flow cytometry analysis of cells from Figure 2.1E. Synchronized wild-type swarmer cells were released into PYE and allowed to proceed through one cell cycle with samples collected every 10 min for FACS analysis. To better characterize the cell cycle function of SciP, we created a depletion strain in which the chromosomal copy of sciP was deleted and a wild-type copy of sciP was placed at the chromosomal xylX locus under the control of the xylose-inducible, glucoserepressible promoter Pyz. When grown in PYE supplemented with xylose, the depletion strain exhibited morphology, chromosome content, and SciP protein levels similar to wild type (Fig. 2.4A-C). Upon shifting to medium supplemented with glucose, SciP was rapidly depleted, with no protein detected after one hour (Fig. 2.4B). We then examined the morphology and chromosomal content of cells following a shift from xylose to glucose. After two hours, the culture showed a significant increase in cells with one chromosome, suggesting that the loss of sciP may cause a transient arrest in GI (Fig. 2.4C). After 12 hours, the depletion strain had overcome this delay and reached a steadystate in which the chromosomal content profile was nearly identical to the sciP deletion strain. By 12 hours, cells also exhibited an unpinched, filamentous morphology similar to the AsciP strain (Fig. 2.4A). To further examine whether sciP affects the Gi-S transition, we synchronized the depletion strain grown in xylose and then released swarmer cells into medium containing either xylose or glucose to maintain or inhibit SciP production, respectively. Cell cycle progression for each population of cells was monitored by flow cytometry (Fig. 2.3) and SciP levels examined by Western blotting (Fig. 2.4E). As expected, for cells released into xylose, SciP was initially abundant but eliminated at the G1-to-S transition (30 min. time point), as seen with wild-type cells. SciP accumulated again in these cells during the latest stages of the cell cycle, at a time coincident with cell division and the generation of new swarmer cells. Although sciP is constitutively expressed, SciP levels still drop after GI (Fig. 2.4E), further demonstrating that sciP expression is post-transcriptionally regulated. Following cell division, daughter cells from the culture grown in xylose initiated a new round of DNA replication, manifest in the flow cytometry profiles by a shift of the entire IN chromosomal peak toward 2N by the 100-minute time point. PY:scIP sciP depletion xylose glucose synchronize, release Into xylose or glucose 'I time (min.) 0 20 B glucose hrs post-shift: 0 1 2 3 xylose 4 4 SciP C P xY,:Scip Ir wash, release into glucose IN 2N --- glucose time post-synchronization (min.) 0 30 90 xylose IN 2N glucose Figure 2.4 SciP depletion phenotype. (A) Cellular morphology of the sciP depletion strain grown in PYE supplemented with xylose or shifted to PYE with glucose for 12 hrs to deplete SciP. (B) Western blot analysis showing SciP levels post-shift to glucose or maintained in xylose at the times indicated. (C) Flow cytometry analysis of chromosomal content in the sciP depletion strain grown in PYE supplemented with xylose and then shifted to PYE with glucose. (D) Flow cytometry analysis of synchronized swarmer cells from the depletion strain released into PYE supplemented with glucose or xylose. Arrows indicate the delay in DNA replication initiation seen after the first cell division for the strain depleted of sciP. (E) Western blot analysis of SciP levels in cultures synchronized as in panel D,at the time points indicated. When the swarmer cells of the depletion strain were released into glucose, SciP was also initially abundant and then rapidly eliminated at the Gl-S transition (Fig. 2.4E). However, in these cells SciP remained undetectable throughout the remainder of the first cell cycle and after cell division, indicating a complete repression of sciP expression. These cells divided at approximately the same time as the control cells grown in xylose, demonstrating that SciP is not required for progression through the late stages of the cell cycle or for cell division (Fig. 2.4D). However, following the first cell division (80 min time point), approximately half of the daughter cells were delayed in initiating DNA replication for up to 60 minutes, relative to the control cells, (Fig. 2.4D). This result supports our conclusion that sciP is important for the Gl-S transition and proper cell cycling. We also resynchronized the culture of cells released into glucose after the first cell division. However, this double synchrony produced a low yield of swarmer cells in contrast to cells released into xylose. Based on flow cytometry (Fig. 2.4D) and time-lapse microscopy (data not shown) we concluded that the lack of swarmer cells was not due to a defect in cell division. Instead, cells lacking sciP likely do not undergo the change in buoyant density that facilitates synchronization by centrifugation. The swarmer cells that were harvested, though, showed a significant delay in initiating DNA replication. Depletion of sciP affects CtrA-dependent gene expression Next, we tested whether the lack of sciP in G1 swarmer cells affected gene expression, particularly the CtrA regulon. Again, we synchronized the depletion strain and released swarmer cells into media supplemented with either glucose or xylose to repress or maintain SciP levels, respectively. After 100 minutes, once all cells in each culture had divided, we isolated RNA from each culture and compared the samples by competitive hybridization on DNA microarrays. These post-division samples include both swarmer and stalked cells because, as noted, the yield of swarmer cells following a second synchronization of the depletion strain is low. However, because SciP is only abundant in swarmer cells (see above), differences in gene expression will only reflect differences in swarmer cell gene expression. Nearly all of the genes showing significant changes in expression level were CtrAdependent genes (GEO GSE22062). Figure 5 shows the changes in expression for genes that are directly regulated by CtrA. Strikingly, nearly every CtrA-activated gene was expressed at significantly higher levels in the cells depleted of sciP. In contrast, the expression of CtrA-repressed genes were unchanged. These data indicate that SciP is required to silence the expression of CtrA-activated genes in swarmer cells, thereby restricting their expression to predivisional cells. Overexpressing sciP disrupts the cell cycle and drives the down-regulation of CtrAdependent genes The data thus far are consistent with SciP being a G1-specific inhibitor of CtrA. To test whether SciP is sufficient to inhibit CtrA transcriptional activity, we examined the consequence of forcing the synthesis of SciP in predivisional cells when it is normally absent. For this experiment we used pHXM-sciP, a high-copy vector for expressing sciP with an N-terminal M2-epitope tag. Although strains overexpressing either sciP or M2sciP gave similar phenotypes, only the latter could be synchronized. Inclusion of the tag also enabled us to distinguish plasmid and chromosomally-encoded SciP, as needed below. The strain harboring pHXM-sciP exhibited normal cellular morphology and chromosome content when grown in glucose to repress expression (Fig. 2.6A). To overproduce SciP, cells were shifted to medium containing xylose. Immunoblots showed that SciP levels increased approximately 7-fold after 4 hours of growth in xylose. After four hours postinduction, cells had become filamentous and accumulated up to four chromosomes per cell, phenotypes consistent with a disruption of cell division (Fig. 2.6A). Immunoblot analysis of synchronized cells demonstrated that SciP was now abundant throughout the cell cycle, overcoming the regulatory mechanisms that usually limit it to swarmer cells (Fig 2.6B). To test whether the overproduction of SciP was sufficient to down-regulate CtrAactivated genes, we again used whole genome DNA microarrays. Cells harboring pHXMsciP were shifted to xylose for 2 hours and mRNA from these cells was compared to mRNA from wild-type cells grown in identical conditions. Nearly every CtrA-activated gene showed significant down-regulation in the cells overproducing SciP (Fig. 2.5). The magnitudes of these drops in expression were comparable to, although slightly less than, those seen in ctrAs and cckA"s strains [20, 26] grown at the restrictive temperature relative to wild type (Fig. 2.5). As with the sciP depletion strain, genes repressed by CtrA were not significantly affected by the overproduction of SciP. * /O * CtrA regulon protein functlon gene 2.monoygomeoe Lno CU270 hypoteticalprotein ein 0027012 bypotbeilcolppro C027114 hoerypotticalpro 0C1211 hpohiolpo, CC3474 osridibn. CC3475 siliT ONA polynravensign. factor. ECF family CC3476pi CC48 pm e po09 CC14628 VT oill Plou" CC14993InA re8F lopren" CC03641 A Coal=A .CCO4 ine n i CC# CC13 potei lrwlo. 1nA CC2620 M poteinri modp CCO2329 CC23 lne flac n mod s protn CC238 c A DNAm rylborom CC2430 -cpA mobyioooplingobonotaxls protein obomoteols proof CC0831 regulator CC0432 -h.3 obectaxlero CC0433 -isA obemotai le CC10434 cho mocaimeplorprotein itblnsiams n bsemote prosan CC 80 -0.0 ,bsmosudi liermi hte urt 0,l.0.es 000436Chas rulo 1CO37 ch*pP obnts response P bnmotae poin CCO42 cpaV CC319 hell bmotaxisProte 000440 0 otnloteei rospnoeresgu etur COO41 protein CC251m ur chloEdhvotisn COMIS W9 fsgefr 11t opqoWln P. W CC2312 606 lEG fiegellarmotorpoite protn AM o fleg rver protein CCOUUY CO11OROD repose eguitor 0C953 M fiuoellerblesel~body rodprotein CCOSO4 ft ngskrben-body rod proteio CCOW51 fiageller hoobkbeea body coropis protein o prot n CC1457006 Opetenmodificabit 00140ODT flegeiler bloweniels rngolotor 0CC145 Wg flagoller 0020614 flo goilbosabbody rodproteio C=204pn stbd-call dlflimetol regolotor dlsiC o dilafridlellonioll C02402 h000 ODflg l pro, cyclereponseregulator 002541 0.A selydiison protein niiblonp it r old C002042 002001 murG pop Idoglwn Synthesis onoyrn sell d~lnproino 002002 MlW COU IMI&Aboldlboyntemlprotein 002620 ltB W~ldtsyntesis protein 002060 noul8 no0,protein'putatire C2M hypothetical noel hypo0nolical protein CC2950 CC2051 yb.leprtl lntre eooO factor 000000 mom hypothe.tical protein 00022 CO04Sypoarelcal protein 000713 f lgnl lP promn = asovdbypothetical 001101' CC1102 bypot Vi protein 001000 GI 00060 b y protein 00106113 sloP ATPdspeod*Mprotbe. prolotic subuit 002S24 hybridbintdbkw blenn bypolhnoftsl protein C3 0054 Op pus ... rte protein protein CC2543opeE PMioassembly 0C2344 Spe pilesaesemblyprotein Coano ope pum- mmooeiblv poten 002544 pp0 usilo eeeoblyprotein pilo asemnbly protein 002-dyopA 00214 pNA plus ebntrti 0021,.ds hypothrecl eprotlnh CC0903scAP CtrAtruoelpionl Inhbitor lografto) -1 -0. 0 0. I Figure 2.5 Effects of sciP overexpression and depletion on CtrA-dependent gene expression. Whole-genome DNA microarrays were used to examine gene expression changes in the strains indicated. The cckAts and ctrAt s mutants were grown in PYE at 28*C, shifted to 370C for two hours, and compared to wild-type grown under the same conditions (data are from [20, 26]). For sciP overexpression, strain ML1749, which harbors pHXM-sciP, was grown in PYE supplemented with xylose for 2 hrs and compared to wild type grown under identical conditions. For sciP depletion, strain ML1750 was grown in PYE supplemented with xylose, synchronized, and released into PYE supplemented with glucose or xylose. Each population of cells were grown for 100 min, and then compared to each other. Expression changes for each gene are colorcoded according to the legend shown at the bottom. The genes shown include those previously reported to be direct CtrA targets based on ChIP-chip analysis and whose expression changed significantly in both cckAt' and ctrAt ' strains after a shift to the restrictive temperature [20, 26]. Note, the divK/pIeD operon was not identified by ChIP-chip analysis, but was subsequently confirmed as a direct CtrA target [17]. sciP is included at the bottom of the list. 61 pHXM-sciP pHXM-scIP - glu = 2.4 t 1.2 (n = 372) xyl = 6.8 T3.0 (n = 319) -0nm s0 xyiose aeMnIA&A i 0 % 40 20 0 2 4 8 6 10 12 14 16 18 20 length (sm) C time(min.) 0 10 20 30 40 80 60 70 80 g0 100 110 78 34 12 1 2 3 4 5 6 --sses01en - 7 8 Mi,.ausa-M2-SciP # of chromosomes D 35 30 25 15 10 E A pHXM4cIP(glu) * pHXM-scIP(xyl) 5 0 20 40 60 80 *wt *AsciP 100 time (min.) 30 0 60 90 time (min.) glucose xylose CtrA-P :0% c f CtrA c o" 0.75 Gr 0.5 a. AscIP pHXM-sc/P (gu) 0.25 pHXM-cIP (xyl) 0 av. 0 10 20 30 40 s0 60 Reaction time (min) Figure 2.6 Phenotypic consequences of sciP overexpression. (A) Cellular morphology (top) and flow cytometry analysis (bottom) of cells overexpressing sciP from a high-copy plasmid (strain ML1749). A culture was grown in PYE glucose and sciP expression induced by the addition of xylose for 4 hrs. (B) Westem blot for SciP in samples taken from a synchronous population of cells overexpressing sciP. (C) The overexpression strain was synchronized and released into glucose or xylose to repress or overexpress, respectively, sciP. ccrM mRNA levels as a function of cell cycle time were measured using qRT-PCR and expressed relative to a control, rho. (D) Pulse-chase analysis of CtrA stability in the strains indicated. For the overexpression strain harboring pHXM-sciP, cells were grown in the M2G or shifted to M2G supplemented with xylose for 1 hour to induce sciP expression. Each experiment was performed in triplicate except for wild type which was done in duplicate; error bars represent standard error of the mean. (E) Phosphorylation levels of CtrA in vivo. Cells harboring pHXM-sciP were grown in media supplemented with glucose and then shifted to media with xylose for two hours. In each case, CtrA was immunoprecipitated from cells after labeling with [y P]-ATP and then separated by SDS-PAGE followed by exposure to a phosphorimage screen (top panel), with CtrA levels measured by Western blotting (bottom panel). (F) CtrA occupancy of the chromosomal origin, Cori, and pilA and ccrM promoters was measured by chromatin immunoprecipitation of CtrA from DsciP and cells overexpressing sciP. Each measurement was done in triplicate and divided by the average triplicate percent input for wild type; error bars represent the standard error of the mean. (5) Dephosphorylation of CtrA by posphorelay reversal in the absence or presence of 1 1iM SciP. Notably, the expression of sciP itself was strongly decreased in ctrA'' and cckA's (Fig. 3) and overproducing M2-SciP from a high-copy plasmid led to a significant drop in the levels of native, chromosomally-encoded SciP (see Fig. 2.8B). Previous ChIP-chip studies did not include the sciP promoter on the microarrays used, but the predicted regulatory region has a near-consensus CtrA binding site, suggesting sciP is a direct CtrA target. To confirm that the down-regulation of CtrA-activated genes was due to the ectopic production of SciP in predivisional cells, we synchronized the overexpression