Valorization of Boiler Ash in Alkali Activated Materials
ARCHIVES
By
MASSA C HuLSETTS INSTITU TE
O FTECHNOLOLGY
Michael Edward Laracy
UL 02 2015
B.S. Civil and Environmental Engineering
Merrimack College, 2013
L IBRARIES
Submitted to the Department of Civil and Environmental Engineering
in partial fulfillment of the requirements for the degree of
MASTER OF SCIENCE IN CIVIL AND ENVIRONMENTAL ENGINEERING
at the
MASSACHUSETTS INSTITUTE OF TECHNOLOGY
June 2015
C 2015 Massachusetts Institute of Technology. All Rights Reserved.
The author hereby grant to MIT permission to reproduce and to distributepubliclypaper and electronic
copies of this thesis document or in part in any medium now known or hereafter created
Signature redacted
Signature of Author:
Department of Civil and EnvirodrmntalE gi ering
0, 9 2015
Certified by:
___________
Signature redacted
_
J-t1n Ochsendorf
Class of 1942 Professor of Civil and Environmental Engineering and Architecture
Thesis Supervisor
Accepted by:
Signature redacted.Nepf
I/' I ediM N
Donald and Martha Harleman Professor of Civil and Environmental Engineering
Chair, Departmental Committee for Graduate Program
Valorization of Boiler Ash in Alkali Activated Materials
by:
Michael Laracy
Submitted to the Departmentof Civil and Environmental Engineering
On May 20, 2015, in partialfulfillment of the requirementsfor the
Degree of Master of Science in Civil and EnvironmentalEngineering
Abstract
For centuries the clay fired brick has been the most popular building material in India due to its
local availability and low cost. Despite the growing demand for bricks, some negative
environmental and social impacts surrounding its production raise concerns about its future use.
In parallel, a growth in industry is generating a number of industrial wastes, such as boiler ash,
which are often disposed of in ways that are harmful to the population and the environment. Due
to its highly variable physical and chemical properties, boiler ash currently has no viable
applications, providing an opportunity to identify a solution.
This research seeks to partially solve both the brick and the waste problem by recycling boiler ash
into masonry construction materials. This is accomplished using alkali activation, a low energy
approach which relies on a chemical reaction to give the product its strength. The aim is to create
a mix design which is robust enough to account for the variability in the ash and which produces
a high performing masonry unit that is both economically and environmentally sustainable.
This work presents two key contributions in service of this goal. Firstly, the physical, chemical,
and mineralogical properties of three different boiler ashes are characterized to assess their
suitability for alkali activation. Second, a robust mix is developed and the mechanical properties
of the resulting products are studied.
The boiler ash has many undesirable characteristics for alkali activation, including varying shape,
large particle sizes ranging from 5-600 micron, loss on ignition between 8-35%, and less than 4%
alumina. However, when combined with supplementary materials in the form of clay and lime,
high compressive strengths are observed in the bricks made with all three ashes, demonstrating the
robustness of the mix design. The final brick formulation with a solids phase of ash/clay/lime =
70/20/10, liquid to solid ratio = 0.45, and NaOH concentration = 2M produced bricks with
compressive strengths between 11-15 MPa after 28 days curing at 30'C. Furthermore, early
strength development is observed as more than 55% of the 28 day strength is achieved after one
day curing.
Thesis Supervisor: John Ochsendorf
Title: Class of 1942 Professor of Civil and Environmental Engineering and Architecture
Acknowledgements
I first want to thank the MIT Tata Center for Technology and Design for allowing me to embark on
this amazing adventure. Without their generous support, this work would have never been possible.
A special thanks to my advisor, Professor John Ochsendorf, for providing me with the opportunity to
join the Tata Center. I could not have asked for more out of this experience. Your guidance over the
last two years has been invaluable, and I feel my personal growth as a researcher and as a person is a
product of your wisdom.
