Biological Psychology A smaller
advertisement

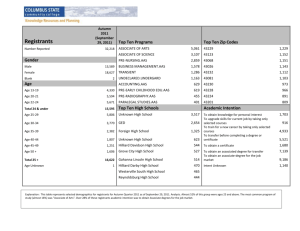
Biological Psychology 113 (2016) 46–51 Contents lists available at ScienceDirect Biological Psychology journal homepage: www.elsevier.com/locate/biopsycho A smaller magnitude of exercise-induced hypoalgesia in African Americans compared to non-Hispanic Whites: A potential influence of physical activity Masataka Umeda a,∗,1 , Laura E. Kempka a , Brennan T. Greenlee b , Amy C. Weatherby c a Department of Kinesiology and Sport Management, Texas Tech University, Lubbock, TX, USA Department of Biological Sciences, Texas Tech University, Lubbock, TX, USA c Honors College, Texas Tech University, Lubbock, TX, USA b a r t i c l e i n f o Article history: Received 1 July 2015 Received in revised form 22 September 2015 Accepted 15 November 2015 Keywords: Race and ethnicity Central pain modulation Minority health a b s t r a c t This study compared exercise-induced hypoalgesia (EIH) between African Americans (AAs, n = 16) and non-Hispanic Whites (NHWs, n = 16), and examined the potential influence of physical activity (PA) on the racial/ethnic difference in EIH. The PA levels were quantified using a questionnaire, and intensity of electrical stimulus to produce moderate pain was individually determined. Participants squeezed a hand dynamometer at 25% of their maximal strength for three minutes, followed by a three-minute post-exercise rest. Numeric ratings to electrical stimulus at the pre-determined intensity were recorded every one minute during and after exercise. Compared to NHWs, AAs reported less lifestyle PA. Both AAs and NHWs showed EIH, but AAs exhibited a smaller magnitude of EIH than NHWs. However, this difference in EIH disappeared after controlling for the lifestyle PA levels. The results suggest that AAs exhibit less efficient pain modulation than NHWs, and AAs’ reduced PA could potentially explain the observed difference in EIH. © 2015 Elsevier B.V. All rights reserved. 1. Introduction Research has been conducted examining racial/ethnic difference in sensitivity to experimental pain stimuli in African Americans (AAs) and non-Hispanic Whites (NHWs), and results from this research generally indicate that AAs are more sensitive to a variety of experimental pain stimuli compared to NHWs (e.g., heat, cold, ischemic stimuli) (Campbell & Edwards, 2012; Edwards, Fillingim, & Keefe, 2001; Rahim-Williams, Riley, Williams, & Fillingim, 2012). Also, physiological thresholds of spinal reflex responses are found to be lower in AAs compared to NHWs (Campbell, France et al., 2008). Together, these observations suggest an increased sensitivity to experimental pain stimuli among AAs compared to NHWs. Muscular contraction has been found to produce naturallyoccurring pain in the exercising muscles (Cook, O’Connor, Eubanks, Smith, & Lee, 1997; Umeda, Newcomb, Ellingson, & Koltyn, 2010). It has been suggested that the localized muscle pain during exer- ∗ Correspondence author at: Department of Kinesiology and Sport Management, Texas Tech University, 3204 Main St Box 43011, Lubbock, TX 79409, USA. Fax: +806 742 1688. E-mail address: masataka.umeda@ttu.edu (M. Umeda). 1 (http://www.depts.ttu.edu/hess/) http://dx.doi.org/10.1016/j.biopsycho.2015.11.006 0301-0511/© 2015 Elsevier B.V. All rights reserved. cise may occur due to stimulation of Type III and Type IV afferent fibers via increased intramuscular pressure and biochemical products produced during muscular contraction (Ellingson & Cook, 2013; O’Connor & Cook, 1999), and research indicates that even submaximal exercise involving smaller muscles can produce a mild-to-moderate intensity of muscle pain (Umeda et al., 2010). Consistent with the increased sensitivity to experimental pain stimuli among AAs, our recent study then demonstrates that AAs report a greater intensity of muscle pain during submaximal isometric exercise compared to NHWs (Umeda, Williams, Marino, & Hilliard, 2015). It has been suggested that pain sensitivity is determined by the complex interactions of endogenous pain modulatory mechanisms that can either inhibit or facilitate nociceptive transmission (Millan, 2002); therefore, it is possible that the consistent observations on the increased pain sensitivity among AAs potentially suggest a functional difference in central pain modulatory processing between AAs and NHWs. Interestingly, evidence shows that exercise does not only produce localized muscle pain, but also reduces sensitivity to experimental pain stimuli in healthy adults (Koltyn, 2000; Naugle, Fillingim, & Riley, 2012). This hypoalgesic phenomenon has been termed as exercise-induced hypoalgesia (EIH) in the literature, and previous research shows that hypoalge- M. Umeda et al. / Biological Psychology 113 (2016) 46–51 sia occurs during and immediately after exercise with a variety of experimental pain stimuli (e.g., pressure, electrical, thermal stimuli) in a systemic manner (e.g., contralateral to exercised muscles) (Koltyn, 2000; Naugle et al., 2012). Also, more recent research indicates that exercise reduces the magnitude of temporal summation of pain (Koltyn, Knauf, & Brellenthin, 2013; Vaegter, Handberg, & Graven-Nielsen, 2014) and that endocannabinoid system may be involved in EIH (Koltyn, Brellenthin, Cook, Sehgal, & Hillard, 2014). Therefore, these data collectively demonstrate that pain modulatory processing within the central nervous