strain, released cells into media with either glucose or xylose, and monitored cell cycledependent expression of ccrM, a CtrA-activated gene, using quantitative PCR. Cells grown in glucose showed the expected peak in ccrM expression in predivisional cells, while cells grown in xylose never induced ccrM above background levels (Fig. 2.6C). In sum, our gene expression studies demonstrate that SciP is sufficient to inhibit CtrA from activating its target genes. SciP does not affect CtrA degradation orphosphorylation How does SciP inhibit CtrA-dependent transcription? To begin addressing this question, we first examined the steady-state levels of CtrA following both sciP depletion and overexpression and found that they did not change significantly in either case (data not shown). We also measured the stability of CtrA in the sciP deletion strain and in a strain overproducing SciP. In AsciP, the half-life of CtrA was 62 minutes, similar to the value measured in wild-type cells (Fig. 2.6D). With the overexpression strain, CtrA was more stable in cells grown in xylose to induce SciP than in cells grown in glucose (50 versus 173 minutes) (Fig. 2.6D). These data indicate that SciP does not down-regulate CtrA activity by stimulating degradation but may stabilize it. To test the effect of SciP on the phosphorylation of CtrA, we first reconstituted CckA-ChpT-CtrA phosphorelay in vitro. The addition of SciP had no significant effect on the rate or steady-state levels of CtrA phosphorylation (data not shown). SciP also had no substantial effect on the dephosphorylation of CtrA-P by phosphorelay reversal (Fig. 2.6H). We also measured the phosphorylation level of CtrA in vivo in cells overexpressing sciP by immunoprecipating CtrA after growth in the presence of [y3 2 P]-ATP. CtrA-P levels did not change significantly after overexpressing sciP for two hours (Fig. 2.6E). Finally, we tested whether sciP affects DNA-binding by CtrA using chromatinimmunoprecipitation (ChIP) to examine the occupancy of CtrA at two target gene promoters and at the origin of replication in vivo. Neither the overexpression nor depletion of sciP had a significant effect on CtrA occupancy of the origin of replication, or the pilA and ccrM promoters (Fig. 2.6F). These findings also corroborate our global gene expression data showing that overexpressing and depleting sciP affects CtrAactivated, but not CtrA-repressed genes. Additionally, these data are consistent with the observation that cells overproducing SciP exhibited relatively moderate chromosomal accumulation. If SciP disrupted CtrA phosphorylation or DNA-binding, we would have expected more significant chromosomal accumulation, as seen with ctrA' and cckA's mutants [10, 18]. The mild accumulation of chromosomes following sciP overexpression is likely due to the disruption of cell division that results from SciP's inhibition of CtrA target genes, many of which are important for cell division. Consistent with this interpretation, we found that cells depleted of the essential cell division geneftsZ showed a similar mild chromosomal accumulation phenotype (Fig. 2.7). PxyrftsZ xylose 1 2 3 4 5 6 7 8 glucose 1 2 3 4 5 6 7 8 # of chromosomes Figure 2.7. Morphology and chromosomal content of tsZ depletion strain. Cells were grown in either xylose to maintain expression of ftsZ or in glucose to repress ftsZ. Cells were examined by light microscopy (top) or flow cytometry (bottom). SciP binds directly to CtrA Our data suggest that SciP could bind to CtrA to prevent it from interacting productively with RNA polymerase to drive transcription of target genes. To test whether SciP regulates CtrA via a direct protein-protein interaction, we first tested whether CtrA and SciP interact in a yeast two-hybrid system. CtrA was fused to the activating domain of Gal4 and SciP to the DNA-binding domain of Gal4. Together, these constructs reconstituted Gal4 activity and drove expression of GAL1-HIS3 at a level sufficient to enable growth on minimal medium lacking histidine (Fig. 2.8A). Neither CtrA nor SciP alone was sufficient to activate HIS3 expression. Also, SciP did not interact with the response regulator PhoB, supporting the notion that SciP binds specifically to CtrA. To identify amino acids important for the CtrA-SciP interaction, we constructed a series of alanine substitutions in SciP at sites that are (i) perfectly conserved in sciP orthologs and (ii) solvent-exposed in an NMR structure of an ortholog from R. sphaeroides solved by a structural genomics project (PDB: 2JRT) (Fig 2.9). In total we made five mutants, R35A, R40A, E57A, Y62A, and Y68A, and found that each mutation, except E57A, completely disrupted the CtrA-SciP interaction in the yeast two-hyrbid assay (Fig. 2.8A). We then tested the activity of these alanine mutants in vivo by overexpressing them in Caulobacter cells using the high-copy vector pHXM. Each alanine mutation, with the exception of E57A, completely eliminated the sciP overexpression phenotype. (Fig. 2.8C). Immunoblots of the overexpression strains indicated that only the R35A and R40A mutants accumulated to levels equivalent to overexpressed wild-type sciP suggesting the other mutations may affect SciP stability (Fig. 2.8B). These results suggest that the severe phenotype of sciP overexpression requires a direct interaction between SciP and CtrA, and that residues R35 and R40 are critical for this interaction. B UO - His AD BD dIvJ dvK ctrA - ctrA SCIP phoB sciP -. sciP % ctrA sciP(R35A) ctrA sclP(R40A) sCIP G6 X + His scP(R35A) G X sc/P(R40A) G X XG M2-ScIP ScIP C sclP sc/P(R35A) sclP(R40A) ---glucose ---- # of chromosomes xylose # of chromosomes # of chromosomes Figure 2.8. SciP and CtrA interact directly in a yeast two-hybrid assay. (A-B) Yeast strain PJ69-4A was co-transformed with plasmids harboring fusions of the indicated genes to either the activation or DNA-binding domain of Gal4, as indicated, and spotted onto histidine deplete or replete solid media. Each row contains serial 10-fold dilutions of overnight cultures normalized to OD 0.5. Growth after 72 hours is shown. Growth on media lacking histidine indicates reconstitution of Gal4 activity by protein-protein interaction. (C)Cellular morphology (top) and flow cytometry analysis (bottom) of Caulobacter strains overexpressing sciP or the indicated alanine mutants of sciP. Cultures were induced with xylose for 4 hrs. (D) Western blot analysis of plasmid-encoded M2-SciP and chromosomally-encoded SciP for the strains from panel C grown in glucose or xylose to repress or overexpress, respectively, the plasmid-borne M2-sciP. Figure 2.9 Model of SciP structure. (A) NMR structure (PDB: 2JRT) of a SciP ortholog from R. sphaeroides. (B) Residues R35 and R40, which are important for inhibiting CtrA-dependent gene expression are highlighted in magenta with spacefilling. SciP binds CtrA without disrupting DNA-binding Next, we used electrophoretic mobility shift assays to test whether SciP could interact with CtrA when CtrA is bound to target promoters. We added purified CtrA~P to radiolabeled, 50-base pair fragments of the ccrM, pilA, andfliFpromoters, which contain CtrA-binding sites [9, 19, 27]. As expected, CtrA bound these DNA fragments, leading to a decrease in unbound DNA and the appearance of lower mobility bands (Fig. 2. 1A-C). The intensity of these latter bands increased with increasing CtrA concentration and were correlated with decreases in unbound DNA. CtrA did not bind to a DNA fragment from the vanA promoter which lacks a CtrA-binding site (data not shown). We then added SciP to each reaction and found that it led to a dramatic enhancement of DNA-binding as evidenced by the nearly complete disappearance of free, unbound DNA at the lowest concentration of CtrA and the appearance of low mobility bands at lower concentrations of CtrA than seen without SciP (Fig. 2.1OA-C). We found that SciP alone did not bind any of the probes tested, even at concentrations up to 10 [M suggesting it does not bind DNA alone or does so only in the presence of CtrA. We also found that SciP(R35A) and SciP(R40A) did not supershift the fliF promoter as observed with wild-type SciP (Fig. 2.101D). In sum, these data support the conclusion that SciP interacts directly with CtrA, but without disrupting CtrA's ability to bind DNA. SciP thus likely inhibits CtrAdependent gene activation by preventing CtrA from interacting productively with RNA polymerase. Pe,,' PPUA -ScIP CtrA (pM) 0 0.2 0.4 0.6 0.8 1.0 0 0.2 0A 0.6 0.8 [pee 1.0 0 0.2 0.4 0.6 0.8 1.0 0 0.2 0.4 0.6 0.8 1.0 D POF CtrA (pM) 0 0.2 OA 0.6 0.8 1.0 0 CtrA (pM) Complexes - ScIP + ScIP -ScIP + ScIP pM POW + ScIP SCIP -4+-. 0.2 0.4 0.6 0.8 1.0 - - - +4+ + - - + - + -+ - +-+ - +4+ OKAA DNA 60 bp 60 bp 25 bp 26 bp PmIF ScIP - ScIP CtrA - CtrA RNAP S- 2.0 - 0.4 0.4 - - - + . 0.2 2.0 - - - - 1.0O + + 0.2 0.8 1A OA OA 0.4 0.4 + . + + RNAP-DNA -OR ICtrA4CP.ONA ## CtrA-ONA AAA& VWWWWWW I* - DNA Figure 2.10. Electrophoretic mobility shift assays with CtrA and SciP. CtrA-P binding to a fragment of the (A) ccrM, (B) pilA, or (C) fiiF promoter containing a CtrA-binding site. SciP, if present, was added at a final concentration of 1.0 RM. The concentration of CtrA in each lane is indicated. (D) Binding of CtrA-P to the fliF promoter in the presence of wild type SciP or the mutant indicated. CtrA was present at 1 [LM and SciP, or mutant SciP, at 1 [tM. Discussion As with all organisms, cell cycle progression in Caulobacter requires the just-in-time transcription of large batteries of genes [28]. At the heart of this transcriptional program is CtrA, which directly controls the expression of nearly 100 genes [20] and indirectly many more. CtrA both activates and represses genes; for activated genes, CtrA binds near the -35 site and probably recruits or binds RNA polymerase, while for repressed genes, CtrA binds near the +1 site and presumably occludes RNA polymerase [16, 29]. A longstanding conundrum is why CtrA represses genes and the origin of replication in both swarmer and predivisional cells, but activates most of its target genes only in predivisional cells. Here, we identified SciP and showed that it temporally restricts the transcriptional activity of CtrA by inhibiting CtrA-activated transcription in G1 swarmer cells. SciP plays a crucial role in regulating the activity of CtrA and, consequently, in regulating the cell cycle and the establishment of asymmetric daughter cells in Caulobacter. CtrA and SciP collaborate to create three distinct states corresponding to the three major Caulobactercell types (Fig. 2.11). In GI swarmer cells, CtrA-P and SciP levels are both high such that CtrA can repress genes and silence the origin of replication but cannot activate gene expression. At the G1-S transition stalked cells are cleared of CtrA and SciP thereby permitting DNA replication and the expression of CtrA-repressed genes. As cells progress through S-phase, CtrA-P again accumulates to high levels but SciP levels remain low, enabling CtrA to activate the expression of target genes and propel cells through the ensuing cell division. CtrA-P O0 O0 G2 Sc CtrA-activated gene expression iP CtrA-P CtrA-P CtrA-activated genes = OFF CtrA-P CtrA-repressed genes CtrA-P a a Figure 2.11 Regulation of CtrA-dependent gene expression during cell cycle progression. CtrA transcriptional activity is controlled by temporally-regulated proteolysis, phosphorylation, and protein-protein interaction with SciP. The timing of each regulatory event is shown beneath a schematic of the Caulobacter cell cycle. These layers of regulation restrict CtrA-activated gene expression to the predivisional stage. A model for how SciP and CtrA combine to regulate gene expression during cell cycle progression is shown at the bottom. Why block transcriptional activition by CtrA in GI cells? The majority of CtrA target genes are involved in polar morphogenesis, including flagellar biogenesis, chemotaxis, and pili biogenesis. CtrA also activates the expression of key cell division genes and ccrM, an essential DNA methyltransferase. These processes - polar morphogenesis, cell division, and DNA methylation - occur exclusively, or at least primarily, in predivisional cells. Constitutive expression of many of these genes, such as ftsA and ccrM, has deleterious consequences to cell cycle progression [30, 31]. SciP thus helps fulfill the important task of preventing the execution of these cell cycle processes at the wrong time. Some CtrA-activated genes produce transcripts that are abundant in swarmer cells [20] and seemingly exceptions to our model for SciP. However, DNA microarrays measure steady-state mRNA levels and reflect both transcription and mRNA decay. Thus, some genes may be transcribed in predivisional cells but yield mRNAs that are unstable until the swarmer phase, as is the case for some flagellin filament genes [21]. Alternatively, some genes could have promoter architectures that prevent SciP from inhibiting CtrA. Regulation of SciP Given the importance of SciP to cell cycle progression and the consequences of misexpression, it comes as no surprise that SciP itself is tightly regulated and restricted to G1. sciP mRNA is detectable in predivisional and swarmer cells. However, SciP protein only accumulates in swarmer cells, suggesting either SciP is rapidly degraded in predivisional cells or the translation of sciP is blocked in predivisional cells and/or activated in swarmer cells. The rapid disappearance of SciP at the G1-S transition suggests it could be subject to temporally-regulated degradation. Notably, CtrA is rapidly degraded by the ClpXP protease at precisely this stage of the cell cycle [32]. Consistent with post-transcriptional regulation of sciP, cells constitutively expressing sciP from a xylose-inducible promoter on the chromosome still only accumulated protein during Gi (Fig. 2.4E). However, the regulation could be overcome by constitutively expressing sciP from a high-copy number plasmid (Fig. 2.6C), and importantly, this overexpression was sufficient to disrupt CtrA-dependent gene activation and, consequently, the cell cycle. Our results outline a new feedback loop important for cell cycle progression in Caulobacter.sciP appears to be a direct transcriptional target of CtrA as sciP expression drops significantly in ctrAt" strains and the regulatory region upstream of sciP has a nearconsensus CtrA binding site. In predivisional cells, active CtrA likely drives the expression of sciP. Following cell division SciP accumulates in swarmer cells, where it feeds back to inhibit CtrA as a transcriptional activator. This feedback is reminiscent of two other feedback loops, involving the cell cycle regulators RcdA and DivK. Both rcdA and divK are direct transcriptional targets of CtrA [20] and each feeds back to inhibit CtrA. RcdA somehow contributes to the regulated degradation of CtrA at the Gl-S transition [33], although its function is unknown, while DivK inhibits the phosphorelays that drive the phosphorylation and proteolytic stabilization of CtrA [34, 35]. The combination of multiple feedback loops