My additional advisors have been instrumental in furthering this research in a number of ways. I thank
Professor Hamlin Jennings for his creative and brilliant ideas, his persistent positive attitude, and most
importantly his passion for the project. I thank Professor Elsa Olivetti for her knowledge and wisdom,
for her commitment to the project, and for furthering the project with her many connections. I thank
Professor Charlie Fine for establishing this project and challenging our team to think about the business
aspect of the project. I also want to thank Professor Rob Stoner for his insight into implementing our
technology. This project has progressed more than I could have imagined thanks to the productive
group dynamic and individual contributions of each advisor, and I greatly appreciate everyone's
dedication.
I can't begin to express my gratitude to my mentor and friend, Dr. Thomas Poinot. He was the
mastermind behind much of the work on this project and the hundreds of papers he read helped guide
our team in the right direction. I especially want to thank him for mentoring me in a new and unfamiliar
field, for building my confidence as a researcher, and for making the work fun even during the times
of unexpected and unfortunate results.
I would also like to thank members of the MIT staff for their assistance in my research. First, Kathleen
Ross, for always taking care of anything I needed. Patrick Boisvert and Charlie Settens for their
assistance in the lab. Finally, Stephen Rudolph, for providing us with space to work and for helping
to fabricate our brick molds.
I'd like to acknowledge a couple more members of the Tata Center. First, Mohit Kansal, who provided
guidance during the 100K and was a huge help during our trips to India. Ben Miller, for providing our
project with exposure to the world. Finally, Chris Porst, for being a great roommate, officemate,
teammate, and friend and for making the last two years a lot more fun.
I am grateful to our partners in India who have been gracious hosts and provided invaluable knowledge
to our project. In particular I'd like to thank Mr. Pankaj Aggarwal, Development Alternatives, and the
National Metallurgical Laboratory.
Most of all I'd like to thank my loved ones for always supporting me and believing in me. Mom and
dad, you've always put the best interest of your children first and instilled in us the necessary values
to be successful. We are a product of your upbringing and you should be proud of yourselves, because
I know I am. My sister, Jen, who is always there to talk and has grown so much as a person over the
last few years, I'm so proud of you. And of course my fiance, Maggie Jacques, who has always
challenged me to be the best I can be and to step outside my comfort zone and try new things. Without
your encouragement and belief in me this chapter of our life would have never been written.
Contents
I
Introduction .........................................................................................................................
1.1
Population Growth and the Demand for Building M aterials ......................................
12
1.2
Industrial Developm ent and W aste Generation ..........................................................
15
1.3
Waste in Building Materials - Existing Solutions and Limitations ............................
18
1.4
2
1 .3 .1
F iring .......................................................................................................................
18
1.3.2
Cem enting ...............................................................................................................
19
1.3.3
Alkali Activation..................................................................................................
19
Thesis Outline ................................................................................................................
Literature Review - Alkali Activation Technology ......................................................
20
21
2.1
Historical Background and Developm ent ...................................................................
22
2.2
Definition and Term inology ........................................................................................
23
2.3
Reaction M echanism s and Products Form ed ............................................................
25
2.3.1
Low calcium alkali activated materials (or 'geopolymers') ................
25
2.3.2
High calcium alkali activated materials..................................................................
27
2.4
3
11
Barriers to Implem entation.........................................................................................
27
2.4.1
Fundam ental Understanding ...............................................................................
27
2.4.2
Long Term Durability Results ................................................................................
28
2.4.3
Standards and Testing Procedures .....................................................................
28
2.4.4
Developm ent of Suitable Adm ixtures.................................................................
29
2.4.5
Raw M aterials Sources .......................................................................................
29
2.4.6
Handling of Alkaline Source ..............................................................................
29
2.4.7
Custom er Acceptance ..........................................................................................
30
2.5
Existing Commercialized Products ............................................................................
30
2.6
Research Objective......................................................................................................
31
Boiler Ash Characterization..........................................................................................
33
3.1
Desired Properties of Boiler Ash .................................................................................
34
3.2
M ethodology ..................................................................................................................
37
-7-
3.2.1
M aterials Procurem ent ........................................................................................
37
3.2.2
Characterization Techniques................................................................................
37
3.3
Results ............................................................................................................................
3.3.1
Physical Properties...............................................................................................
39
3.3.2
Chem ical Properties.............................................................................................
39
3.3.3
M ineralogical Properties......................................................................................