system is involved in EIH, suggesting that EIH can be used as a laboratory test to examine central pain modulatory processing. However, no study has been conducted to date to compare the EIH responses between AAs and NHWs. Very little is currently known regarding the factors that influence the function of central pain modulatory processing; however, the increasing body of evidence from recent research appears to suggest that the function of central pain modulatory processing may be influenced by physical activity (PA) levels. For example, there is some evidence that physically active individuals show reduced sensitivity to experimental pain stimuli compared to less physically active individuals (Andrzejewski, Kassolik, Brzozowski, & Cymer, 2010; Ellingson, Colbert, & Cook, 2012; Freund et al., 2013; Johnson, Stewart, Humphries, & Chamove, 2012), and exercise interventions successfully reduce pain sensitivity among healthy adults (Anshel & Russell, 1994; Jones, Booth, Taylor, & Barry, 2014). Furthermore, several studies have been conducted examining the potential influence of regular exercise on central pain modulatory processing using the other laboratory test termed as conditioned pain modulation (CPM), which has been suggested to be mediated by descending pain modulation (Le Bars, 2002; van Wijk & Veldhuijzen, 2010). Results from the studies then show the potential benefits of regular exercise on central pain modulatory processing using this experimental paradigm (Geva & Defrin, 2013; Naugle & Riley, 2014; Umeda, Lee, Marino, & Hilliard, 2015). In contrast, one study indicates that both physically active and less active adults show the comparable EIH responses (Vaegter, Handberg, Jorgensen, Kinly, & Graven-Nielsen, 2014), whereas the other study indicates that endurance athletes show a smaller magnitude of CPM responses compared to healthy adults (Tesarz, Gerhardt, Schommer, Treede, & Eich, 2013). However, the null findings from these studies may be due to several methodological factors, including operational definition of active individuals and conditions of experimental testing. Therefore, although more research is needed in this area, it appears that there is some empirical evidence in the literature suggesting that PA levels may influence the function of central pain modulatory processing. This potential influence of PA on central pain modulatory processing is important to consider the functional difference in central pain modulatory processing in AAs and NHWs because it has been shown that AAs are less physically active compared to NHWs (Hillier, Tappe, Cannuscio, Karpyn, & Glanz, 2014; Trost, Owen, Bauman, Sallis, & Brown, 2002) and spend more time for sedentary behaviors (Cohen et al., 2013). Therefore, the purpose of this study was to compare the functional difference in central pain modulatory processing between AAs and NHWs using the EIH paradigm, and examine the potential influence of PA on the racial/ethnic difference in the EIH responses. 2. Materials and methods 2.1. Participants Healthy adults who identified themselves as AA or NHW were recruited to participate in the study. The inclusion criteria for this study were (1) 18–30 years of age, (2) self-identification as 47 African American or non-Hispanic White, (3) no medical conditions diagnosed by their physician, and (4) no medication use. The participants were excluded from the study if (1) they indicated medical contraindications for exercise, (2) they were currently pregnant or breastfeeding, or (3) they planned to be pregnant or breastfeed in the near future. The two groups were matched based on age (±3 years) and gender. The study protocol was fully approved by an institutional review board, and all participants signed a consent form before participating in the study. Power analysis was performed to estimate a sample size to accurately detect significant difference in EIH between AAs and NHWs. Effect size was first calculated using our pilot data, and the analysis was performed with a large effect size, an ˛ = 0.05, and a power = 0.80. The analysis indicated that approximately 12–17 AAs and 12–17 NHWs would be needed for this study. 2.1. Measures 2.1.1. Physical activity levels To quantify the PA levels, the Baecke Physical Activity Questionnaire (BPAQ) was used in this study. The BPAQ consists of three subscales to estimate work, sport, and leisure related PA, and reliability and validity of the BPAQ have been well-investigated in the previous studies (Baecke, Burema, & Frijters, 1982; Jacobs, Ainsworth, Hartman, & Leon, 1993). The work-related PA subscale assesses the type of occupation and the activity levels associated with their occupation. The sport-related PA subscale quantifies the amount of PA that the participants are recreationally engaging in (e.g., playing basketball, swimming), whereas the leisure-related PA subscale quantifies the amount of lifestyle PA associated with routine, daily activities (e.g., walking, riding a bike), except for the recreational PA. Scores from the three subscales are summed to compute the total PA levels, with the higher scores indicative of more PA. Although the BPAQ does not estimate the actual time spent for different type or intensity of PA, previous research has shown that the BPAQ scores correlate to other measures of PA and physical fitness (e.g., PA history, VO2 max, % body fat, etc.) in expected directions (Jacobs et al., 1993). 