is likely critical to maintaining robust, sustained cell cycling in Caulobacter. Mechanism of inhibiting CtrA We found no evidence that SciP affected the steady-state levels, stability, or phosphorylation of CtrA. Instead, our data suggest that SciP directly binds to and inhibits CtrA. SciP does not, however, prevent CtrA from binding to DNA, consistent with our finding that sciP mutations do not affect CtrA-repressed genes. The simplest model to account for our data is that SciP blocks CtrA from binding to RNA polymerase and activating transcription of target genes. The interaction of SciP and CtrA in the presence of DNA was particularly striking and suggestive of a highly cooperative interaction. At concentrations of CtrA that yield only minimal DNA-binding, the addition of SciP stimulated very strong binding. The shifted bands in the presence of CtrA and SciP were also sharper than with CtrA alone suggesting the ternary complex is more stable than the CtrA-DNA complex. SciP did not bind DNA on its own, although it may bind DNA nonspecifically in the presence of CtrA. In fact, sequence analysis and the NMR structure of a SciP ortholog (PDB: 2JRT) indicate some similarity to helix-turn-helix proteins (Fig 2.11 A-B). Regulating response regulators SciP represents a novel mechanism for modulating the output of a two-component signaling pathway. Response regulators are usually regulated at the level of phosphorylation by cognate kinases and phosphatases. There are also regulatory proteins that can bind and stabilize the aspartyl-phosphate linkage. For example, in Salmonella enterica the protein PmrD binds the response regulator PmrA to block dephosphorylation [36]. There are only a few examples of proteins that regulate response regulators without affecting phosphorylation, such as the B. subtilis proteins RapC and RapG which prevent ComA and DegU, respectively, from binding DNA [37, 38]. The E. coli protein TorI was reported to inhibit transcriptional activation by the regulator TorR in vitro, but torI null mutants were not shown to affect TorR-dependent gene expression [39] and TorI is not expressed during exponential phase growth (M. Ansaldi and V. Mejean, personal communication). The role of TorI is also unclear as recent data indicate TorI's primary function is as a prophage excionase [40]. Perhaps the protein most analogous to SciP is Spx in B. subtilis. Spx is a small protein that binds to the alpha-subunit of RNA polymerase at the same site contacted by the response regulators ComA and ResD [41]. Spx thereby prevents these regulators from binding RNA polymerase to stimulate the transcription of target genes. This may not be a common regulatory strategy though as binding to RNAP polymerase runs an inherent risk of having pleitropic effects on transcription. SciP's inhibition of CtrA-dependent expression thus represents a novel mechanism for regulating gene expression in bacteria. However, this mechanism may be prevalent throughout the bacterial kingdom. First, SciP exhibits strict coconservation with CtrA and has an ortholog in nearly every a-proteobacterial genome. More generally, SciP shows moderate homology to helix-turn-helix proteins; many bacterial genomes encode for dozens of small, uncharacterized helix-turn-helix proteins and we speculate that some of them could inhibit other response regulators by a similar mechanism to SciP. Concluding remarks SciP's function underscores the notion that bacterial gene expression is often not dictated simply by the activation of a single transcription factor, but instead involves the combinatorial action of multiple regulators. The identification of SciP also highlights the importance of regulated protein-protein interactions to cell cycle control in organisms from bacteria to metazoans. As noted earlier, the master transcription factor in metazoans, E2F, is regulated by the binding of Rb, while in budding yeast the cell cycle transcription factors SBF and MBF are regulated through the binding of Whi5. Other key cell cycle regulators, such as cyclin-dependent kinases, are also regulated by protein- protein interactions [42, 43]. For instance, SICI in S. cerevisiae accumulates to maximal levels in GI cells where it inhibits S-phase-specific CDKs while Rumlp and Rux play similar roles in S. pombe and D. melanogaster, respectively. In mammalian cells, two major classes of inhibitors, exemplified by p27 and pl6mK, directly inhibit different classes of CDKs. Regulated protein-protein interactions involving master regulators are thus critical to cell cycle progression at many levels and in all organisms. Experimental Procedures Bacterial strains and media E. coli and C. crescentus strains were grown as previously described [44]. PYE was supplemented with 0.3% xylose or 0.2% glucose when indicated. M2G was supplemented with 0.3% xylose when indicated. Xylose inductions were performed by diluting cultures grown to mid-log phase into PYE supplemented with xylose or adding 0.3% xylose to M2G. For SciP depletion, a culture of ML1750 was grown to mid-log phase, pelleted at 6,800 g for 1 minute, washed with PYE, and resuspended in PYE plus glucose. Synchronizations were performed on mid-log phase cells using Percoll (GE Healthcare) density gradient centrifugation. Cloning and mutagenesis All expression vectors were constructed using the Gateway cloning system (Invitrogen) as previously described [44]. The CC0903 (sciP) open reading frame was amplified using the primers listed in Table 2 and cloned into pENTR-D-TOPO to create pENTR-sciP. Alanine mutations were made using the QuikChange kit (Stratagene) with pENTR-sciP as template. The pENTR-sciP plasmids were recombined with pHXM-DEST, pHIS- DEST, pAD-DEST and pBD-DEST to construct the expression plasmids pHXM-sciP, pHIS-sciP, pAD-sciP and pBD-sciP, respectively. Entry vectors for divJ (cytoplasmic domain), divK, ctrA, and phoB were recombined with pAD-DEST and pBD-DEST. pAD-DEST was constructed by ligating the Rfa cassette (Invitrogen) into pGAD-c1 linearized with SmaI. pBD-DEST was made by ligating Rfa into pGBDU-c3 that had been digested with Clal and blunted with the End-It DNA End-Repair kit (Epicentre). The orientation of the Rfa cassettes was verified by EcoRI digestion. pNPTS-spec-DEST was constructed by inserting the Rfa cassette into the SmaI site of pNPTS138 followed by replacement of the kanamycin resistance cassette (digestion with XbaI and NscI followed by end repair) with the spectinomycin resistance cassette from HP45Q (excised with SmaI). The MultiSite Gateway vectors pEl-CC0903_LFR, pE3-CC0903_RFR, and pE2-FRT-tet were constructed by amplifying a 600 bp region upstream of CC0903 (including 15 nucleotides into the coding sequence), a 600 bp region downstream of CC0903 (including the last 30 nucleotides of coding sequence), and the tetracycline resistance cassette from pKOC3 using the primers specified in Table 2 and cloning the PCR products into pDONR221 Pl-P4, pDONR221 P3-P2, or pDONR221 P4r-P3r, respectively, by BP recombination (Invitrogen) in a reaction consisting of 40 ng PCR product, 0.5 ptL pDONR vector, and 1 pL of BP clonase in a 5.5 pL reaction volume. The BP reactions were incubated overnight at room temperature and then transformed into chemically competent TOP1O cells and plated on LB with kanamycin. The pKO-CC0903 plasmid was then constructed with MultiSite Gateway Pro three fragment recombination between pEl-CC0903_LFR, pE3-CC0903_RFR, pE2-FRT-tet, and pNTPS-DEST in a reaction containing 40 ng of each entry plasmid, 100 ng of the destination vector, and 2 pLL of Clonase II enzyme in 10 tL total reaction volume. The recombination reaction was incubated at room temperature overnight and transformed into chemically competent DH5ca and plated on LB supplemented with spectinomycin and tetracycline. The integrating plasmid pX-sciP was created by excising GFP from pXGFPN-2 with NdeI and KpnI to replace the GFP cassette with the full sciP coding sequence derived by digesting a PCR fragment amplified with primers harboring NdeI and KpnI restriction sites as indicated in Table 2. Deletion and depletion of sciP SciP was deleted through a two-step homologous recombination method previously described [44] using the pKO-CC0903 integrating plasmid. The sciP deletion was verified by PCR using one primer outside the region used for homologous recombination and one primer in the tetracycline resistance cassette (Table 2). The tetracycline-marked deletion was transduced into a clean CB15N background using $CR30. To generate a depletion strain, the integrating plasmid pX-sciP was transformed into CB 15N by electroporation and integrants of sciP at the xylX locus were selected on PYE plates containing kanamycin and glucose. The tetracycline-marked sciP deletion was then transduced from ML1749. The depletion strain was selected on PYE plates containing kanamycin, oxytetracycline, and xylose and verified by PCR to contain sciP only at the xylX locus. Protein purification and antibody production His6-SciP was expressed from pHIS-sciP plasmid and purified, along with His 6-CtrA, as described previously [44]. His6-RpoD was expressed from pHIS-rpoD and purified as with His6-SciP and His6-CtrA but with the addition of 6 M GuHCl to the lysis buffer. RpoD was bound to Ni-NTA resin under denaturing conditions and subsequently renatured by washing in wash buffer with no GuHCl. Refolding was confirmed by CD spectroscopy. TAP-tagged RNA polymerase was purified from ML1799 as previously described [45] and dialyzed overnight at 4'C against 500 volumes of TGED buffer (10mM Tris-HCL pH 7.9, 50% glycerol, 0.1 mM EDTA, 0.1 mM DTT, 500 mM NaCl). Coomassie staining was used to verify that purified TAP-tagged RNA polymerase contained alpha, beta, beta', and sigma subunits. Rabbit polyclonal antisera were generated from purified His6-SciP (Covance). Rabbit polyclonal antisera were generated from purified His6 -SciP (Covance). Immunoblots Cell pellets were collected at indicated time points. Cells were resuspended in 1x SDS sample buffer and resolved on 15 %,Tris-HCl gels (Bio-Rad) run at 150 V for 1 hr at room temperature, transferred to PVDF membrane and probed with a 1:2,000 dilution of SciP antisera, 1:1,000 anti-M2 affinity-purified antibody (Sigma), or 1:10,000 of CtrA antisera in lx TBS plus 0.05% Tween-20. Microscopy Cells were fixed with 0.5% paraformaldehyde, centrifuged at 6,800 g, washed with PBS and resuspended in a small volume of PBS. Differential interference contrast images were taken with a Zeiss Axiovert 200 microscope with a Zeiss aPlan-Fluar 100x/1.45 oil objective and an Orca II camera (Hamatsu) controlled by MetaMorph 7.1.3.0 software. Flow cytometry Samples for flow cytometry were prepared and analyzed as described previously [35]. DNA microarray analysis Cultures were grown in to mid-log phase, harvested by centrifugation at 20,000 g, and frozen in liquid nitrogen. For the sciP depletion experiment, ML1750 was grown to midlog phase in PYE supplemented with xylose, synchronized and released into fresh PYE containing either glucose or xylose. Cell pellets were collected after 100 minutes, immediately after cell division in each culture. RNA was isolated using the RNeasy kit (Qiagen). 30 [tg of total RNA was directly labeled using the Superscript-II kit (Invitrogen) and either Cy3- or Cy5-dCTP. RNA template was degraded with NaOH. Cy3- and Cy5-labeled cDNA samples for comparison were mixed and unincorporated nucleotides removed using the QIAquick PCR purification kit (Qiagen). Labeled cDNAs were hybridized to Agilent arrays, arrays scanned using an Agilent scanner, and data extracted using the Agilent feature extraction scripts within the Matlab Bioinformatics Toolbox. Analysis of the depletion and overexpression strains were done in duplicate and triplicate, respectively, and results averaged in each case. Yeast two-hybrid assays Protein-protein interactions were assayed in the yeast two-hybrid system described previously [46] except using the Gateway modified pAD-DEST and pBD-DEST vectors. S. cerevisiae PJ69-4A was co-transformed with combinations of pAD and pBD vectors using LiAc. Double transformants were selected on solid CSM-Leu-Ura. Yeast strains were grown at 30 0C in liquid SD minimal media supplemented with 2% glucose and CSM-Leu-Ura or CSM-His-Leu-Ura plus 2% bactoagar for solid media. Electrophoretic mobility shift assays EMSAs were performed using His 6 -SciP and His 6-CtrA purified protein. 50 base pair PAGE purified primers (Sigma-Genosys) were annealed in TEN buffer (10 mM Tris-HCl (pH 8.0), 1 mM EDTA, and 50 mM NaCl) at 95*C for 10 minutes and slowly cooled to room temperature. The double-stranded DNA was labeled with [y32P]-ATP using T4 polynucleotide kinase (NEB). 10 liM CtrA was phosphorylated using 1jxM MBP-EnvZ in HKEG buffer (10 mM HEPES-KOH [pH 8.0], 50 mM KCl, 10% glycerol, 0.1 mM EDTA, 1 mM DTT) plus 5 mM MgCl2 and 1 mM ATP for 20 min at 37 C. The CtrA-P was diluted into HKEG plus MgCl 2 to lOX before being added to the gel shift reactions. Proteins were pre-incubated at room temperature for 30 min in gel shift buffer (50 mM KCl, 5mM MgCl 2 , 20 mM Tris HCl (pH 8.0), 100 [M EDTA, 1 mM DTT, 1 mg/ml BSA, 10% glycerol, 100 ng/ml competitor DNA (Poly (I)-Poly(C))) and then incubated reaction volume. 