42
3.4
4
D iscussion ......................................................................................................................
M echanical Properties of Products Form ed .................................................................
4.1
M ethodology ..................................................................................................................
43
49
50
4.1.1
M aterials Procurem ent .........................................................................................
50
4.1.2
Sam ple Preparation.............................................................................................
50
4.1.3
Sam ple Testing....................................................................................................
51
4.2
D evelopm ent of Brick Form ulation ...........................................................................
51
4.3
Robustness of Brick Form ulation................................................................................
53
4.3.1
Compressive Strength...........................................................................................
53
4.3.2
Durability................................................................................................................
54
4.4
5
39
Discussion ......................................................................................................................
Conclusions and Future W ork .........................................................................................
55
61
5.1
Conclusions ....................................................................................................................
62
5.2
Future Work ...................................................................................................................
63
A ppendix A
-
A dditional Experim ental R esults..................................................................
67
Supplem entary Materials ....................................................................................................
68
Ash Content ..........................................................................................................................
69
M olar Concentration and Curing Tem perature..................................................................
70
Liquid to Solid W eight Ratio.............................................................................................
71
A dditional W aste Products ...............................................................................................
73
M ixing Tim e and Consistency of M ixture.........................................................................
75
Prem ixing ..............................................................................................................................
76
-8-
Dry versus W et Clay.............................................................................................................
78
Appendix B - Im proving W ater Absorption........................................................................
79
Appendix C - Environm ental and Econom ic Im pact...........................................................
83
Environm ental Impact ...............................................................................................................
84
Economic Impact and Im plem entation Strategies.................................................................
87
Appendix D - Detailed Procedure for Sample Preparation ...............................................
93
References....................................................................................................................................
97
-9-
-
10-
CHAPTER 1
Introduction
-
- 11
M. LARACY 1 2015
1.1 Population Growth and the Demand for Building Materials
India is the home to over 1.26 billion people, making it the second most populous nation in the
world. In the coming decades, population growth in India is expected to rise and studies predict
India will surpass China for the largest population by 2030 (Chandramouli 2011; "India IData"
2014; James 2011). It is anticipated that this spike in population will increase the demand for
buildings and other infrastructure at a growth rate of 6.6% per year between 2005 and 2030, thus
multiplying India's housing stock by five times its current value (Maithel and Uma 2012).
Furthermore, India's urban population which is projected to be more than 50% of the total
population by 2050 (see Figure 1.1) is going to make the demand for materials such as steel,
concrete, and masonry substantial, as they are the primary construction materials used in urbanized
areas.
While concrete and steel are becoming increasingly popular in urbanized areas of India, the
traditional clay fired brick still remains the most used building material in India ("World Bank to
Revolutionise Brick Making in India" 2006).
Accounting for approximately 11% of global
production, India's brick industry produces over 200 billion bricks per year and generates revenues
of over 5 billion US dollars annually (Maithel and Uma 2012). The clay fired brick is primarily
used in load bearing masonry or as infill for reinforced concrete frames, but can also be seen in
walls and road construction. The mass appeal for these bricks comes from their local availability
and most importantly their low cost (Yadav 2015). The price ranges anywhere from 3.00-4.00
rupees per brick in Northern India and 4.50-8.00 rupees in southern and western India where poor
soil quality leads to less brick making and higher transportation costs (Maithel and Uma 2012).
Despite the clay fired bricks long standing dominance of the building material industry, a number
of environmental and social concerns surrounding its production have raised concern about its
future use.