2.1.2. Pain catastrophizing The Pain Catastrophizing Scale (PCS) was used in this study to assess pain catastrophizing, a psychological trait regarding one’s negative thoughts and feelings related to pain experience (Sullivan, Bishop, & Pivik, 1995). The PCS consists of the rumination, magnification, and helplessness subscales, and scores from the three subscales were summed to obtain the total score, with the higher scores indicative of greater pain catastrophizing. Previous studies have examined reliability and validity of the PCS (Osman et al., 1997; Sullivan et al., 1995), and it has been shown that pain catastrophizing is a psychological factor that may influence the EIH responses in healthy adults (Naugle, Naugle, Fillingim, & Riley, 2014). 2.2. Procedures The participants were asked to visit our laboratory for two sessions. Upon arrival at our lab for the first session, the participants were first asked to sign a consent form, and then to complete a questionnaire regarding demographics and general health, the BPAQ, and the PCS. The participants were then asked to squeeze a hand dynamometer as hard as possible with their dominant hand to measure the maximal voluntary contraction (MVC). The MVC assessment was conducted twice, and the average MVC was used to calculate a target force (25% MVC) for the exercise protocol in the next session. The MVC assessment was followed by a familiar- 48 M. Umeda et al. / Biological Psychology 113 (2016) 46–51 ization session of electrical pain protocol. The participants were first asked to remove a sock and shoe of the contralateral foot to the participants’ dominant hand, and place the foot on a foot rest. The researcher then attached a small stimulation electrode (50 mm × 15 mm) to the participants’ ankle on the sural nerve using surgical tape and wrapped the electrode up using a strap tape. Next, the researcher delivered very brief electrical stimulations (constant pulse duration = 0.2 s) to the ankle through the electrode using a constant current stimulator (Digitimer DS7AH, Hertfordshire, England). To make sure that the electrode was placed on the sural nerve, the researcher confirmed with the participants that electrical sensation was felt in the anatomical area innervated by the sural nerve (lateral foot toward a pinky toe). The participants were then asked to verbally provide a numeric rating to each stimulation using a 0–100 pain rating scale, with the following six pairs of numbers and corresponding descriptors: 0 = no sensation, 25 = uncomfortable, 50 = painful, 75 = very painful, and 100 = maximum tolerable (France & Suchowiecki, 2001; Umeda, Lee et al., 2015). The stimulus intensity was determined using a similar stair-case method that was used in previous studies examining the exercise effects on sensitivity to noxious electrical stimuli (Ring, Edwards, & Kavussanu, 2008; Umeda, Lee et al., 2015). The stimulus intensity was increased by 4-mA from 0 mA until the participants rated a stimulation as 50 ± 5. If the participants provided a rating of 50 ± 5 to the stimulation during this ascending series of stimulations, the assessment was completed at the point. If the participants rated the stimulation higher than 55, the stimulus intensity was then decreased by 2-mA until the participants rated the stimulation as 50 ± 5. This protocol was repeated one more time to familiarize the participants with the protocol. Lastly, the participants were asked to refrain from (1) strenuous exercise for 12 h, (2) alcohol consumption for 12 h, (3) caffeine intake for 3 h, and (4) smoking for 3 h before the next session to assess blood pressure (BP) responses during the nest session (Ring et al., 2008; Shapiro et al., 1996). This concluded the first session, which took approximately 40–45 min to complete. The participants visited our lab for the second session at least 24 h after the first session. After confirming the participants’ compliance with the above guidelines for BP assessment, the researcher prepared the participants for assessment of their resting BP by placing three electrodes on their chest and attaching a BP cuff around their non-dominant upper arm (Tango+, SunTech Medical, NC). The participants were asked to remain seated in a comfortable chair for several minutes, and the researcher then assessed the participants’ resting BP. Resting BP assessment was followed by electrical pain testing, conducted using the same procedure as the first session, to determine electrical stimulus intensity that was required to elicit a rating of 50 ± 5. After electrical pain testing, the participants were asked to squeeze a hand dynamometer with their dominant hand at 25% MVC, and hold the force as well as possible for three minutes. This submaximal isometric exercise was chosen in this study because previous research shows that this exercise protocol successfully produces EIH (Koltyn et al., 2013; Umeda et al., 2010). The researcher then assessed muscle pain ratings in the exercising forearm using the muscle pain rating (MPR) scale (Cook et al., 1997), and exertion using the rating of perceived exertion (RPE) scale (Borg, 1998) every one minute during exercise. The MPR scale is constructed in a vertical alignment with 12 numbers (0, ½, 1–10), and has corresponding pain descriptors next to the nine numbers. The MPR scale ranges from 0 (no pain) to 10 (extremely painful), and also allows the participants