2 with 3.5 nM labeled DNA at room temperature for 30 min in a 18 1A tl of 0.2% bromophenol buffer was added to each reaction immediately before the samples were loaded onto a Novex 6% DNA retardation gel (Invitrogen) and run at 300 V in chilled 0.5x TBE buffer for approximately 17 min or until the dye front migrated sufficiently far through the gel. The gel was then transferred to Whatman paper, covered on top with Saran wrap, dried on a gel drier for 1 hr, and exposed to a phosphor screen for 1 hour. Screens were scanned with a Typhoon scanner. Chromatin immunoprecipitation Bacterial ChIP was performed as described previously using 2.5 ptL of polyclonal c-CtrA antibody for each pull-down [47]. The % input values for the ChIP samples were calculated using the average Ct values from triplicate measurements and a standard curve generated from five two-fold serial dilutions of the input samples. Each ChIP experiment was performed on three independent colonies to calculate standard error bars. The % input values for the ChIP samples were calculated using the average Ct values from triplicate measurements and a standard curve generated from five two-fold serial dilutions of the input samples. Each ChIP experiment was performed on three independent colonies to calculate standard error bars. The relative expression of genes was calculated using the following formula: ratio = ((Etarget)^ACPtarget(control-sample))/ ((Erho)AACPrho(control-sample)). For expression analysis, 1 [tg of total RNA was reverse transcribed into cDNA using Superscript-II, RNA template degraded was with RNAse H, and nucleotides were removed using the QIAquick PCR purification kit (Qiagen). Quantitative real-time PCR Quantitative real-time PCR for ChIP and expression analyses was performed using the DNA Engine Opticon 2 system (MJ Research) and the QuantiFast SYBR Green PCR Kit (Qiagen). Each reaction contained 2.5% of total ChIP sample or 2% total cDNA for expression analysis, 10 pmol of forward and reverse primer, lx Quantifast SYBR Green PCR master mix (Qiagen) in a 25 pl reaction. Samples were amplified by incubating at 504C for 2 min, 95'C for 15 min followed by 45 cycles of 95'C for 15 sec, and 60'C for 30 sec followed by fluorescence data collection. Cycle threshold (Ct) values were calculated using Opticon Monitor software (MJ Research). The relative expression of genes was calculated using the following formula: ratio = ((Etarget)AACPtarget(controlsample))/ ((Erho)AACPrho(control-sample)). For expression analysis, 1 pg of total RNA was reverse transcribed into cDNA using Superscript-II, RNA template degraded was with RNAse H, and nucleotides were removed using the QlAquick PCR purification kit (Qiagen). Quantitative real-time PCR was performed using the DNA Engine Opticon 2 system (MJ Research) and the QuantiFast SYBR Green PCR Kit (Qiagen). Each reaction contained 2.5% of total ChIP sample or 2% total cDNA for expression analysis, 10 pmol of forward and reverse primer, lx Quantifast SYBR Green PCR master mix (Qiagen) in a 25 I reaction. Samples were amplified by incubating at 50'C for 2 min, 95'C for 15 min followed by 45 cycles of 95*C for 15 sec, and 60*C for 30 sec followed by fluorescence data collection. Cycle threshold (Ct) values were calculated using Opticon Monitor software (MJ Research). Pulse-chase analyses CtrA stability was assayed as described previously [8]. Cultures grown in M2G were and for ML1748, pre-induced with xylose for one hour and labeled with [ 35S]-methionine and chased with 1 mM methionine and 0.3% casmino acids. I mL aliquots were removed at indicated time points, cells were pelleted by centrifugation at 20,000 x g, and frozen in liquid nitrogen. Cell pellets where resuspended in 50 pL of lysis buffer (10 mM Tris pH 8, 1% SDS, 1mM EDTA), boiled for 2 minutes to lyse, and diluted with 800 pL chilled IP buffer (50 mM Tris pH 8, 150 mM NaCl, 0.5% Triton X-100). Samples were precleared with 20 pL Pansorbin Cells (Calbiochem) immunoprecipitated with 1.5 pL CtrA antibody at 4 C over night, and immune complexes were collected with 30 pL protein A beads (Invitrogen). The samples were washed 3x with chilled IP buffer and eluted in 30 pL of 2x SDS sample buffer by boiling for 5 min. The samples were loaded onto 10% Tris-HCl gels (Bio-Rad) and run at 150 V for 40 min at room temperature. Gels transferred to Whatman paper, dried for 1 hr and exposed to a phosphor screen for 72 hours. In vitro phosphorelay dynamics In vitro phosphorylation and phosphorelay reversal assays were done as previously reported [48]. In vivo phosphorylation Analysis of CtrA phosphorylation in vivo was performed essentially as described previously [8]. Briefly, one colony was inoculated into M5G medium containing 0.05 mM phosphate and grown overnight at 30'C. The cultures were grown at 30'C until the optical density of the culture measured at 660 nm was in the range 0.1 to 0.2. Cultures were then split with one maintained in glucose and the other grown in xylose for 2 hours. Equal numbers of cells (based on OD) were labeled for 5 minutes with 30 pCi/mL [y32 P]ATP before immunoprecipitating CtrA. Table S1 - Strains and Plasmids Strain or Organism or Plasmid Plasmild Category Strain Plasmid Strain or Plasmid Name C. crescentus CB15N ML1748 ML1749 ML1750 E. coli DH5a BL21-Tuner One Shot ccdBSurvival Ti TOP10 PJ69-4A S. cerevisiee pHXM-DEST destination vectors pHIS-DEST pNPTS-spec-DEST pAD-DEST pBD-DEST pHXM-sciP expression vectors pHXM-sciP(R35A) pHXM-sdP(R40A) pHXM-sciP(E57A) pHXM-sdP pHXM-sciP(R35A) pHXM-sciP(R40A) pHXM-sdP(E57A) pENTR/D-TOPO vectors general purpose pKOC3 pNPTS138 HP45Q P1-P4 pDONR221 P3-P2 pDONR221 P4r-P3r pDONR221 pXGFPN-2 pGBDU-c3 pGAD-cl pEl-CC0903_LFR donor vectors pE3-CC0903_RFR pE2-FRT-tet pENTR-sciP entry vectors pENTR-sciP(R35A) pENTR-sciP(R40A) pENTR-sciP(E57A) pENTR-phoB pENTR-divK pENTR-divJ pENTR-ctrA pKO-CC0903 plasmids Integrating pX-sclP vectors pAD-phoB yeast two hybrid pBD-phoS pAD-divK pBD-divJ pAD-ctrA pBD-crA pBD-sciP pBD-sciP(R35A) pBD-sciP(R40A) pBD-sciP(E57A) Table 2.1. Strains list. Descprtion Source or Reference Evinger and Agabian, 1977 this study this study this study Invitrogen Novagen Invitrogen Invitrogen James at a, 1996 Skerker at al, 2005 Biondi et al, 2005 this study this study this study this study pJS71X-M2-sciP (specp) this study pJS71-M2-sciP(R35A)(specR) this study pJS71-M2-sc/P(R40A)(spec") this study pJS71-M2-sc/P(E57A)(spec") this study pET-His.-sdP (amp") this study pET-His-sciP(R35A) (ampR) this study pET-Hise-sciP(R40A) (amp") this study pET-His-srdP(E57A) (ampR) Invitrogen ENTRYvector forGateway cloning system (kan") integrating vector with FRTflanked tetracycline resistance cassette (chlorR) Hamilton at al, 1989 lab collection integration vector with sacB (kan") lab collection source of omega cassette (spec") Invitrogen DONR vector forGateway multisite cloning system invitrogen DONR vector forGateway multisite cloning system invitrogen DONR vector forGateway multisitecloning system Thanbichler at al, 2007 integrating plasmld(kan") James at al, 1996 for fusions to C-terminaldomainof GAL4binding domain (LEU2,amp') James at al, 1996 for fusions to C-terminaldomainof GAL4activating domain (URA3,amp") this study 600 base pairs upstreamof CC0903in pDONR221 P1-P4 (kanR) this study 600 base pairs downstream of CC0903in pDONR221 P3-P2 (kan") this study pDONR221 P4r-P3rwithFRT flanked tetracycline resistance cassette (kanR) this study CC0903 in pENTR/D-TOPO (kane) this study (kan") CC0903(R35A) InpENTR/D-TOPO this study CC0903(R40A) InpENTR/D-TOPO (kanR) this study CC0903(E57A) in pENTR/D-TOPO (kan") Skerker et al, 2005 CC0294 in pENTR/D-TOPO (kan") 0 Skerker et al. 2005 (kan ) CC2463 in pENTR/D-TOPO Skerker et al, 2005 (kan") CC1067 (cytoplasmic domain) in pENTR/D-TOPO 0 Skerker at al. 2005 CC3035 in pENTRID-TOPO (kan ) this study In-frame deletion construct for sciP (tet") Thanbichler et al, 2007 integrating plasmidfor sciP at Pxyl (kan") this study (carbR,LE2) pGAD-phoB this study pGSDU-phoB (carb", URA3) this study pGAD-divK (carbR, LEU2) this study pGBDU-divJ (carb",URA3) this study pGAD-crA (carb, LEU2) this study pGBDU-ctrA (carbR,URA3) this study pGBDU-sciP (carb", URA3) this study pGBDU-sciP(R35A) (carb", URA3) this study pGBDU-sciP (R40A)(carb", URA3I this study pGBDU-sciP (E57A)(carbR,URA3) Synchronizable derivative of wild-type CB15 CB15N+ pHXM-sciP (spec") CB15NAsciP (tet") 0 CB15N AscP xyl::sciP(te", kan ) General cloning strain Strain for protein expression andpurification of destination vectors Strain for propagation clones General cloning strain for pENTR/D-TOPO GAL7-IacZ strain for yeast two-hybrid with GALI-HIS3, GAL2-ADE2, pJS71X-M2; high-copy, P. M2tag (spece,chlore) pET-His.(amp') pNPTS1 38 derivativemodifiedwith Rfacassette (spec") pGAD-CIGa4 AD fusion plasmid (carb", LEU2) Gal4 BD fusion plasmid (carb", URA3) pGBDU-C3 Table S2 - Primem R- FO-Nd ENM pft.1ft pENM-F $ft OW.Md Mutw CACCTTGTTGCAGCACCAGCOCAC OGG"GTCACATCAACTCA pd- l. GCGGAGACCCAGQCrrGGGrrAT=T ACG"TAAC4CX GCCTGWTCT=GC PENM-.ORR40A) TGC4"ATCCGTGCGMaGCCGAWTC GACCTMGCCTrCOCACGOATAACCCA PENM-F(EVA) CTMOCTCQACGCG=TGCGATMT pENM--FYRW) ACOATCGCACG=CG=AGCGAW canobvft DoUtion PEI-CCOW3 R pE3-CCODOM _LHR OC0903 KO cw* PE2.FRT4@1 .ftgr.u.d=p..YDr bon TTACTGACOCAGTACAACA= GOGGACAAGTMTACAAAAAAGCAGr CATGr.C r4C.ACAACrTTGTATAGAAAAGTTCG.TGCTGCTGCTGC GOGOACAACTrrOTATAATAAAGTrOCTGGAOCACCCGGATCCAOC G<VX2ACCACTrn3TACAAGAAAGCTGGGTACCGArGGAGCCOGAOCYCACOAG GACOCCCTGOOCATCOAC GAArGrrQAAGM00GAr.A GOMACMCMA7TATA'TTGTGAAGTTCCTATC CQCGACAACTTTTCTATACAAA(MGCTAAGAAG"CCTATACTTTC'rAGAGAATAGGAACTTCTCMTOOTCAM-.MCTOGGTCC TCCAGG- WTCCAG CAGCGATCGGCTOGTTGCO GGGCGGTCAC;ATCMCTCA PCM P (hWW. K -Q GOTACCOMAArOGTACTOCTOGA _C AG=GGOGAGACOACCATATGiTTOCAOCAOCAGCOCAC up Ar-W ACTCGCACAGCCCGTCCCGG TCCCCAAGCCTGCGCCGCAT MIPpXA GCCAGCATCACM)C:-MGG CGACTGCACTrAATGGCCAG 3'r-- GTCAACGGCGAGCTAGACCT CAGTCrCCGTGGATGATGGT q RT4CR MW PAP CCGATCCGCCCrATAOLTCTG CCTACCGGCAAGCTGATra GITGTGATAGCTGCCGATCA AACrGGACCAGGMCGCCAC w shift .w CTCAAAArCOCCTGAMQGCCrTGGTTAACGGOCCGCTAACCACGT=T AGACs OGTGGTrAGCGGOCCGTTAACCACrGC4TrTrAGGCOCTtTTrAG pAA 'rTOWAGCGATCGC4"OTGCATOGTTAAGAACAAATAACOGTAAATACA TOTATTTACZGTTATTTOTTCT'rAACCATOCACTCCGCGATCOCTOCCAA W GGAAAOCCrGACOATCGGCAGATATAAACOCCIrGTTTACCTrGTACTOG CCAGTACAAGGTAAACOAC-3rGMATATCTGWGATCGTCAWrMCC MM AGGTCOCOGAACCACGATGCGAGOAAAOrACGATGGGTAOCGAAACCGTGCACGGTrTrGGTACCCATOOTOGMOCTCGrAYCGTOGTTCGMaACCT Table 2.2. Primers. Acknowledgements We thank J. Modell, K. Milaninia, and B. Pando for help with experimental techniques. We thank F. Solomon, M. Neiditch, A. Fan, A. Grossman, and members of the Laub lab for comments on the manuscript. M.T.L. is an Early Career Scientist at the Howard Hughes Medical Institute. This work was supported by an NIH grant (5R01GM082899) to M.T.L. References 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. 12. 13. 14. 15. 16. 17. Breeden, L.L., Periodic transcription:a cycle within a cycle. Curr Biol, 2003. 13(1): p. R31-8. Dyson, N., The regulationof E2F by pRB-family proteins.Genes Dev, 1998. 12(15): p. 2245-62. Ross, J.F., X. Liu, and B.D. Dynlacht, Mechanism of transcriptionalrepressionof E2F by the retinoblastomatumor suppressorprotein. Mol Cell, 1999. 3(2): p. 195-205. Iyer, V.R., et al., Genomic binding sites of the yeast cell-cycle transcription factors SBF and MBF. Nature, 2001. 409(6819): p. 533-8. Costanzo, M., et al., CDK activity antagonizes Whi5, an inhibitor of Gl/S transcriptionin yeast. Cell, 2004. 117(7): p. 899-913. Laub, M.T., L. Shapiro, and H.H. McAdams, Systems biology of Caulobacter. Annu Rev Genet, 2007. 41: p. 429-41. Ryan, K.R. and L. Shapiro, Temporal and spatial regulationin prokaryoticcell cycle progressionand development. Annu Rev Biochem, 2003. 72: p. 367-94. Domian, I.J., K.C. Quon, and L. Shapiro, Cell type-specificphosphorylationand proteolysis of a transcriptionalregulatorcontrols the Gl-to-S transition in a bacterialcell cycle. Cell, 1997. 90(3): p. 415-24. Quon, K.C., G.T. Marczynski, and L. Shapiro, Cell cycle control by an essential bacterialtwo-component signaltransductionprotein. Cell, 1996. 84(1): p. 83-93. Quon, K.C., et al., Negative controlof bacterialDNA replicationby a cell cycle regulatorvprotein that binds at the chromosome origin. Proc Natl Acad Sci U S A, 1998. 95(1): p. 120-5. Laub, M.T. and M. Goulian, Specificity in two-component signaltransduction pathways. Annu Rev Genet, 2007. 41: p. 121-45. Stock, A.M., V.L. Robinson, and P.N. Goudreau, Two-component signal transduction.Annu Rev Biochern, 2000. 69: p. 183-215. Reisenauer, A., K. Quon, and L. Shapiro, The CtrA response regulatormediates temporal control of gene expression during the Caulobactercell cycle. J Bacteriol, 1999. 181(8): p. 2430-9. Siam, R. and G.T. Marczynski, Cell cycle regulatorphosphorylationstimulates two distinct modes of binding at a chromosome replicationorigin. EMBO J, 2000. 19(5): p. 1138-47. Biondi, E.G., et al., A phosphorelaysystem controls stalk biogenesis duringcell cycle progressionin Caulobactercrescentus. Mol Microbiol, 2006. 59(2): p. 386401. Domian, I.J., A. Reisenauer, and L. Shapiro, Feedback control of a master bacterialcell-cycle regulator.Proc Natl Acad Sci U S A, 1999. 96(12): p. 664853. Hung, D.Y. and L. Shapiro, A signal transductionprotein cues proteolytic events criticalto Caulobactercell cycle progression.Proc Natl Acad Sci U S A, 2002. 99(20): p. 13160-5. 18. 19. 20. 21. 22. 23. 24. 25. 26. 27. 28. 29. 30. 31. 32. 33. 34. Jacobs, C., et al., Cell cycle-dependent polar localization of an essential bacterial histidine kinase that controls DNA replicationand cell division. Cell, 1999. 97(1): p. 111-20. Wu, J., N. Ohta, and A. Newton, An essential, multicomponent signal transductionpathway requiredfor cell cycle regulationin Caulobacter.Proc Natl Acad Sci U S A, 1998. 95(4): p. 1443-8. Laub, M.T., et al., Genes directly controlledby CtrA, a master regulatorof the Caulobactercell cycle. Proc Natl Acad Sci U S A, 2002. 99(7): p. 4632-7. Llewellyn, M., et al., The conservedflaF gene has a critical role in coupling flagellin translationandassembly in Caulobactercrescentus. Mol Microbiol, 2005. 57(4): p. 1127-42. Milhausen, M. and N. Agabian, CaulobacterflagellinmRNA segregates asymmetrically at cell division. Nature, 1983. 302(5909): p. 630-2. Srinivasan, B.S., et al., eds. Integratedproteininteraction networksfor 11 microbes. Research in Computational Molecular Biology.Lecture Notes in Computer Science, ed. G.C. Apostiloco A, Istrail S, Pevzner P, Waterman M. Vol. 3909. 2006, Springer: New York, NY. Mullin, D.A., et al., Organization,expression, andfunctionof Caulobacter crescentus genes neededfor assembly andfunction of theflagellarhook. Mol Genet Genomics, 2001. 265(3): p. 445-54. Chen, L.S., D. Mullin, and A. Newton, 1dentification, nucleotide sequence, and control of developmentally regulatedpromoters in the hook operon region of Caulobactercrescentus. Proc Natl Acad Sci U S A, 1986. 83(9): p. 2860-4. Jacobs, C., et al., Functions of the CckA histidinekinase in Caulobactercell cycle control. Mol Microbiol, 2003. 47(5): p. 1279-90. Skerker, J.M. and L. Shapiro, Identification and cell cycle control of a novel pilus system in Caulobactercrescentus. EMBO J, 2000. 19(13): p. 3223-34. Laub, M.T., et al., Global analysis of the genetic network controllinga bacterial cell cycle. Science, 2000. 290(5499): p. 2144-8. Spencer, W., et al., CtrA, a global response regulator,uses a distinct second category of weak DNA bindingsitesfor cell cycle transcriptioncontrol in Caulobactercrescentus. J Bacteriol, 2009. 191(17): p. 5458-70. Zweiger, G., G. Marczynski, and L. Shapiro, A CaulobacterDNA methyltransferase thatfunctions only in the predivisionalcell. J Mol Biol, 1994. 235(2): p. 472-85. Martin, M.E., M.J. Trimble, and Y.V. Brun, Cell cycle-dependent abundance, stability and localization of FtsA andFtsQ in Caulobactercrescentus. Mol Microbiol, 2004. 54(1): p. 60-74. Jenal, U. and T. Fuchs, An essentialprotease involved in bacterialcell-cycle control. EMBO J, 1998. 17(19): p. 5658-69. McGrath, P.T., et al., A dynamically localizedproteasecomplex and a polar specificityfactor control a cell cycle master regulator.Cell, 2006. 124(3): p. 53547. Biondi, E.G., et al., Regulation of the bacterialcell cycle by an integratedgenetic circuit. Nature, 2006. 444(7121): p. 899-904. 35. 36. 37. 38. 39. 40. 41. 42. 43. 44. 45. 46. 47. 48. Chen, Y.E., et al., Dynamics of two Phosphorelays controllingcell cycle progressionin Caulobactercrescentus. J Bacteriol, 2009. 191(24): p. 7417-29. Kato, A. and E.A. Groisman, Connectingtwo-component regulatory systems by a protein thatprotects a response regulatorfrom dephosphorylationby its cognate sensor. Genes Dev, 2004. 18(18): p. 2302-13. Solomon, J.M., B.A. Lazazzera, and A.D. Grossman, Purificationand characterizationof an extracellularpeptidefactor that affects two different developmentalpathways in Bacillus subtilis. Genes Dev, 1996. 