- 12-
CHAPTER 1: INTRODUCTION
-
1,800,000,000
-
1,600,000,000
-
1,400,000,000
1,000,000,000
-
-
1,200,000,000
-
-
400,000,000
200,000,000
-
600,000,000
-
800,000,000
0
1955 1960 1965 1970 1975 1980 1985 1990 1995 2000 2005 2010 2015 2020 2025 2030 2035 2040 2045 2050
0 Total Population
i Urban Population
Figure
1.1:
Expected population and urbanization growth in India
(Adaptedfrom http://www.worldometers.info/world-population/india-population/)
The brick manufacturing industry is extremely energy intensive and highly dependent on natural
resources such as clay, coal, and sand. Much of the outdated technology used in this industry is at
fault for these problems, and can also be blamed for the significant air pollution associated with
the industry ("Eco Brick" 2012). The combustion of coal and biomass (see Figure 1.2a) to fire the
bricks at high temperatures of 700-1 100C creates air pollution (see Figure 1.2b) in the form of
carbon dioxide, carbon monoxide, sulphur dioxide, nitrogen oxides, black carbon, and particulate
matter (Maithel and Uma 2012). It is estimated that more than 24 million tons of coal are
consumed each year producing over 42 million tons of CO 2 emissions ("Eco Brick" 2012). In
addition to the issues with coal consumption and air pollution, another pressing environmental
concern associated with the brick making industry is the degradation of 600 million tons of topsoil
each year to make the bricks (see Figure 1.2c) ("Alternative Building Materials" 2015). According
to local sources on the ground in India, the government has attempted to place a restriction on top
soil use which limits the depth of excavation, but a lack of enforcement has allowed brick kiln
owners to ignore this law. Additionally, these kilns have a lifetime of about 10 brick making
seasons, after which the site is abandoned and remains unusable due to the high temperatures at
which the land is subjected too (Status and Development Issues of the Brick Industry in Asia 1993).
These two issues related to top soil depletion may pose a future threat for food security in India,
as the amount of irrigable land continues to decrease ("Eco Brick" 2012).
-
- 13
M. LARACY
1 2015
Figure 1.2: Negative impacts of the clay fired brick a) energy consumption
b) air pollution c) top soil depletion d) working conditions
Beyond these environmental concerns stems another dimension of social problems (see Figure
1.2d). Each year nearly 10 million workers migrate their families from poorer regions of the
country to these brick kiln clusters. For the six to seven months that the industry is running (brick
kilns do not operate during the rainy season) these men, women, and children are subjected to
inhumane working and living conditions (Yadav 2015). Often these families are offered no shelter
and a lack of access to clean drinking water and sanitation (Maithel and Uma 2012). Inhalation of
irrespirable suspended particulate matter from all the air pollution is one significant health problem
these workers face (Jitendra 2015). Furthermore, women are forced to carry bricks to the kiln by
head load leading to serious neck issues. During a recent trip to a brick kiln in Hyderabad the BBC
news reported seeing pregnant women working 12-18 hour days and four year olds hitting each
other with coal in order to break it up. At a rate of $2.50 per 12 hour day, many protesters consider
the labor to be slave work and are now labeling the bricks they make as "blood bricks" (Hawksley
2014). These problems suggest there is a great need for automated machinery to change the way
bricks are produced and to improve the quality of life of these workers.
Thus far the brick industry's importance in the livelihoods of the poor and the low cost of these
bricks have outweighed the environmental and social concerns regarding its production. However,
clay fired bricks are not a sustainable solution for the future. If the demand for housing continues
to rise as predicted, there may come a time when the supply of top soil cannot meet this demand.
After all, top soil is a limited natural resource. The government of India is aware of the need for
a change and is seeking more environmentally friendly building materials.
14
-
-
CHAPTER 1 : INTRODUCTION
1.2
Industrial Development and Waste Generation
In parallel with the growing population has been a growth in industry. This rapid industrialization
in India is resulting in the generation of huge quantities of unused industrial byproducts, provoking
researchers to identify ways of converting these wastes into resources. These industrial wastes,
which are both solid and liquid, are generated in industrial sectors such as pulp and paper, sugar,
steel, mining, fruit and food processing, starch, distilleries, dairies, tanneries, etc. Despite the
government's requirements for pollution control, much of these wastes are dumped in landfills or
local bodies of water without any treatment, creating a number of environmental hazards (Pappu
et al. 2007; "Industrial Waste Generation and Management in India" 2012). Among the different
wastes generated, one which has not yet found any practical application is boiler ash.
Boiler ash is a byproduct generated during the combustion of raw materials to produce energy at
small to medium sized factories. Many of these factories are forced to produce their own energy
in this way as they operate 24/7 and the electricity in India is not always reliable (Black 2014).