to respond with greater numbers than 10 if the participants experience a greater intensity of muscle pain than extremely intense pain. The RPE scale is also constructed in a vertical alignment with 15 numbers that range from 6 to 20 and corresponding descriptors to the nine numbers (e.g., 6 = no exertion, 20 = maximal exertion). The participants were asked to verbally provide a number that best corresponded to the intensity of muscle pain and the level of exertion, respectively, at each time point during exercise. Also, BP was recorded every minute during exercise. After completion of BP assessment at every one minute, an electrical stimulus was delivered at the intensity that was determined during the baseline electrical pain testing to the participant’s ankle. The participants were then asked to verbally provide ratings to the stimulation using the 0–100 pain rating scale. After completion of the submaximal isometric exercise, numeric rating to electrical stimulus at the same intensity was obtained every one minute during the next three minutes. This concluded the second session, and the second session took approximately 45 min to complete. 2.3. Data analyses Both BPAQ and PCS were scored based on the established scoring guidelines (Baecke et al., 1982; Sullivan et al., 1995). BP data were converted into mean arterial pressure (MAP) and body mass index (BMI) was calculated using the following formulas: MAP = diastolic BP + (systolic BP–diastolic BP)/3 and BMI = weight (kg)/(height (m))2 . One-way ANOVAs were then performed to examine group differences between AAs and NHWs in the following participants’ characteristics: age, BMI, MVC, resting MAP, BPAQ scores, PCS scores, baseline electrical stimulus intensity that was required to elicit a rating of 50 ± 5, and baseline electrical pain ratings. MPR, RPE, and BP were recorded every one minute during 3min isometric exercise; therefore, 2 (groups: AAs and NHWs) × 3 (times: every minute during 3-min exercise) multivariate repeated measures ANOVAs were performed to examine group differences in changes in the psychophysiological responses to exercise. Pain ratings were obtained before exercise, and every one minute during 3-min exercise and 3-min post-exercise rest. Therefore, 2 (groups: AAs and NHWs) × 6 (times: every one minute during 3-min exercise and 3-min post-exercise rest) multivariate repeated measures ANCOVA was performed to examine group difference in pain ratings, with the baseline pain ratings as a covariate. When there was a significant group difference in the participants’ characteristics, the variable was entered into the above multivariate repeated measures ANOVA for MPR and ANCOVA for pain ratings as a covariate, to test if the variable could potentially explain the group difference in muscle pain and EIH responses. To support this mediation analyses, correlation analyses were also performed using Pearson’s correlation to test if the variable was associated with average MPRs and EIH responses, calculated as baseline pain ratings—average pain ratings during and after exercise. Furthermore, correlation analyses were performed using Pearson’s correlation to test if average MPRs and changes in MAP by exercise were associated with EIH responses. These analyses were performed as secondary analyses of interests to examine the potential association of muscle pain and BP with EIH responses. The significance level was set at ˛ = 0.05 for all statistical analyses. 3. Results 3.1. Participants Sixteen healthy AAs (8 men and 8 women) and 16 NHWs (8 men and 8 women) were recruited from campus and completed this study. The participants completed the two sessions, with approximately 3 days on average between the two sessions (mean = 2.875, M. Umeda et al. / Biological Psychology 113 (2016) 46–51 Table 1 Characteristics of study participants. 49 Table 2 Perceived exertion and mean arterial pressure responses during isometric exercise. African Americans (n = 16) Non-Hispanic Whites (n = 16) Age (years) BMI (kg/m2 ) MVC (kg) Resting MAP (mmHg) BPAQ-Total BPAQ-Work BPAQ-Sport BPAQ-Leisure* PCS-Total PCS-Rumination PCS-Magnification PCS-Helplessness 20.75 (2.79) 22.52 (2.97) 36.52 (15.66) 79.82 (6.04) 8.17 (2.27) 2.91 (1.63) 3.01 (1.24) 2.43 (0.53) 14.43 (8.37) 5.50 (3.10) 3.00 (2.76) 5.94 (3.57) 20.56 (1.97) 23.38 (2.60) 34.95 (9.84) 81.27 (10.37) 8.93 (1.55) 2.55 (0.68) 3.13 (0.94) 3.25 (0.57) 10.69 (7.90) 4.44 (3.67) 2.50 (2.25) 3.88 (3.22) Time Minute 1 Minute 2 Minute 3 RPE African Americans (n = 16) Non-Hispanic Whites (n = 16) 9.69 (2.21) 9.28 (2.13) 11.94 (2.77) 11.47 (2.38) 13.75 (2.93) 13.19 (2.90) MAP African Americans (n = 15) Non-Hispanic Whites (n = 16) 90.38 (6.54) 85.78 (11.37) 92.84 (6.31) 90.65 (16.14) 95.96 (7.19) 92.19 (14.91) Numbers are means, and numbers in parentheses are standard deviations. RPE; Rating of Perceived Exertion. MAP; Mean Arterial Pressure. MPR Scores Numbers are means, and numbers in parentheses are standard deviations. BMI; Body Mass Index. MAP; Mean Arterial Pressure. MVC; Maximal Voluntary Contraction. BPAQ; Baecke Physical Activity Questionnaire. PCS; Pain Catastrophizing Scale. * Denotes a significant group difference at p < 0.001. Extremely Intense Pain African Americans Non-Hispanic Whites 50 Pain Ratings 10 9 8 7 6 5 4 3 2 1 0 60 Painful 40 30 20 African Americans 10 Strong Pain Non-Hispanic Whites 0 BL 1 2 Exercise 3 1 2 3 Post-Exercise Time (minutes) 1 2 Time (minutes) 3 Fig. 1. Muscle pain responses during 3 min of isometric handgrip exercise at 25% MVC. The data indicate group means ± SE at each time point. SD = 2.791). Results indicated no group differences in the participants’ characteristics (F1, 30 = 0.048-2.945, p > 0.05), except that NHWs had higher scores on the BPAQ leisure subscale compared to AAs (F1, 30 = 17.931, p < 0.001). These results are summarized in Table 1. 