10(16): p. 201424. Ogura, M., et al., Binding of response regulatorDegU to the aprEpromoter is inhibitedby RapG, which is counteractedby extracellularPhrG in Bacillus subtilis. Mol Microbiol, 2003. 49(6): p. 1685-97. Ansaldi, M., L. Thdraulaz, and V. Mdjean, Tor], a response regulatorinhibitor of phage origin in Escherichiacoli. Proc Natl Acad Sci USA, 2004. 101(25): p. 9423-8. Panis, G., V. Mejean, and M. Ansaldi, Control and regulation of KplEI prophage site-specific recombination:a new recombinationmodule analyzed. J Biol Chem, 2007. 282(30): p. 21798-809. Nakano, S., et al., A regulatoryprotein that interferes with activator-stimulated transcriptionin bacteria.Proc Natl Acad Sci U S A, 2003. 100(7): p. 4233-8. Morgan, D.O., The Cell Cycle: Principlesof Control2007, London, UK: New Science Press Ltd. Sherr, C.J. and J.M. Roberts, CDK inhibitors:positive and negative regulatorsof G1-phaseprogression.Genes Dev, 1999. 13(12): p. 1501-12. Skerker, J.M., et al., Two-component signal transductionpathways regulating growth and cell cycle progression in a bacterium:a system-level analysis. PLoS Biol, 2005. 3(10): p. e334. Charity, J.C., et al., Twin RNA polymerase-associatedproteins control virulence gene expression in Francisellatularensis.PLoS pathogens, 2007. 3(17571921): p. e84. James, P., J. Halladay, and E.A. Craig, Genomic librariesand a host strain designedfor highly efficient two-hybrid selection in yeast. Genetics, 1996. 144(4): p. 1425-36. Radhakrishnan, S.K., M. Thanbichler, and P.H. Viollier, The dynamic interplay between a cellfate determinantand a lysozyme homolog drives the asymmetric division cycle of Caulobactercrescentus. Genes Dev, 2008. 22(2): p. 212-25. Chen, Y.E., et al., Dynamics of Two Phosphorelays ControllingCell Cycle Progressionin Caulobactercrescentus. Journal of Bacteriology, 2009. 191(24): p. 7417-7429. Chapter 3 The Caulobacter cell cycle regulator SciP is proteolyzed at the G1-to-S transition by Lon This work is a manuscript in progress by Kasia G Gora, Matt Wohlever, Barrett S Perchuk, and Michael T Laub. K.G.G and M.T.L designed the experiments, analyzed data, and wrote the manuscript. K.G. performed all the experiments with the exception of in vitro degradtion performed by M.W and microarrays perfomed by B.S.P. Introduction In the previous chapter I described the identification and characterization of SciP, a new cell cycle regulator in Caulobacter crescentus. I demonstrated that SciP prevents the activation of CtrA-dependent genes through a direct interaction with CtrA. SciP accumulates to maximal levels in G1-phased swarmer cells, is rapidly eliminated during the Gl-S transition, and then begins accumulating again shortly before cell division. The rapid elimination of SciP during the G1-S transition is reminiscent of the rapid degradation of CtrA by the protease ClpXP during the same cell cycle [1] In this chapter I examine the regulation of SciP and demonstrate that (1) cell cycle changes in SciP abundance involve regulated proteolysis by Lon and (2) an inability to degrade SciP disrupts normal cell cycle progression. Lon in an AAA+ protease whose homologs are widely distributed in both eukaryotic and prokaryotic organisms [2]. Like other bacterial AAA+ proteases, Lon is functional as a hexamer but unlike ClpXP, Lon consists of an ATPase regulatory domain fused to a proteolytic domain [3]. In bacteria, Lon important for the proteolysis of unfolded proteins through the recognition exposed hydrophobic residues in unfolded structures in addition to other native key regulators [4]. In Caulobacter Lon is a non-essential protein and has been shown to degrade the cell cycle methyltransferase CcrM in late predivisional cells [5]. Here we demonstrate that Lon also plays a key role in the degradation of SciP specifically at the G 1-to-S transition. Results SciP is regulated primarily at a post-transcriptional level Consistent with its proposed role as a swarmer-specific inhibitor of CtrA-activated gene expression, SciP abundance is maximal in swarmer cells and then drops rapidly during the G1-to-S transition [6]. This pattern suggests that cells may tightly regulate SciP levels. The transcription of sciP depends on CtrA and is consequently strongly cell cycleregulated, peaking in predivisional cells [6, 7]. To test whether regulated transcription contributes to the cell cycle changes in SciP, we measured SciP abundance in a strain harboring a deletion of the native sciP and expressing sciP from the chromosomal xylX locus, i.e. the sciP depletion strain [6]. Growth in the presence of xylose leads to constitutive expression from the PsY promoter [8]. Synchronized swarmer cells from this strain were isolated and released into rich media containing xylose or glucose. Consistent with previous results, SciP levels were high in swarmer cells, but decreased approximately 30 minutes post-synchronization, despite the constitutive expression of sciP (Fig. 3.1) [6]. Immunoblotting for CtrA in the same samples indicated that the disappearance of SciP was coincident with the proteolysis of CtrA (Fig. 3.1). These results suggested that changes in SciP abundance may be driven by proteolysis during the G1-to-S transition in addition to changes in transcription. minutes post 0 15 30 45 60 76 90 105 synchrony y 4 CtrA SciP PxyrsciP OAW CtrA SSciP Figure 3.1 Post-transcriptional regulation of SciP. Cell cycle abundance of SciP under constitutive expression. The sciP depletion strain was grown in PYE plus xylose, synchronized, and released into PYE plus xylose or glucose as indicated. Samples were taken at 15-minute intervals for Western blot analysis with CtrA and SciP antisera. SciP is an unstable protein, but is not degraded by CIpXP, CIpAP, or HslUV To measure the stability of SciP we used pulse-chase analysis on wild-type cells expressing sciP from a low-copy plasmid and grown in a minimal medium, M2G. Under these conditions in which the cell cycle is -140 minutes, SciP had a half-life of only -23 minutes indicating it is a relatively unstable protein (Fig. 3.2A). We therefore sought to identify the protease(s) responsible for SciP degradation. Because SciP normally disappears during the Gi -to-S transition, concurrent with the proteolysis of CtrA, we first investigated ClpXP, the primary CtrA protease [1]. As both ClpP and ClpX are essential proteins, we assayed SciP levels in cells depleted of ClpP [1]. Strain UJ199 harbors a deletion of the native, chromosomal clpP gene with a single copy of clpP under control of the P,,/ promoter at the xylX locus [1]. This strain was grown in rich medium containing glucose for four generations (-6 hours) to deplete ClpP. We then isolated swarmer cells, released them into glucose or xylose to repress or induce, respectively, c/pP expression, and sampled cells every 30 minutes for immunoblot analysis (Fig. 3.2B). As expected, cells released into glucose failed to proteolyze CtrA, whereas cells released into xylose eliminated CtrA after -60 minutes. In contrast to CtrA, the same samples, from both xylose and glucose, showed a nearly complete elimination of SciP by the 60-minute time point. These data indicate that cells lacking ClpP can still degrade SciP during the Gl-to-S transition. Because ClpP is the peptidase subunit for both ClpXP and the protease ClpAP, these data argue against the involvement of either protease in SciP proteolysis. To confirm these results, we also monitored SciP abundance in synchronized Ac/pA and AcpdR strains [9, 10] ClpA is the ATPase subunit of ClpAP and is not essential for viability [10]. CpdR is a non-essential protein that is required for the ClpXP-dependent degradation of CtrA at the G1-to-S transition [9]. In AclpA cells, SciP was eliminated during the GI-to-S transition, as in wild-type cells (Fig 3.2C). In AcpdR cells, SciP levels dropped significantly in the middle of the cell cycle though not with the same timing as in wild-type cells (Fig 3.2C). This delay may result from the fact that AcpdR has a longer cell cycle at least in part because these cells fail to clear CtrA at the Gl-to-S transition [9]. Alternatively, ClpXP may make a minor contribution to SciP proteolysis that was not detected in our analysis of the ClpP depletion strain. We also tested the role of HslUV, another ATP-dependent protease [11]. We synchronized a strain harboring a transposon within the hslV coding region, hslV::Tn5, and monitored SciP levels by Western Blot. However, SciP was still eliminated during the Gi -to-S transition, indicating that HslUV does not degrade SciP (Fig 3.2D). 0 100%1 5 ScIP In Alon minutes post synchrony 0 30 60 90 120 *ScP-M2 1 10% CtrA * M2-SciP ASCIP Pxyr.CIpP 4 CtrA 0% 0 30 60 Time Post Pulse 90 SciP - C. D. minutes post synchrony 0 30 60 90 120 minutes post synchrony SclP AcpdR 0 30 90 120 SclP hs+n *%gyp 4 AcipA[egas 60 4 CtrA SciP E. minutes post synchrony 0 30 60 1.-14 90 120 SciP Alanr11 1 CtrA Figure 3.2 SciP stability in various protease mutant strains. (A) Pulse-chase analysis of SciP stability in the strains indicated. Wild type or Dion cells harboring the indicated allele of SciP on a low copy plasmid grown in M2G and pre-induced with xylose for 30 minutes before the pulse chase. Each experiment was performed in duplicate or triplicate and error bars indicated standard error of the mean. (B) The c/pP depletion strain was grown in PYE plus xylose and switched to PYE plus glucose to deplete SciP for 6 hrs. Cells were synchronized and released into PYE plus glucose or xylose as indicated and samples were taken at 15-minute intervals for Western blot analysis with CtrA and SciP antisera. The Ac/pA, AcpdR (C), hsIV::tn5 (D), Alon strains (E) were grown in PYE, synchronized, and analyzed as in (B). SciP is degraded by Lon We also tested SciP proteolysis in cells harboring a disruption of the gene encoding the Lon protease [5]. We synchronized Alon cells and monitored both SciP and CtrA levels during cell cycle progression. Whereas CtrA disappeared, as expected, at the 30 minute time point, SciP was still present after 30 minutes and was maintained at significant levels throughout the cell cycle (Fig. 3.2E) We also measured SciP stability in a mixed population of Alon cells using pulse-chase analysis. We found that wild-type SciP protein had a half-life of -230 minutes in the Alon mutant compared to just 23 minutes in the wild type (Fig. 3.2A) Taken together, these data strongly suggest that SciP is degraded by Lon. Next we tested the ability of purified E. coli Lon to proteolyze SciP in vitro. We incubated 10 [M SciP with 500 nM E. coli Lon hexamer and observed rapid and almost complete degradation within 30 minutes at 30 C (Fig. 3.3). Given the high degree of sequence similarity between E. coli and Caulobacter Lon (62% identical), these data further support the conclusion that SciP is a direct substrate for Lon in Caulobacter. reaction time (min) 0 10 20 30 all-e +Lon *-Pyruvate kinase 4-SciP Degradation Products Figure 3.3 In vitro proteolysis of SciP by E. col Lon. 10 [tM SciP was incubated with 500 nM Lon hexamers from E. coli and degraded for 30 min at 30C. Samples were taken every 10 min for SDS-PAGE gel analysis. Pyruvate kinase was added to generate ATP from phosphoenolpyruvate present in the reaction. Addition of a C-terminal tag interferes with SciPproteolysis To test the importance of regulated SciP degradation to cell cycle progression, we sought to create a proteolytically stable version of SciP. Because proteases often rely on N- or Cterminal sequences for substrate recognition, we tested the effects of placing an M2 epitope at the N- or C-terminus of SciP. We transformed wild-type cells with low copy plasmids expressing sciP, M2-sciP, or sciP-M2 under the control of Pxyl. Mixed populations of cells were grown in rich media supplemented with glucose to repress the plasmid-borne copy of sciP, synchronized, and released into medium supplemented with xylose to induce sciP, M2-sciP, or sciP-M2 expression. Samples were collected every 15 minutes for immunoblot analysis (Fig. 3.4). Note that we did not induce expression of the plasmid-borne copies of sciP prior to synchronization as such pre-induction significantly decreased the yield of swarmer cells for the sciP-M2 strain during synchronization. 0 minutes post synchrony PYrsciP 15 30 45 60 75 90 105 120 j SciP 4 PxyrM2 -sciP _________________________4 PxyrsciP-M2_4 M2-SciP SciP ScIP-M2 Figure 3.4 Stabilization of SciP through an C-terminal M2 tag. Wild-type cells harboring a low copy plasmid with the indicated sciP allele were grown in PYE plus glucose, synchronized, and released into PYE plus xylose to induce sciP expression at time 0. Samples were taken every 15 minutes for Western blot analysis with SciP antisera. For cells synthesizing untagged SciP, we found that SciP was high in swarmer cells, almost completely eliminated after ~30-45 minutes, and then accumulated again during later stages of the cell cycle (Fig. 3.4), a pattern similar to wild-type and cells in which the only copy of sciP is expressed from the chromosomal xylX locus (see Fig. 3.1). By contrast, for cells expressing M2-sciP, we found that M2-SciP protein accumulated during the early stages of the cell cycle as significantly higher levels of M2-SciP were seen at the 30 and 45 minute time points compared to untagged SciP (Fig. 3.4). An even more pronounced effect was seen in the cells expressing sciP-M2, with high levels of SciP-M2 detectable throughout the time course examined (Fig. 3.4). Consistently, the SciP-M2 that accumulated blocked expression of the chromosomally-encoded sciP, a CtrA-activated gene, during late stages of the cell cycle; as the native SciP is slightly smaller than SciP-M2 the two forms were distinguishable on immunoblots using SciPspecific antibodies (Fig. 3.4). These results suggest that the C-terminus, and to a lesser extent the N-terminus, of SciP is important for the degradation of SciP. The presence of a C-terminal M2 epitope likely stabilizes SciP by interfering with its proteolysis. We also assayed the stability of M2-SciP and SciP-M2 using pulse-chase analyses of mixed populations of cells grown in minimal medium. In cells producing M2-SciP or SciP-M2, we measured a protein half-life of 36 and 58 minutes, respectively, compared to 23 minutes for the untagged protein (fig. 3.2A). These results further indicate that adding a C-terminal tag to SciP interferes with its degradation. Proteolysis of SciP is necessary for proper cell cycle progression To investigate the phenotypic consequences of producing stable SciP, we expressed sciPM2 from a low-copy plasmid under the control of a xylose-inducible promoter in otherwise wild-type cells. This strain was grown in rich medium supplemented with glucose and then shifted to medium containing xylose, leading to a significant growth defect (Fig. 3.5A). After four hours, cells were also moderately filamentous and flow cytometry analysis indicated that cells had accumulated up to four chromosomes per cell, consistent with a defect in cell division (Fig. 3.5B). In contrast to sciP-M2, cells expressing untagged sciP or M2-sciP from the same low-copy plasmid exhibited no significant defects in growth, morphology, or chromosome content (data not shown). The phenotypic consequences of producing SciP-M2 from a low-copy plasmid are reminiscent of those previously reported for cells producing M2-SciP from the same xylose-inducible promoter on a high-copy plasmid. 