The raw materials that these factories use are constantly changing as they aim to use the cheapest
materials on the market. Some of the materials burned include petroleum coke (petcoke), coal,
and biomass in the forms of rice husk, bagasse, mustard straw, and wood chips. The continuous
changes in the quantity and quality of raw material sources, produces ash with high variability in
its physical and chemical properties. Also, the inefficiency of the boiler where the raw materials
are combusted produces ash with a large amount of unburnt material. These two issues have
prevented boiler ash from finding any practical application. Therefore, all of this boiler ash is
being dumped into landfills (Figure 1.4) or disposed of illegally which wastes valuable farmland
and poses serious hazards to both the environment and human health. Furthermore, landfilling
this ash comes at a large expense to factory owners who need to purchase land, transport the ash
to the site, and finally wet and level the ash. A complete flowchart describing boiler ash production
is shown in Figure 1.3.
-
- 15
M. LARACY
J 2015
Boiler
Byproduct: Ash
Lancilling
Figure 1.3: A flowchart depicting how boiler ash is generated and disposed
The differences between boiler ash and fly ash should be clearly stated. Although their production
process is similar, fly ash is a byproduct of combustion of coal only. Also, fly ash is typically
produced at large thermal power plants and in massive quantities. Furthermore, approximately
50% of fly ash is already being utilized in India, with the majority being in the cement and concrete
industry (Bhattacharyya et al. 2012), making it a more difficult market to enter.
Due to boiler ashes small scale local production in comparison with large thermal power plants
that produce fly ash, there has been low interest among entrepreneurs to utilize it, despite its large
collective impact.
If we focus on just one industry generating boiler ash, paper mills, there are
approximately 800 spread throughout India.
According to local sources, each of these are
producing between 25-100 tons per day for a combined yearly production of between 10-30
million tons. This is enough ash to cover the entire city of Cambridge, MA at least 2 feet deep!
While it is possible that boiler ash is being utilized somewhere, a thorough literature review
revealed no papers on this topic, presenting a great opportunity to identify an application.
-16-
CHAPTER 1 : INTRODUCTION
Figure 1.4: A landfill of boiler ash in the city of Muzaffarnagar, Uttar Pradesh, India.
- 17-
M. LARACY 1 2015
1.3 Waste in Building Materials - Existing Solutions and Limitations
A good way to solve the two problems mentioned above is by using industrial byproducts as a raw
material or aggregate in building materials. Despite the complexity of utilizing these byproducts
due to their often heterogeneous characteristics, a lot of progress has been made over the years.
Solid wastes from organic, inorganic, hazardous, and non-hazardous sources have been recycled
to produce cement, concrete, bricks, tiles, ceramics, polymer composites, as well as a number of
other building materials (Pappu et al. 2007). Zhang did a critical review which focused on ways
that waste materials could be used in bricks (Zhang 2013). He suggests there are three general
strategies for producing masonry with industrial byproducts: firing, cementing, and alkali
activation.
1.3.1
Firing
This method follows the same procedure necessary for the traditional clay fired brick, the only
difference being a partial substitution of clay with the industrial waste. Some wastes that have
been investigated using this technique include fly ash, biomass ash, slag, waste marble powder,
kraft pulp production residue, among others. For a complete list of studies performed using these
methods over the last 20 years please refer to the following critical review (MunFoz Velasco et al.
2014).
The substitution percentage in these studies ranges from 0-100% and tests typically
included compressive strength, water absorption, and bulk density, although other tests were
performed in isolated studies. In general the results showed that as the substitution percentage of
the waste increased, the compressive strength and bulk density decreased while the water
absorption increased. Similar statements were made from owners of brick kilns in India who tried
to substitute boiler ash in their bricks but were observing cracking and a decrease of strength.
Apart from the negative effects on the bricks mechanical properties, this technique does not have
a significant environmental benefit. The bricks still need to be fired at high temperatures using
traditional kiln technology, thus the energy consumption and air pollution are equal to that of
traditional clay fired brick production (Zhang 2013). The only environmental savings come from
the reduction in top soil depletion and avoidance of landfilling the waste. Furthermore, the cost
of transporting the waste to the brick kiln has prevented large scale commercialization of this
technology.