3.2. Psychophysiological responses to exercise Results for MPRs indicated a significant time effect (F2, 29 = 45.228, p < 0.001), and a significant group effect (F1,30 = 4.621, p = 0.040). However, there was not a significant time × group interaction (F2 , 29 = 0.604, p > 0.05). These results suggest that MPRs generally increased during exercise in both groups, but AAs consistently reported higher ratings during exercise compared to NHWs. The MPR data are illustrated in Fig. 1. Results for RPE indicated a significant time effect (F2,29 = 63.519, p < 0.001); however, neither a group effect (F1,30 = 0.318, p > 0.05) nor a time × group interaction (F2,29 = 0.030, p > 0.05) was found to be significant. These results suggest that RPE generally increased during exercise in AAs and NHWs in a similar manner. The RPE data are summarized in Table 2. Due to a technical error in BP assessment during exercise, we were unable to obtain BP data during exercise from one AA participant. Therefore, the MAP data analysis was conducted with 15 AAs and 16 NHWs. The results for MAP responses indicated that there was a significant time effect (F2,28 = 8.314, p = 0.001); however, Fig. 2. Pain ratings at each time point during and after 3 min of isometric handgrip exercise at 25% MVC in comparison to baseline (BL). The data indicate group means ± SE at each time point. neither a group effect (F1,29 = 0.885, p > 0.05) nor a time × group interaction (F2,28 = 0.584, p > 0.05) was found to be significant. These results suggest that MAP generally increased during exercise in AAs and NHWs in a similar manner. The MAP data are summarized in Table 2. Together, the results indicated that AAs consistently reported a greater intensity of muscle pain during exercise compared to NHWs; however, other psychophysiological responses to exercise were similar in AAs and NHWs. 3.3. EIH Responses Results for the baseline electrical pain testing indicated that there were no baseline differences between AAs and NHWs in the stimulus intensity that was required to elicit a rating of 50 ± 5 (AAs: 43.38 mA ± 18.51 & NHWs: 48.75 mA ± 22.76, F1,30 = 0.537, p > 0.05) and baseline electrical pain ratings (AAs: 49.56 ± 0.89 & NHWs: 49.19 ± 1.62 SD, F1 , 30 = 0.657, p > 0.05). Results for EIH indicated no significant time effect (F5,25 = 0.438, p > 0.05). In addition, neither a significant time × group interaction (F5,25 = 1.025, p > 0.05) nor a significant time × baseline pain ratings interaction (F5,25 = 0.397, p > 0.05) was found. However, the results indicated a significant group effect (F1,29 = 6.046, p = 0.020), suggesting that pain ratings during and after exercise were generally higher in AAs compared to NHWs. These results indicate that a magnitude of EIH responses were generally smaller among AAs compared to NHWs. These EIH data are illustrated in Fig. 2. 50 M. Umeda et al. / Biological Psychology 113 (2016) 46–51 3.4. Covariate analyses Since our analyses showed that NHWs scored higher on the BPAQ leisure subscale compared to AAs, the follow-up analyses were conducted with the subscale scores as a covariate to test if the BPAQ leisure subscale scores would account for the racial/ethnic differences in MPRs and EIH. The results for the correlational analyses indicated that the BPAQ leisure subscale scores marginally correlated with average MPRs and EIH responses in expected directions (MPRs: r = −0.324, p = 0.070 & EIH: r = 0.345, p = 0.053). The results for the covariate analyses then indicated that after controlling for the racial/ethnic difference in the BPAQ leisure subscale scores, there were no longer significant differences in MPRs and EIH between AAs and NHWs (MPRs: F1,29 = 1.516, p > 0.05 & EIH: F1,28 = 2.232, p > 0.05), suggesting that the racial/ethnic differences in MPRs and EIH may be explained by the racial/ethnic difference in the BPAQ leisure subscale scores. Correlational Analyses for Secondary Interests Results for the correlational analyses indicated that neither average MPRs nor changes in MAP by exercise correlated with EIH responses (MPRs: r = −0.152, p > 0.05 & MAP: r = −0.084, p > 0.05). 4. Discussion The present study compared the EIH responses to submaximal isometric handgrip exercise between healthy AAs and NHWs, and examined the potential influence of PA on the racial/ethnic difference in the EIH responses. The results indicated that both AAs and NHWs experienced EIH, but AAs exhibited a smaller magnitude of EIH in comparison to NHWs. Furthermore, AAs reported reduced lifestyle PA during their leisure time compared to NHWs, and the observed racial/ethnic difference in EIH disappeared after statistically controlling for the lifestyle PA levels between the two groups. Together, the present study collectively shows that AAs exhibit a less efficient function of central pain modulatory processing compared to NHWs, and the reduced activity among AAs may potentially explain their less efficient function of central pain modulatory