100 PxytsciP-M2 1pxyj scIP-M2 .8 glucose 0 -*-glucose -O-xylose 1.4- 1.2 0 0 90 180 270 360 45( lime (minutes) 1 2 3 4 5 6 70 15 45 1 2 3 4 5 6 7 8 1J A ReF ICtri mprud goe* minutes post synchrony 0 F 30 60 a I 75 90 105 120 4 ScIP- M2 *~ ~ +CtrA PxytsciP-M2 CtrA-sctvh1 gal- -1 0.5 0 0.5 4 SciP L ctrA 1 Figure 3.5 Phenotypic consequences of stable SciP. (A) Growth curve of wildtype harboring PxyrsciP-M2 on a low copy plasmid grown in PYE supplemented with glucose or xylose. (B) Morphology (top) and FACS analysis (bottom) of PxyrsciP-M2 induction for 4 hrs. (C) Microarray analysis of CtrA regulon in cells expressing SciP-M2 for 4 hrs vs. wildtype grown in similar conditions. Changes in gene expression are compared to previously published data sets in reference [6]. (D) CtrA dynamics in synchronized wild type cells harboring PxyrsciP-M2 grown in PYE plus glucose, synchronized, and released into PYE plus glucose or xylose as indicated. Samples were taken every 15 minutes for Western blot analysis with SciP or CtrA antisera. To further compare the phenotypes that result from synthesizing the non-degradable SciP-M2 from a low-copy plasmid or M2-SciP from a high-copy plasmid, we examined changes in gene expression in these strains using DNA microarrays. Wild-type cells harboring the low-copy sciP-M2 expression plasmid were induced with xylose for 4 hours; mRNA was isolated from these cells and compared to mRNA from identically 101 treated wild-type cells using whole genome microarrays (Fig. 3.5C). We then directly compared the results to those obtained for cells expressing sciP from a high-copy plasmid and to data for ctrA's and cckA" strains grown at a restrictive temperature [6]. The lowcopy expression of sciP-M2 gave rise to an -3-fold increase in sciP mRNA relative to wild-type cells while high-copy expression of sciP resulted in an -18-fold increase. Despite the lower induction of sciP-M2, the consequent down-regulation of CtrA target genes was very similar to that seen in cells expressing M2-sciP from a high-copy plasmid (Fig. 3.5C). In both cases, CtrA-repressed genes were generally unaffected. To determine whether the down regulation of CtrA-activated genes was due simply to changes in CtrA levels, we measured CtrA abundance in synchronized cells expressing sciP-M2 from a low copy plasmid. Cells were grown initially in the presence of glucose, synchronized, and then released into media containing xylose or glucose, and sampled every 15 minutes for immunoblot analysis. In both conditions CtrA levels fluctuated as in wild type cells, indicating that the accumulation of SciP-M2 likely down regulates CtrAtarget genes through a direct interaction with CtrA at target promoters (Fig 3.5D). Taken all together, our results suggest that SciP is normally proteolyzed at the Gl-S transition and that the failure to properly degrade SciP prevents the activation of CtrA-dependent genes in predivisional cells, thereby disrupting cell cycle progression. 102 Discussion Post-translational Regulation ofSciP In our initial report identifying SciP, we observed the importance of its tight regulation for proper cell-cycle progression. In wild-type cells SciP protein is present predominantly in swarmer cells and either its ectopic expression or depletion results in a dysregulation of the cell cycle. Consistent with the tight temporal regulation of SciP, previous reports have found that the sciP mRNA is strongly cell-cycle regulated [7, 12]. In this study, I demonstrated that SciP is also subject to post-transcriptional regulation. Specifically, SciP protein is proteolyzed at the G 1-S transition by the Lon protease. Why proteolyze SciP at the G 1-S transition? A priori, one might expect that fluctuations in sciP mRNA levels are sufficient to restrict SciP to swarmer cells. Several studies, including ours, have shown that sciP mRNA is present in swarmer cells, disappears during the Gl-S transition, and is transcribed again in predivisional cells following the synthesis of CtrA [6, 7, 12]. However, we observed that fluctuations in SciP levels still occur when sciP is constitutively expressed from a chromosomal locus. As with CtrA, proteolysis may allow cells to quickly and irreversibly clear SciP from cells ensuring a robust transition from the GI/swarmer state to the S/stalked state. Regulated Proteolysis by Lon The Lon protease is an AAA+ protein that is widely conserved throughout the bacterial kingdom. CaulobacterLon is highly similar to the E. coli Lon protein that has been well characterized experimentally. In E. coli, Lon is responsible for degrading unfolded 103 proteins as well as several key regulators like the transcription factor SoxS and the division inhibitor SulA [2, 13, 14]. The rules for Lon substrate specificity are variable but Lon is thought to recognize hydrophobic patches in unfolded proteins and the N- or Ctermini of the substrates SoxS and SulA, respectively [4, 15, 16]. In Caulobacter,Lon is known to degrade the cell-cycle methyltransferase CcrM [5]. The Lon deletion strain exhibits moderate filamentation and accumulation of up to six chromosomes per cell which has been attributed to a failure of degradation of CcrM [5]. However the Alon phenotype is also similar to the phenotypic consequences of expressing non-degradable sciP-M2 in wild type cells suggesting that failure to clear SciP at the G 1-to-S transition may also contribute to the pleotropic effects of a Ion mutant. In this study, we observed that SciP is proteolyzed specifically at the Gl-to-S transition and is only allowed to accumulate in early predivisional cells when constitutively expressed. Previous reports suggested that Lon protease is preferentially synthesized at the stalked pole of predivisional cells and that stalked cells have approximately twice as much Lon as swarmer cells immediately after cell division [5, 17]. Perhaps newly differentiated stalked cells are also slightly enriched in Lon to provide a boost to the proteolysis of SciP during the Gl -S transition. Alternatively some as of yet unidentified adapter protein may mediate SciP degradation at the Gl-to-S. In addition, it is possible that differential localization of Lon during the cell cycle regulates its activity similar to what has been reported for ClpXP [9]. While SciP has no obvious Lon degrons, we found that SciP is stabilized by the addition of a C-terminal M2 epitope (DYKDDDDK). One possibility is that some of the many 104 positively charged aspartic acid residues of the M2 epitope may form very stable interactions with several negatively charged arginine residues at the C-terminus of wildtype SciP (RTTRIQQYR). Such a secondary structure could obstruct normal substrate recognition by Lon by forming a secondary structure at a normally floppy C-terminal end or by preventing the C-terminal from interacting with Lon. Concluding Remarks Our analysis of the post-transcriptional regulation of SciP provides another key example of the importance of regulated proteolysis in bacterial cell cycle control. As with other important regulatory proteins such as CtrA, and CcrM, Caulobacter has evolved redundant mechanisms for ensuring their correct expression during the cell cycle [1, 5]. Bacterial cells exert transcriptional control in addition to other post-transcriptional regulation such as phosphorylation, sub-cellular localization, and proteolysis to ensure robust, stable progression through the cell cycle. 105 Experimental procedures Bacterial strains and media E. coli and Caulobacter strains were grown as previously described [18]. PYE was supplemented with 0.2% glucose (PYEG) and 0.3% xylose (PYEX) as indicated. MG2 was supplement with 0.3% xylose as indicated. ML1750 was grown in PYEX, synchronized, and released into PYEG or PYEX. KG91, KG313, KG314 were grown in PYEG supplemented with oxytetracyline, synchronized, and released into PYEG supplemented with oxytetracyline or PYEX with oxytetracyline. UJ838, UJ199, LS2382, AcpdR, and hslV: :tn5 were grown in PYE. UJ199 was depleted and synchronized as previously reported [1]. All synchronies were preformed in a 50% Percoll gradient in 50 mL tubes (Falcon). Cloning and mutagenesis The plasmid pENTR-Pxyl-sciP was constructed by TOPO cloning a translational fusion of sciP to the Pxyl promoter. The translational fusion was made by S.O.E. PCR as previously described [18]. The Pxyi promoter was amplified from pHXM-DEST using the primers Pxyl F and Pxyl R. SciP was amplified from genomic DNA using the primers sciP F and sciP R. The plasmid pENTR-Pxyl-sciPM2 was constructed by TOPO cloning a PCR fragment amplified from pLX-sciP using the primer Pxyl F and sciP M2 R. The low copy plasmids with PxI-sciP (pLX-sciP) and Pxyi-sciP-M2 (pLX-sciPM2) were constructed by LR recombination of pENTR-Pxyl-sciP and pENTR-Pxyl-sciPM2 into the destination vector pMR20-DEST respectively as previously described [18]. The low copy 106 plasmid with Pxy-M2-SciP (pLXM-sciP) was constructed by LR recombination of pENTR-sciP into pLXM-DEST. The expression plasmids were electroporated into CB 15N and Alon electrocompetent cells as indicated. The pBAD-lon plasmid was constructed by digesting pBAD33-lon with NdeI and Sbft and ligating in a PCR product amplified from CB15N genomic DNA using the primers lon F and R and digested with NdeI and SbfL. Immunoblots Cell pellets were resuspended in the equivalent of 50 pL 1 X SDS sample buffer per 1 mL of OD 0.15 culture. 10 ptL of lysate was resolved on a 12% gel (Bio-Rad) run at 130 V for 1 hr at room temperature. Samples were transferred to PVDF membrane and probed as previously described [6]. Flow cytometry Samples were analyzed as previously described [19]. Pulse-chase SciP stability was assayed as previously described with the following modifications [6]. Expression of various sciP alleles in KG91, KG313, KG314, and KG336 expression was pre-induced for 30 minutes immediately before the pulse-chase. Samples were immunoprecipitated with 2 RL of SciP anti-sera for 2 hrs at 4 C and immune complexes were collected for 1 hr at 4 C. 107 DNA microarrayanalysis Microarrays were conducted as previously reported on Agilent arrays [20]. Experiments were run in duplicate and the average results are reported. Protein purifications CtrA, SciP, and Lon were purified as previously described [4, 6]. In vitro degradation In vitro degradation assays were run in 25 mM Tris pH 8.0, 10 mM MgC12, 100 mM KCl buffer with 5 mM DTT, 20 mM phosphoenolpyruvate, pyruvate kinase (0.01 units/pL), 10 iM SciP, and 500 nM E. coli Lon (hexamer) at 30 C. 10 RL aliquots were collected at indicated time points and quenched in 4x SDS PAGE loading buffer. Reaction products were separated on 4-20% gradient gel (Biorad). 108 Table S1 - Strains and Plasmids Strain or Organism or Plasmid Plasmid Category Strain C.crescentus E coli Plasmid destination vectors entry vectors expression vectors Strain or PlasmidName Descprition Synchronizable derivativeof wild-type CB15 CB15N AsciPxylX::sciP (tetRkanR) CB15N pLX-scP (tet", chlor") (tot", chlor") CB15N pLXM-scIP CB15N pLX-sciPM2 (tet". chlor*) CB15N Alon pLX-sc/P (tetR,chor,spec") CB15N AcipA(spec") CB15N AcIpP xylX::cfpP (spec", tet") CB15N &cpdR (tet") CB15N hs/1Vtn5 (kan") 5 CB15N Alon (spec ) LS2382 General cloning strain DH5a of destination vectors One Shot ccdB Survival TV Strain for propagation clones General cloning strainfor pENTRID-TOPO TOP1O of transgenic Lon Alonstrain for purification ER2566 0 (tete,chlor ) pMR20, tow-copy pMR20-DEST pMR20-M2; low-copy, Pxyl,N' M2tag (tetRchiorR) pLXM-DEST Pxyl,N' M2 tag (spec", chlor") pJS71X-M2;high-copy, pHXM-DEST ENTRY vector forGateway cloning system (kan") pENTR/D-TOPO (kanR) CC0903in pENTR/D-TOPO pENTR-sciP (kan") In pENTRID-TOPO Pxyl fused to CCO903 pENTR-Pxyi-sc/P (kanR) Pxyl fused to CC0903with M2 epitope a C' in pENTR/D-TOPO pENTR-Pxyl-sciPM2 pMR20-X-sc/P (tet", chlor") pLX-scIP pMR20-X-M2-sciP (tet", chlor") pLXM-sciP pMR20-X-sc/P-M2 (tet", chior") pLX-sclPM2 wild-type ctrA promoterincludingP1and P2 fused to lacZ pctrA290 pctrA290 with muatedP1 (tot") pctrA-P1 pctrA290 with mutatedP2 (tet") pctrA-P2 pctrA290 with muated-39 to -48 putative SciPbinding site (tet") pctrA-bs1 pctrA290 with musted-70 to -79 putatitve SciPbinding site (let") pctrA-bs2 pctrA290 with both-39 to -48 and -70 to -79sites mutated (tet") pCtrA-bsl +2 pBAD33-ion with Caulobacter tonreplacing E. colt on (chlor") pBAD-lon Ionexpression plasmid(chlor") pBDA33-lon CB15N ML1750 KG314 KG91 KG313 KG336 UJ838 UJ199 Source or Reference Evinger and Agabian, 1977 Gora et al, 2010 this study this study this study this study Grunenfelder et al, 2004 Jenal et al, 1998 Skerker et al, 2005 Josh Modell, unpublished Wright et al, 1996 invitrogen Novagen invitrogen New England Biolabs Chen et al, 2009 Skerker st al, 2005 Skerker et al, 2005 Invitrogen Gora et a, 2010 this study this study this study this study this study Domian et aL.1999 Domian et al. 1999 Domian et al. 1999 this study this study this study this study Christensen st al. 2004 Table 3.1. Strains. TableS2 - Primers PusionPCR Pxyl sciP sciPM2 Gel Shift Ion Forward primer CACCGGTACCTCGAACAGGGCCGTCAGGTCGCG AGTTTGGGGAGACGACCATATGTTGCAGCAGCAGCGCAC TTTCATATGTCCGAACTACGTACGCTTCCTG Reverseprimer ATGGTCGTCTCCCCAAAACT AGTTTTGGGGAGACGACCATATGTTGCAGCAGCAGCGCAC TTACTTGTCGTCGTCGTCCTTGTAGTCACGGTACTGCTGGATCCGGG TTCCTGCAGGTrAGTGCGTCAGCAT Table 3.2. Primers. 109 Acknowledgements We thank J. Modell for his generous gift of the hslV::tn5 strain and U. Jenal for providing published strains. M.T.L. is an Early Career Scientist at the Howard Hughes Medical Institute. This work was supported by an NIH grant (5R01GM082899) to M.T.L. 110 References 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. 12. 13. 14. 15. Jenal, U. and T. Fuchs, An essentialprotease involved in bacterialcell-cycle control. The EMBO journal, 1998. 17(19): p. 5658-69. Melderen, L.V. and A. Aertsen, Regulation and quality control by Lon-dependent proteolysis. Research in Microbiologoy. 160(9): p. 645-651. Sauer, R.T. and T.A. Baker, AAA + Proteases:A TP-fueled Machines of Protein Destruction. Annual Review of Biochemistry, 2010. 80(1): p. 587-612. Gur, E. and R.T. Sauer, Recognition of misfoldedproteins by Lon, a AAA(+) protease. Genes & Development, 2008. 22(16): p. 2267-2277. Wright, R., et al., CaulobacterLon protease has a critical role in cell-cycle control ofDNA methylation. Genes & Development, 1996. 10(12): p. 1532-42. Gora, K.G., et al., A cell-type-specific protein-proteininteractionmodulates transcriptionalactivity of a master regulatorin Caulobactercrescentus. Molecular Cell, 2010. 39(3): p. 455-67. Tan, M.H., et al., An essential transcriptionfactor, SciP, enhances robustness of Caulobactercell cycle regulation.Proceedings of the National Academy of Sciences of the United States of America, 2010. 107(44): p. 18985-90. Meisenzahl, A.C., L. Shapiro, and U. Jenal, Isolation and characterizationof a xylose-dependentpromoterfrom Caulobactercrescentus. Journal of Bacteriology, 1997. 179(3): p. 592-600. Iniesta, A.A., et al., A phospho-signalingpathway controls the localizationand activity of a protease complex criticalfor bacterialcell cycle progression. Proceedings of the National Academy of Sciences of the United States of America, 2006. 103(29): p. 10935-40. Grunenfelder, B., et al., Identification of the Protease and the Turnover Signal Responsiblefor Cell Cycle-Dependent Degradationof the CaulobacterFliF Motor Protein. Journal of bacteriology, 2004. 186(15): p. 4960-4971. Rohrwild, M., et al., HslV-HslU: A novel A TP-dependentproteasecomplex in Escherichiacoli relatedto the eukaryoticproteasome. Proceedings of the National Academy of Sciences of the United States of America, 1996. 93(12): p. 5808-13. Chen, L.S., D. Mullin, and A. Newton, Identification,nucleotide sequence, and control of developmentally regulatedpromoters in the hook operon region of Caulobactercrescentus.Proceedings of the National Academy of Sciences of the United States of America, 1986. 83(9): p. 2860-4. Mizusawa, S. and S. Gottesman, Proteindegradationin Escherichiacoli- The Lon gene controls the stability of SulA protein. Proceedings of the National Academy of Sciences of the United States of America-Biological Sciences, 1983. 80(2): p. 358-362. Griffith, K.L., I.M. Shah, and R.E. Wolf, Proteolytic degradationofEscherichia coli transcriptionactivators SoxS and MarA as the mechanismfor reversing the induction of the superoxide (SoxRS) and multiple antibiotic resistance (Mar) regulons. Molecular Microbiology, 2004. 51(6): p. 1801-1816. Shah, I.M. and R.E. Wolf, Sequence requirementsfor Lon-dependent degradation of the Escherichiacoli transcriptionactivator SoxS: Identification of the SoxS residues criticalto proteolysis and specific inhibition of in vitro degradation by a 111 16. 17. 18. 19. 20. peptide comprisedof the N-terminal 21 amino acid residues. Journal of Molecular Biology, 2006. 357(3): p. 718-73 1. Ishii, Y., et al., Regulatory role of C-terminal residues of SulA in its degradation by Lon protease in Escherichiacoli. Journal of Biochemistry, 2000. 127(5): p. 837-844. Reuter, S.H. and L. Shapiro, Asymmetric segregationof heat-shockproteins upon cell-division in Caulobactercrescenuts. Journal of Molecular Biology, 1987. 194(4): p. 653-662. Skerker, J.M., et al., Two-Component Signal TransductionPathways Regulating Growth and Cell Cycle Progressionin a Bacterium:A System-Level Analysis. PLoS Biology, 2005. 3(10): p. e334. Chen, Y.E., et al., Dynamics of Two Phosphorelays Controlling Cell Cycle Progressionin Caulobactercrescentus. Journal of Bacteriology, 2009. 191(24): p. 7417-7429. Laub, M.T., et al., Global analysis of the genetic network controllinga bacterial cell cycle. Science, 2000. 290(5499): p. 2144-8. 112 Chaper 4 Conclusions and Future Work 112 In this thesis I have presented work that furthers our understanding of the complex regulation of CtrA, a master regulator of the Caulobacter cell cycle. In Chapter 2, I described the identification of SciP, a swarmer-specific inhibitor of CtrA transcriptional activity and showed that SciP binds CtrA at CtrA-dependent promoters and likely prevents CtrA from recruiting RNA polymerase to these promoters. I showed that SciP, although not essential, is critical for cell cycle progression and either the absence or overproduction of SciP caused severe cell cycle defects through misregulation of the CtrA regulon. In Chapter 3, I investigated the post-transcriptional regulation of SciP and showed that SciP is specifically proteolyzed at the Gl-S transition by Lon protease. I created a stable allele of sciP by epitope-tagging the C-terminus and demonstrated that a failure to clear SciP during the G 1-S transition disrupts cell cycle progression. The regulation of CtrA by SciP constitutes a novel mechanism for regulating the activity of a response regulator. It is the first protein shown to block transcriptional activation by a response regulator without affecting phosphorylation or DNA-binding. Like CtrA, SciP homologs are present throughout alpha-proteobacteria and are highly conserved, with orthologs typically exhibiting more than 75% amino acid identity. Given the coinheritance of SciP and CtrA, it seems likely that SciP is an important regulator of CtrA throughout the alpha-proteobacteria. However it remains to be seen whether these SciP orthologs do in fact regulate CtrA orthologs or if even small changes in the amino acid sequence allow them to perform different functions. 113 Furthercharacterization of the interaction between CtrA and SciP Although the biochemical experiments in chapter 2 support the idea that SciP and CtrA form a complex at CtrA activated promoters, several questions remain about the molecular nature of this interaction. Does SciP contact DNA and if so, how? In chapter 2, I used electrophoretic mobility assays (EMSA) to demonstrate that SciP alone cannot bind DNA, but that it does form a complex with DNA-bound CtrA. Our analyses further showed that complex formation requires free DNA adjacent to that bound by CtrA; 25 base pair probes that are likely completely covered by CtrA did not experience a super-shift upon addition of SciP, in contrast to 50 basepair probes. Our analyses did not uncover any sequence requirements for this putative SciP-DNA interaction suggesting that any interaction between SciP and DNA is non-specific. In contrast, a recent study suggested that SciP can bind DNA on its own and that it specifically binds the motif GTCGC [1]. However, the binding of SciP reported was weak and not concentration-dependent. Moreover, the motif GTCGC is unlikely to provide binding specificity within a genome that has nearly 70% GC content. Nevertheless, I tested the importance of this putative motif for SciP/CtrA/DNA complex formation by mutating the core nucleotides of the motif in a pilA promoter probe used in Chapter 2. We mutated TGTCGCG to TGTTTTG and tested the ability of CtrA and SciP to bind the mutant probe (Fig. 4.1A). As expected, purified CtrA~P alone bound both the wild-type and mutant probes, producing nearly identical shifts that correspond to a CtrADNA complex (Fig. 4. 1B). We then added purified SciP along with CtrA-P and observed 114 a similar pattern of super-shifting with both probes (Fig. 4.1 B). These results suggest the TGTCGG motif is not required for a SciP-CtrA-DNA complex to form on this probe. This conclusion is also consistent with our previous report that SciP and CtrA form a complex on a 50 base-pair probe taken from the fliF promoter that does not include the TGTCGG motif. To corroborate these results in vivo, we examined a Pet,.A-lacZ reporter; this construct contains both the P1 and P2 promoters of ctrA fused to lacZ and two instances of the TGTCGG motif (Fig. 4.1 C) [1, 2] We mutated each motif independently and in a double mutant to create PctrAbs1, PetrAbs2, and Pct,.Absl+2 respectively. The PetrAbs2 reporter exhibited activity comparable to the wild-type reporter suggesting that the distal TGTCGG motif is not important for the regulation of the ctrA promoter (Fig 4.1D). The PetrAbs] and PetrAbs1 +2 reporters both exhibited a 25% increase in activity relative to the wild-type reporter. As a control, we compared the differences in activity to two additional PetrA mutants, PctrA-P1 and PctrAP 2 that preserve only the P1 and P2 promoters of ctrA, respectively. In our hands, eliminating P1 (PctrA-P2) resulted in a -25% decrease in activity and eliminating P2 (PctA-PI) reduced activity by ~60% in general agreement with previously published data (Fig. 4, 1D) [2]. To test if mutating the TGTCGG motifs affects SciP-dependent regulation of ctrA we examined expression from PctrAbsl, PctrAbs2, and PctrAbsl+2 in cells overexpressing sciP. As shown in chapter 2, overproducing SciP downregulates ctrA expression. We induced expression of sciP from a high copy plasmid for 2 and 4 hrs and then measured promoter activity. We found that sciP overexpression reduced the activity of all three reporters to similar extents (Fig. 4. 1E). Therefore although one of the TGTCGG motifs has a modest 115 effect on ctrA expression, that effect does not depend on SciP raising the possibility that that motif may be important for the binding of a yet unknown regulator. A. be 5 0 ppp.A aca C. s' ACGCTGAAAA e-2L CACCCGCCTG CGACGCCTGC GACCAATGTG PpILAbs bel x XXX A TAT TTCAGGGCT CCGACGGC TTGCACCCGA TTCGCAAATC consensus A/C TGTCGCA AGMKC3 CTCClAC GGG 3' crA bei CGC crA b2 A/C consensus B. PpILA ScIP (1IpM) CtrA (pM) + 0 0.5 1 D. PpIlAbs + 0 0.5 1 + + 0 0.5 1 1.75 + 1.50 0 0.5 1 1.25 1 TGTCGCA 1.00 - - - - - 0.75 complexes 0.50 0.2 0.25 -P kesDNA E. P2 bsl bs2 bs1+2 Reporter Construct 1.00- o S0.75 21 Fiue0.50 wt bel bs2 bs1+2 Reporter Construct Figure 4.1. Dispensability of the putative SciP motif in vitro and in vivo. (A) Mutation of the putative SciP motif (arrow) in a PpiA probe. CtrA binding sites are boxed. The motif is compared to the reported consensus sequence below. (B) Gel shift analysis with wt PpiiA vs PpilAbs. CtrA-P was added at the indicated final concentration and SciP was at luM when present. (C) Mutation of two putative SciP motifs (arrows) in the PctrA-lacZ reporter with annotations as in A. Only a subset of the promoter fragment is shown. (D) Beta-galactosidase activity of PctrAbsl (bsl), PctrAbs2 (bs2), PctrAbsl+2 (bsl+1) relative to wt PctrA in CB15N shown in grey. The PctrAP1 and PctrAP2 mutants are shown for reference. (E) Activity of PctrA (wt), PctrAbsl (bsl), PctrAbs2 (bs2), PctrAbsl+2 (bsl+2) in CB15N harboring sciP on a high copy xylose inducible plasmid, induced for 2 hrs (dark grey) or 4 hrs (light grey) vs. uninduced wt. 116 Why does SciP require free DNA for complex formation? Recent homology searches suggest that SciP has a helix-turn-helix motif and an NMR structure of the SciP homolog from Rhodobacter sphaeroides allows us to model the three-dimensional structure of SciP [3]. Based on the R. sphaeroides structure, SciP consists of two beta sheets followed by four distinct alpha helices with unstructured N and C-terminal tails (Fig 4.2A, B). A recent study has speculated that SciP interacts with the major groove of DNA through the third alpha helix [1]. In this model the residues R35 and R40 are adjacent to the DNA backbone but are not within the canonical DNA recognition helix. This model is consistent with our mutational analysis in Chapter 2 in which the mutations SciP(R35A) and SciP(R40A) disrupt the interaction between SciP and CtrA in both the yeast two-hybrid and EMSA assays suggesting these residues mediate a direct interaction between SciP and CtrA. If helix three is the main determinant of the SciP DNA interaction, then R35 and R40, which are located at the beginning of helix one and are exposed to adjacent DNA, might be free to interact with CtrA. Interestingly, our attempts to mutate residues in helix one, two, and three destabilized the SciP protein based on the failure of these mutants to accumulate when overexpressed in vivo as well as poor purification yields (unpublished data). 117 ED ED MLQQQRTNSRGEKYVIGPTGAPLTLADLPPAETQRWVIRRKAEVVAAVRGGLLSLDEACDRYKLTNDEFLSWQQSIDRHGLAGLRTTRIQQYR tft B. C. D60A T65M R40A R40S R40 R35 W36A k Figure 4.2. Modeling the three-dimensional structure of SciP. (A) Secondary structure domains superimposed on the SciP primary amino acid structure. R35 and R40 are indicated by arrows. SciP was modeled onto the R. sphaeroides SciP homolog 2jRT (B). (C) Proposed interaction between SciP helix three and DNA. Image taken from [1]. What is the molecular surface that mediates the interaction between CtrA and SciP? In Chapter 2, I used alanine scanning to probe SciP for residues necessary for interacting with CtrA. We are also interested in identifying the residues of CtrA that mediate its interaction with SciP. To find candidates, I performed a structural alignment of CtrA with a structure of E. coli OmpR to identify solvent exposed residues. Because SciP interacts with CtrA but not Caulobacter PhoB, I compared the solvent exposed residues of CtrA with PhoB to identify significant differences. This analysis yielded approximately 20 candidate resides for mutational analysis, 11 of which I tested in the yeast two-hybrid assay for interaction with CtrA. Alanine mutations at 4 of these 11 sites led to a disruption of the interaction between SciP and CtrA in the yeast two-hybrid assay. One mutant, CtrA(Y179A), was able to weakly bind DNA in EMSAs but failed to interact with SciP (Fig. 4.3). Residue Y179 is located within the DNA-binding domain, is solvent 118 exposed, and predicted based on a comparison to the OmpR to not contact DNA. This work is still preliminary but it may provide a clue to the CtrA/SciP interface. Ultimately a co-crystal structure of the SciP/CtrA/DNA complex would be most informative. wt CtrA(uM) ScIPiuM 1 + 1 + H177A 1 1 2 2 + + Y179A 1 1 2 2 + + Figure 4.3. Electrophoretic mobility assays with mutant CtrA and wild-type SciP. Wild-type CtrA-P and indicated CtrA mutants binding to a fragment of the fliF promoter containing a CtrAbinding site. SciP, if present, was added at a final concentration of 1.0 RM. The concentration of CtrA in each lane is indicated. Regulated proteolysis and the Caulobacter cell cycle In Chapter 3, I showed that the Lon protease degrades SciP in vivo and in vitro. Our experiments also indicate that SciP is specifically proteolyzed at the Gl-S transition. However, the Lon protease is present throughout the cell cycle and degrades another substrate, CcrM at a different, later stage of the cell cycle [4]. These observations beg the question of how Lon proteolyzes SciP only during the GI-to-S transition. One possible answer is that SciP is targeted for proteolysis during the G 1-S transition by an adaptor protein that is only produced or active during this window of time. There are many examples of adapter proteins that regulate proteolysis of key regulatory proteins in 119 other bacteria. For instance, in E. coli, the sigma factor RpoS is present at low levels during exponential growth because it is proteolyzed by ClpXP in an RssB dependent manner [5]. RssB is a response regulator that acts as an adapter protein binding to a recognition sequence on RpoS [6]. RpoS interacts with the phosphorylated form of RssB leading to the speculation that dephosphorylation of RssB under stress conditions stabilizes RpoS [6]. One strategy for identifying a SciP adaptor(s) would be to perform a yeast two-hybrid screen. One could use a bait plasmid containing SciP and co-transform with a random genomic library of prey plasmids and select for interactors using the appropriate nutritional markers. Because SciP is highly soluble and readily interacts with CtrA