processing. The present study demonstrated that AAs exhibited a smaller magnitude of EIH compared to NHWs. Although the present study was the first to test racial/ethnic difference in the function of central pain modulatory processing between AAs and NHWs using the EIH paradigm, several previous studies to date have suggested a less efficient function of central pain modulatory processing among AAs using different experimental paradigms. For example, Campbell et al., examined the functional difference in central pain modulatory processing in AAs and NHWs using CPM, and found that AAs exhibited a smaller magnitude of pain modulation compared to NHWs (Campbell et al., 2008). Furthermore, research indicates an inverse association between pain sensitivity and BP (Bruehl & Chung, 2004; Koltyn & Umeda, 2006), and it has been suggested that elevations in BP lead to reduced sensitivity to pain stimuli via descending pain modulation initiated in several brain sites that are involved in both cardiovascular and pain modulatory controls (e.g., rostral ventromedial medulla, nucleus tractus solirarius) (Bruehl & Chung, 2004; Ghione, 1996). Our previous study demonstrated that AAs experienced augmented muscle pain during exercise compared to NHWs, although both AAs and NHWs showed a comparable magnitude of BP elevations during exercise (Umeda et al., 2015). Therefore, the results also suggest a less efficient pain modulation in AAs compared to NHWs. Together, previous research in this area consistently suggest the functional difference in central pain modulatory processing among AAs compared to NHWs, such that AAs typically show a less efficient function of central pain modulatory processing compared to NHWs. The results indicated that the racial/ethnic difference in the EIH responses and augmented muscle pain during exercise between AAs and NHWs disappeared after statistically controlling for the lifestyle PA levels, suggesting that increasing PA may help improve the function of central pain modulatory processing, and reduce the intensity of muscle pain during exercise among AAs. Currently, only small database is available in the literature about the effects of regular exercise on pain sensitivity, but previous studies provide preliminary evidence that regular PA may help reduce sensitivity to a variety of experimental pain stimuli (i.e., pressure, thermal, cold, and ischemic stimuli) (Andrzejewski et al., 2010; Anshel & Russell, 1994; Ellingson et al., 2012; Freund et al., 2013; Jones et al., 2014). Such generalized hypoalgesic effects of regular PA potentially suggest the benefits of regular PA on pain sensitivity and central pain modulatory processing. To support this hypothesized role of regular PA on central pain modulatory processing, there has been emerging evidence using the CPM paradigm suggesting that regular PA may help improve central pain modulatory processing in healthy adults (Geva & Defrin, 2013; Naugle & Riley, 2014; Umeda, Lee et al., 2015). On the other hand, some investigators found that physically active and inactive adults exhibited a comparable magnitude of EIH (Vaegter, Handberg et al., 2014), and others reported that endurance athletes demonstrated a smaller magnitude of the CPM responses compared to healthy controls (Tesarz et al., 2013). It is currently unclear why these studies did not find supportive evidence for the potential benefits of regular exercise on central pain modulatory processing; however, it is possible that methodological factors, such as operational definition of active individuals and conditions of experimental testing, may be responsible for the inconsistent findings in this research. Together, little is known regarding the effects of regular PA on pain sensitivity and central pain modulatory processing, and most of the studies in this area are cross-sectional studies. Therefore, more research is needed to better understand whether regular PA will help minimize muscle pain and improve the function of central pain modulatory processing. There are several limitations in this study. First, the present study was conducted with a small sample size; therefore, the present study could not determine potential gender difference in the EIH responses in AAs and NHWs. More research is needed in this area. Second, we assessed pain ratings to quantify EIH. Although pain ratings has been frequently used in EIH research (Koltyn, 2000; Naugle et al., 2012), there are other methods to quantify pain sensitivity, including pain thresholds and pain tolerances. More comprehensive assessment of pain sensitivity may help describe the racial/ethnic differences in EIH in AAs and NHWs. The third limitation to note is that self-report questionnaire was used to quantify PA levels of the participants in this study. The questionnaire has been well-validated in previous research (Baecke et al., 1982; Jacobs et al., 1993); however, it is important in the future research to use more comprehensive assessment of PA to determine the potential influence of regular PA on EIH. Lastly, the correlational analyses that were performed in conjunction with mediation analyses for MPRs and EIH responses showed statistically marginal correlations. Although the correlations were generally in expected directions, the correlations did not reach conventional statistical significance due partly to small sample size in the present study. Therefore, more research is