in the yeast two-hybrid, such a selection might quickly yield potential adaptor proteins. Another possibility is that the activity of Lon is regulated by some other mechanism such as subcellular localization. As previously discussed, Caulobacter ClpXP proteolyzes CtrA specifically at the Gl-to-S transition despite the fact that ClpP and ClpX subunits are present throughout the cell cycle [7]. ClpXP activity is thought to be controlled by its localization to the stalked pole during the Gl-S transition [8]. An additional adapter protein, RcdA, may be required to bring CtrA to the stalked pole for proteolysis [9]. The subcellular localization of Lon has not been examined so it is possible a similar mechanism might regulate the activity of Lon and the degradation of SciP during the G 1S transition. Finally, it is possible that some form of post-translational modification could play a role in regulating SciP proteolysis at the Gl-to-S transition. Recent whole-proteome mass spec studies have only begun to catalog the full diversity and prevalence of post120 translational modifications in bacteria including proteolytic cleavages, methylation, and acetylation [10, 11]. In contrast to ubiquitin-targeted proteolysis in eukaryotic systems, post-translational modifications have only rarely been implicated in proteolytic control in bacteria. One key example is stabilization through proteolytic cleavage of UmuD, an alternate RNA polymerase subunit induced during the SOS response in E. coli [12]. Upon SOS induction, RecA cleaves the 24 N-terminal amino acids of UmuD which protects the protein against proteolysis by Lon by ostensibly eliminating a degradation signal in that N-terminal domain [13]. Although we have not observed any conspicuous shifts in SciP migration in Western blots, such a proteolytic cleavage might result in a small shift and therefore difficult to detect without mass spec analysis of the Caulobacter proteome. Interestingly, mass spec analysis is not well suited to identifying post-translational modification by phosphorylation in two-component systems because of the lability of phosphoramidate bonds characteristic of His~P and Asp~P modifications [14]. However in our analysis of SciP we were unable to find any conspicuous phosphorylation site motifs suggesting that SciP is not likely subject to regulation by phosphorylation. Outstanding questions in cell cycle regulated transcription in Caulobacter Although the work presented here contributes to our understanding of the fine-tuning of CtrA-activated transcription, questions remain about CtrA and cell cycle regulated transcription in general. For instance, some CtrA-activated genes have abundant transcripts in swarmer cells [15]. It is unclear whether these genes are actively transcribed in swarmer cells and somehow immune to the effects of SciP or if these genes are expressed in predivisional cells with transcripts that are unusually stable and hence still present in swarmer cells, as is known to be the case with some flagellin genes [16]. If the 121 late genes are actively transcribed in swarmer cells, how do they avoid inhibition by SciP? Perhaps the late genes share a distinct promoter architecture that is not inhibited by SciP. Alternately or in addition, these genes may require another co-factor to fine tune their expression pattern. Although CtrA is a key global regulator of cell cycle transcription in Caulobacter, it directly regulates only ~100 out of more than 500 cell cycle-regulated genes [15]. Another transcriptional regulator, GcrA has been implicated in the regulation of an additional 125 genes [17]. However, most of these genes showed very limited, i.e. less than two-fold, changes in expression upon depletion of GcrA [17]. Further, it remains unknown if GcrA directly binds DNA. Regardless, the expression of at most 30% of cell cycle regulated genes can be explained by CtrA and GcrA suggesting that additional factors remain to be discovered. What regulates the remaining 70% of cell-cycle-regulated genes? One possibility is that one or more uncharacterized transcription factors play key regulatory roles. The Caulobacter genome contains approximately 200 predicted transcription factors, 44 of which are response regulators [18]. A previous study from our lab deleted each of the response regulator genes and screened for cell cycle defects [19]. Five were found to be essential, including ctrA and divK [19]. Two of the remaining three, (petR and ntrY) are homologs of transcription factors that regulate metabolic genes, and the third, cenR, regulates genes important for cell envelope metabolism [19]. Two other response regulators exhibited significant cell cycle defects: flbD and tacA [19]. FlbD is a 122 transcriptional regulator of class III and V flagellar genes while TacA controls stalk biogenesis by activating the transcription factor StaR [20, 21]. These two regulators thus regulate some, but not all, of the remaining cell cycle genes. Our lab is currently deleting and characterizing the other remaining predicted transcription factors in the Caulobacter genome. However, preliminary results suggest that few have severe cell cycle phenotypes suggesting there may be no other global regulators of transcriptional activity or that any remaining global regulators are at least partially redundant in function. It is also possible that sigma factors play a significant role in cell cycle transcription. In eubacteria, the RNA polymerase core enzyme consists of five subunits (a2ppa) that must associate with a sigma factor to form the holoenzyme that can recognize promoters and activate transcription [22]. In E. coli, there are seven different sigma factors that can associate with the RNAP core enzyme to direct global transcription patterns of different classes of genes [22]. C70, encoded by the rpoD, directs the expression of genes in exponential growth while as (rpoS) drives expression of genes during the general stress response [22] . Y54 (rpoN) regulates genes involved in nitrogen assimilation and other metabolic functions [23]. F (fliL) directs the expression of late flagellar genes while -2 (rpoH), dE (rpoE), and Fec respond to specific stress conditions [22]. The Caulobacter genome encodes 16 predicted sigma factors, almost three times as many as E. coil [18]. As in E. coli, rpoD encodes the dominant sigma factor, 073, and rpoH encodes &2 [24- 26]. In addition the gene rpoN encodes Y54, which regulates the expression of class II and IV flagellar genes as well as genes involved in stalk morphogenesis analogous with the role of J in E. coli [20, 27]. Of the remaining 13 predicted sigma factors, only three have been described. oJ (sigF) is thought to protect Caulobacter from oxidative stress during 123 stationary phase, while ad (sigT) and d (sigU) may have a more general role in responding to oxidative stress and heavy metals [28-30]. Given the precedence of sigma factors regulating specific stress responses, it seems unlikely that the remaining 10 uncharacterized sigma factors in Caulobacter contribute significantly to cell cycleregulated transcription [22]. One final possibility is that chromosome structure may play an important role in cell cycle-regulated transcription in Caulobacter.In bacteria, the chromosome forms a highly condensed structure of supercoiled DNA called the nucleoid. In E. coli, it has been shown that DNA topology changes in response to different growth conditions and environmental stresses through changes in the activity of topoisomerase, DNA gyrase, and various nucleoid associated proteins [31]. Several studies have shown that changes in DNA supercoiling influences global gene expression [32]. For instance quickly reducing supercoiling changes the expression of about 300 genes in E. coil [33]. In this study, chromosome relaxation was accomplished by inhibiting gyrase and topoisomerase with antibiotics or through temperature sensitive alleles [33]. Although nucleoid structure and dynamics in Caulobacterhas not been as extensively studied as in E. coli, recent work has shown that the physical location of various chromosomal loci with respect the long axis of the cell depends linearly on their distance from the origin suggesting that the Caulobactergenome is also highly ordered and structured [34]. It would be interesting to define nucleoid structure as a function of the cell cycle using new methods such as Hi-C and to then look for correlations between changes in local nucleoid structure and the expression patterns of cell cycle genes [35]. 124 References 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. 12. 13. 14. 15. Tan, M.H., et al., An essential transcriptionfactor, SciP, enhances robustness of Caulobactercell cycle regulation.Proceedings of the National Academy of Sciences of the United States of America, 2010. 107(44): p. 18985-90. Domian, I.J., A. Reisenauer, and L. Shapiro, Feedback control of a master bacterialcell-cycle regulator.Proceedings of the National Academy of Sciences of the United States of America, 1999. 96(12): p. 6648-53. Gora, K.G., et al., A cell-type-specific protein-proteininteractionmodulates transcriptionalactivity of a master regulatorin Caulobactercrescentus. Molecular Cell, 2010. 39(3): p. 455-67. Wright, R., et al., CaulobacterLon protease has a criticalrole in cell-cycle control ofDNA methylation. Genes & Development, 1996. 10(12): p. 1532-42. Pratt, L.A. and T.J. Silhavy, The response regulatorSprE controls the stability of RpoS. Proceedings of the National Academy of Sciences of the United States of America, 1996. 93(6): p. 2488-2492. Becker, G., E. Klauck, and R. Hengge-Aronis, Regulation ofRpoSproteolysis in Escherichiacoli: The response regulatorRssB is a recognitionfactorthat interacts with the turnover element in RpoS. Proceedings of the National Academy of Sciences of the United States of America, 1999. 96(11): p. 64396444. Jenal, U. and T. Fuchs, An essentialprotease involved in bacterialcell-cycle control. The EMBO journal, 1998. 17(2): p. 5658-69. Iniesta, A.A., et al., A phospho-signalingpathway controls the localizationand activity of a proteasecomplex criticalfor bacterialcell cycle progression. Proceedings of the National Academy of Sciences of the United States of America, 2006. 103(29): p. 10935-40. McGrath, P.T., et al., A dynamically localizedprotease complex anda polar specificity factor control a cell cycle master regulator.Cell, 2006. 124(3): p. 535547. Gupta, N., et al., Whole proteome analysis ofpost-translationalmodifications: Applications of mass-spectrometryfor proteogenomic annotation.Genome Research, 2007. 17(9): p. 1362-1377. Hu, L.I., B.P. Lima, and A.J. Wolfe, Bacterialprotein acetylation: the dawning of a new age. Molecular Microbiology, 2010. 77(1): p. 15-21. Gonzalez, M. and R. Woodgate, The "tale"of UmuD and its role in SOS mutagenesis. Bioessays, 2002. 24(2): p. 141-148. Galhardo, R.S., et al., An SOS-regulatedoperon involved in damage-inducible mutagenesis in Caulobactercrescentus. Nucleic Acids Research, 2005. 33(15886391): p. 2603-14. Stock, A.M., V.L. Robinson, and P.N. Goudreau, Two-component signal transduction.Annual Review of Biochemistry, 2000. 69: p. 183-215. Laub, M.T., et al., Genes directly controlledby CtrA, a master regulatorof the Caulobactercell cycle. Proceedings of the National Academy of Sciences of the United States of America, 2002. 99(7): p. 4632-7. 125 16. 17. 18. 19. 20. 21. 22. 23. 24. 25. 26. 27. 28. 29. 30. 31. Llewellyn, M., et al., The conservedflaF gene has a criticalrole in coupling flagellin translationand assembly in Caulobactercrescentus. Molecular microbiology, 2005. 57: p. 1127-42. Holtzendorff, J., et al., Oscillatingglobal regulatorscontrol the genetic circuit driving a bacterialcell cycle. Science, 2004. 304(5673): p. 983-987. Nierman, W.C., et al., Complete genome sequence of Caulobactercrescentus. Proceedings of the National Academy of Sciences of the United States of America, 2001. 98(7): p. 4136-4141. Skerker, J.M., et al., Two-Component Signal TransductionPathways Regulating Growth and Cell Cycle Progressionin a Bacterium:A System-Level Analysis. PLoS Biology, 2005. 3(10): p. e334. Biondi, E.G., et al., A phosphorelaysystem controls stalk biogenesis during cell cycle progressionin Caulobactercrescentus. Molecular Microbiology, 2006. 59(2): p. 386-401. Wu, J., A.K. Benson, and A. Newton, Global regulationof a sigma 54-dependent flagellargene family in Caulobactercrescentus by the transcriptionalactivator FlbD. Journal of Bacteriology, 1995. 177(23): p. 3241-50. Gruber, T.M. and C.A. Gross, Multiple sigma subunits and the partitioningof bacterialtranscriptionspace. Annual Review of Microbiology, 2003. 57: p. 441466. Reitzer, L. and B.L. Schneider, Metabolic context andpossible physiological themes ofsigma(54)-dependentgenes in Escherichiacoli. Microbiology and Molecular Biology Reviews, 2001. 65(3): p. 422-+. Malakooti, J. and B. Ely, Principalsigma-subunitof the caulobacter-crescentus rna-polymerase.Journal of Bacteriology, 1995. 177(23): p. 6854-6860. Reisenauer, A., C.D. Mohr, and L. Shapiro, Regulation of a heat shock sigma(32) homolog in Caulobactercrescentus.Journal of Bacteriology, 1996. 178(7): p. 1919-1927. Wu, J. and A. Newton, Isolation, identification,and transcriptionalspecificity of the heat shock sigmafactor sigma32from Caulobactercrescentus. Journal of Bacteriology, 1996. 178(7): p. 2094-101. Brun, Y.V. and L. Shapiro, A temporally controlledsigma-factor is requiredfor polar morphogenesis and normal-celldivision in caulobacter.Genes & Development, 1992. 6(12A): p. 2395-2408. Alvarez-Martinez, C.E., et al., The ECF sigmafactorsigma(T) is involved in osmotic and oxidative stress responses in Caulobactercrescentus. Molecular Microbiology, 2007. 66(5): p. 1240-1255. Lourengo, R., C. Kohler, and S. Gomes, A two-component system, an anti-sigma factor and two paralogousECF sigma factors are involved in the control of general stress response in Caulobactercrescentus. Molecular microbiology, 2011: p. no-no. Alvarez-Martinez, C.E., R.L. Baldini, and S.L. Gomes, A Caulobactercrescentus extracytoplasmicfunction sigmafactormediating the response to oxidative stress in stationaryphase. Journal of Bacteriology, 2006. 188(5): p. 1835-1846. Rui, S. and Y.C. Tse-Dinh, Topoisomerasefunctionduring bacterialresponses to environmental challenge. Frontiers in Bioscience, 2003. 8: p. D256-D263. 126 32. 33. 34. 35. Travers, A. and G. Muskhelishvili, Bacterialchromatin. Current Opinion in Genetics & Development, 2005. 15(5): p. 507-514. Peter, B.J., et al., Genomic transcriptionalresponse to loss of chromosomal supercoilingin Escherichia coli. Genome Biology, 2004. 5(11). Viollier, P.H., et al., Rapid and sequentialmovement of individualchromosomal loci to specific subcellular locations during bacterialDNA replication. Proceedings of the National Academy of Sciences of the United States of America, 2004. 101(25): p. 9257-9262. Lieberman-Aiden, E., et al., Comprehensive Mapping ofLong-Range Interactions Reveals FoldingPrinciplesof the Human Genome. Science, 2009. 326(5950): p. 289-293. 127