needed to confirm the mediational role of physical activity in the relationship between pain sensitivity and race/ethnicity in the future. In conclusion, the present study demonstrates that AAs exhibit a smaller magnitude of EIH in comparison to NHWs, and that AAs’ reduced lifestyle PA during the leisure time may potentially account for their smaller magnitude of EIH. Together, these results from the present study suggest that AAs exhibit a less efficient function of pain modulatory processing within the central nervous system compared to NHWs, and increasing activity levels may help restore M. Umeda et al. / Biological Psychology 113 (2016) 46–51 the reduced function of central pain modulatory processing among AAs. Future research is warranted in this area to determine the effects of regular exercise on central pain modulatory processing among AAs. Conflict of interest There is no conflict of interest among authors. Acknowledgement We thank Ramona Harwell for her administrative support and Dr. Youngdeok Kim for his statistical consultation for the present study. References Andrzejewski, W., Kassolik, K., Brzozowski, M., & Cymer, K. (2010). The influence of age and physical activity on the pressure sensitivity of soft tissues of the musculoskeletal system. Journal of Bodywork and Movement Therapies, 14(4), 382–390. http://dx.doi.org/10.1016/j.jbmt.2009.07.004 Anshel, M. H., & Russell, K. G. (1994). Effect of aerobic and strength training on pain tolerance, pain appraisal and mood of unfit males as a function of pain location. Journal of Sports Sciences, 12(6), 535–547. http://dx.doi.org/10.1080/ 02640419408732204 Baecke, J. A., Burema, J., & Frijters, J. E. (1982). A short questionnaire for the measurement of habitual physical activity in epidemiological studies. American Journal of Clinical Nutrition, 36(5), 936–942. Borg, G. (1998). The Borg RPE scale Borg’s perceived exertion and pain scales. Champaign, IL: Human Kinetics. Bruehl, S., & Chung, O. Y. (2004). Interactions between the cardiovascular and pain regulatory systems: an updated review of mechanisms and possible alterations in chronic pain. Neuroscience and Biobehavioral Reviews, 28(4), 395–414. http:// dx.doi.org/10.1016/j.neubiorev.2004.06.004S0149-7634(04) 69-7 [pii] Campbell, C. M., & Edwards, R. R. (2012). Ethnic differences in pain and pain management. Pain Management, 2(3), 219–230. http://dx.doi.org/10.2217/pmt. 12.7 Campbell, C. M., France, C. R., Robinson, M. E., Logan, H. L., Geffken, G. R., & Fillingim, R. B. (2008). Ethnic differences in diffuse noxious inhibitory controls. Journal of Pain, 9(8), 759–766. http://dx.doi.org/10.1016/j.jpain.2008.03.010 Campbell, C. M., France, C. R., Robinson, M. E., Logan, H. L., Geffken, G. R., & Fillingim, R. B. (2008). Ethnic differences in the nociceptive flexion reflex (NFR). Pain, 134(1–2), 91–96. http://dx.doi.org/10.1016/j.pain.2007.03.035 Cohen, S. S., Matthews, C. E., Signorello, L. B., Schlundt, D. G., Blot, W. J., & Buchowski, M. S. (2013). Sedentary and physically active behavior patterns among low-income African–American and white adults living in the southeastern United States. Public Library of Science, 8(4), e59975. http://dx. doi.org/10.1371/journal.pone.0059975 Cook, D. B., O’Connor, P. J., Eubanks, S. A., Smith, J. C., & Lee, M. (1997). Naturally occurring muscle pain during exercise: assessment and experimental evidence. Medicine and Science in Sports and Exercise, 29(8), 999–1012. Edwards, C. L., Fillingim, R. B., & Keefe, F. (2001). Race, ethnicity and pain. Pain, 94(2), 133–137. Ellingson, L. D., Colbert, L. H., & Cook, D. B. (2012). Physical activity is related to pain sensitivity in healthy women. Medicine and Science in Sports and Exercise, 44(7), 1401–1406. http://dx.doi.org/10.1249/mss.0b013e318248f648 Ellingson, L. D., & Cook, C. B. (2013). Physical activity and pain. In P. Ekkekakis (Ed.), Routledge handbook of physical activity and mental health (pp. 400–410). New York, NY: Routledge. France, C. R., & Suchowiecki, S. (2001). Assessing supraspinal modulation of pain perception in individuals at risk for hypertension. Psychophysiology, 38(1), 107–113. Freund, W., Weber, F., Billich, C., Birklein, F., Breimhorst, M., & Schuetz, U. H. (2013). Ultra-marathon runners are different: investigations into pain tolerance and personality traits of participants of the TransEurope FootRace 2009. Pain Practice, 13(7), 524–532. http://dx.doi.org/10.1111/papr.12039 Geva, N., & Defrin, R. (2013). Enhanced pain modulation among triathletes: a possible explanation for their exceptional capabilities. Pain, 154(11), 2317–2323. http://dx.doi.org/10.1016/j.pain.2013.06.031 Ghione, S. (1996). Hypertension-associated hypalgesia. Evidence in experimental animals and humans, pathophysiological mechanisms, and potential clinical consequences. Hypertension, 28(3), 494–504. Hillier, A., Tappe, K., Cannuscio, C., Karpyn, A., & Glanz, K. (2014). In an urban neighborhood, who is physically active and where? Women and Health, 54(3), 194–211. http://dx.doi.org/10.1080/03630242.2014.883659 Jacobs, D. R., Jr., Ainsworth, B. E., Hartman, T. J., & Leon, A. S. (1993). A simultaneous evaluation of 10 commonly used physical activity questionnaires. Medicine and Science in Sports and Exercise, 25(1), 81–91. 51 Johnson, M. H., Stewart, J., Humphries, S. A., & Chamove, A. S. (2012). Marathon runners’ reaction to potassium iontophoretic experimental pain: pain tolerance, pain threshold, coping and self-efficacy. European Journal of Pain, 16(5), 767–774. http://dx.doi.org/10.1002/j.1532-2149.2011.00059.x Jones, M. D., Booth, J., Taylor, J. L., & Barry, B. K. (2014). Aerobic training increases pain tolerance in healthy individuals. Medicine and Science in Sports and Exercise, 46(8), 1640–1647. http://dx.doi.org/10.1249/MSS. 0000000000000273 Koltyn, K. F. (2000). Analgesia following exercise: a review. Sports Medicine, 29(2), 85–98. Koltyn, K. F., Brellenthin, A. G., Cook, D. B., Sehgal, N., & Hillard, C. (2014). Mechanisms of exercise-induced hypoalgesia. journal of Pain, 15(12), 1294–1304. http://dx.doi.org/10.1016/j.jpain.2014.09.006 Koltyn, K. F., Knauf, M. T., & Brellenthin, A. G. (2013). Temporal summation of heat pain modulated by isometric exercise. European Journal of Pain, 17(7), 1005–1011. http://dx.doi.org/10.1002/j.1532-2149.2012.00264.x Koltyn, K. F., & Umeda, M. (2006). Exercise, hypoalgesia and blood pressure. Sports Medicine, 36(3), 207–214. Le Bars, D. (2002). The whole body receptive field of dorsal horn multireceptive neurones. Brain Research Brain Research Reviews, 40(1-3), 29–44. Millan, M. J. (2002). Descending control of pain. Progress in Neurobiology, 66(6), 355–474. S0301008202000096 [pii]. Naugle, K. M., Fillingim, R. B., & Riley, J. L., 3rd. (2012). A meta-analytic review of the hypoalgesic effects of exercise. Journal of Pain, 13(12), 1139–1150. http:// dx.doi.org/10.1016/j.jpain.2012.09.006 Naugle, K. M., Naugle, K. E., Fillingim, R. B., & Riley, J. L., 3rd. (2014). Isometric exercise as a test of pain modulation: effects of experimental pain test, psychological variables, and sex. Pain Medication, 15(4), 692–701. http://dx.doi. org/10.1111/pme.12312 Naugle, K. M., & Riley, J. L., 3rd. (2014). Self-reported physical activity predicts pain inhibitory and facilitatory function. Medicine & Science in Sports & Exercise, 46(3), 622–629. http://dx.doi.org/10.1249/MSS.0b013e3182a69cf1 O’Connor, P. J., & Cook, D. B. (1999). Exercise and pain: the neurobiology, measurement:and laboratory study of pain in relation to exercise in humans. Exercise and Sport Sciences Reviews, 27, 119–166. Osman, A., Barrios, F. X., Kopper, B. A., Hauptmann, W., Jones, J., & O’Neill, E. (1997). Factor structure, reliability, and validity of the Pain Catastrophizing Scale. Journal of Behavioral Medicine, 20(6), 589–605. Rahim-Williams, B., Riley, J. L., 3rd., Williams, A. K., & Fillingim, R. B. (2012). A quantitative review of ethnic group differences in experimental pain response: do biology, psychology, and culture matter? Pain Medication, 13(4), 522–540. http://dx.doi.org/10.1111/j. 1526-4637.2012.01336. x Ring, C., Edwards, L., & Kavussanu, M. (2008). Effects of isometric exercise on pain are mediated by blood pressure. Biological Psychology, 78(1), 123–128. http:// dx.doi.org/10.1016/j.biopsycho.2008.01.008. S0301-0511(08) 00029-X [pii] Shapiro, D., Jamner, L. D., Lane, J. D., Light, K. C., Myrtek, M., Sawada, Y., et al. (1996). Blood pressure publication guidelines. Psychophysiology, 33(1), 1–12. http://dx.doi.org/10.1111/j.1469-8986.1996.tb02103.x Sullivan, M. J. L., Bishop, L., & Pivik, J. (1995). The pain catastrophizing scale: development and validation. Psychological Assessment, 7(4), 524–532. Tesarz, J., Gerhardt, A., Schommer, K., Treede, R. D., & Eich, W. (2013). Alterations in endogenous pain modulation in endurance athletes: an experimental study using quantitative sensory testing and the cold-pressor task. Pain, 154(7), 1022–1029. http://dx.doi.org/10.1016/j.pain.2013.03.014 Trost, S. G., Owen, N., Bauman, A. E., Sallis, J. F., & Brown, W. (2002). Correlates of adults’ participation in physical activity: review and update. Medicine and Science in Sports and Exercise, 34(12), 1996–2001. http://dx.doi.org/10.1249/01. MSS. 0000038974.76900.92 Umeda, M., Newcomb, L. W., Ellingson, L. D., & Koltyn, K. F. (2010). Examination of the dose-response relationship between pain perception and blood pressure elevations induced by isometric exercise in men and women. Biological Psychology, 85(1), 90–96. http://dx.doi.org/10.1016/j.biopsycho.2010.05.008. S0301-0511(10) 170-5 [pii] Umeda, M., Williams, J. P., Marino, C. A., & Hilliard, S. C. (2015). Muscle pain and blood pressure responses during isometric handgrip exercise in healthy African American and non-Hispanic White adults. Physiology and Behavior, 138, 242–246. http://dx.doi.org/10.1016/j.physbeh.2014.09.013 Umeda, M., Lee, W., Marino, C. A., & Hilliard, S. C. (2015). Influence of moderate intensity physical activity levels and gender on conditioned pain modulation. Journal of Sports Sciences, 1–10. http://dx.doi.org/10.1080/02640414.2015. 1061199 Vaegter, H. B., Handberg, G., & Graven-Nielsen, T. (2014). Isometric exercises reduce temporal summation of pressure pain in humans. European Journal of Pain, http://dx.doi.org/10.1002/ejp.623 Vaegter, H. B., Handberg, G., Jorgensen, M. N., Kinly, A., & Graven-Nielsen, T. (2014). Aerobic Exercise and Cold Pressor Test Induce Hypoalgesia in Active and Inactive Men and Women. Pain Medicine, http://dx.doi.org/10.1111/pme.12641 van Wijk, G., & Veldhuijzen, D. S. (2010). Perspective on diffuse noxious inhibitory controls as a model of endogenous pain modulation in clinical pain syndromes. journal of Pain, 11(5), 408–419. http://dx.doi.org/10.1016/j.jpain.2009.10.009. S1526-5900(09) 809-8 [pii]