18
-
-
CHAPTER 1 : INTRODUCTION
1.3.2
Cementing
The use of a kiln for high firing temperatures can be avoided by producing cementitious bricks
using waste materials. Depending on the waste source it can either be used alone or mixed with
ordinary Portland cement (OPC) or lime to form calcium-silicate-hydrates (CSH), which give the
compound its high strength (Zhang 2013). One of the more popular cementing technologies named
the "Fal-G" brick to easily describe its constituents, uses fly ash, lime, gypsum, and sand in its
production. The lime and gypsum can come from mineral sources or be procured from industrial
wastes. When the lime and gypsum are taken directly from mineral sources it can be difficult to
produce a brick that is cost competitive with the clay fired brick. Thus this technology's success
often depends on its ability to use waste lime and phosphogypsum, a by-product of phosphoric
acid production (Kumar 2002). Additional energy demands for this technology can sometimes
come from the need to use vibration or a hydraulic press to mold the bricks and from autoclaving
the bricks. The potential for success of this technology comes when all raw materials are all in
close proximity, minimal treatment of wastes are required, and production techniques are not
costly. However, without all these factors working in sync, the costs associated with transportation
and treatment can cause the costs of the bricks to be substantially higher than the clay fired brick
(Kumar 2002).
Furthermore, when cement is used in the mix design, the carbon footprint
associated with the bricks is greatly increased (Komnitsas 2011). In these cases, the environmental
benefit is reduced due to the impacts of using cement.
1.3.3
Alkali Activation
Alkali activation, claimed to be the green building material of the future (Zhang 2013), is a
technology that depends on the chemical reaction between amorphous alumina and silica rich
solids and an alkaline activator (Provis and van Deventer 2014). Waste products are typically used
as the aluminosilicate solid, although additional materials can be added if the waste is lacking in
silica or alumina. The alkaline activator is generally a highly concentrated aqueous solution of
alkali hydroxide, silicate, carbonate, or sulfate (Provis 2013). This strategy uses a low energy
process for making masonry allowing the bricks to gain strength at ambient temperature as
opposed to the high firing temperatures of clay fired bricks. Furthermore, depending on the raw
materials, this strategy can exhibit several advantages over the other methods such as rapid strength
development, fast or slow setting, acid resistance, fire resistance, and low thermal conductivity
-
- 19
M. LARACY 1 2015
(Duxson, et al. 2006). Although global commercialization of alkali activated materials has yet to
occur, leaders in the field believe that with further research, this technology has the potential for
wide scale utilization in the construction industry (Duxson, et al. 2006).
Due to the increased interest in the field of alkali activation and its anticipation as a green building
material of the future, the work in this thesis will focus on this strategy rather than the other
techniques of firing and cementing. More details on alkali activation technology can be found in
Chapter 2 which presents an in-depth literature review on the subject.
1.4 Thesis Outline
In order to solve the problems with boiler ash generation and the impacts of the clay fired brick,
this thesis seeks to determine if boiler ash can be used as a raw material in the production of high
performance, low cost, and environmentally friendly masonry using alkali activation technology.
Chapter 2 presents a thorough literature review on alkali activation technology including its
historical background, definition and terminology, reaction mechanisms and products formed,
barriers to implementation, and commercialized products. Chapter 3 seeks to characterize the
physical, chemical, and mineralogical properties of the ash to assess its suitability as a raw material
for alkali activated masonry. Chapter 4 looks at the experimental work performed and discusses
the pathway to the brick formulation and the mechanical properties of the products formed.
Finally, Chapter 5 summarizes the findings from this work and suggests areas for future work.
Further work is available in the appendices. Appendix A presents the findings of experiments on
individual variables that were essential in shaping the brick formulation. Appendix B explains the
work done to try and improve the water absorption in the bricks.
Appendix C assesses the
environmental and economic tradeoffs between alkali activated bricks and traditional clay fired
bricks, and also looks at strategies for implementation in the Indian market. Appendix D is a
detailed procedure for preparing samples in order to ensure repeatability in future work.
20
-
-
CHAPTER 2
Literature Review - Alkali Activation Technology
This chapter presents existing work in the field of alkali activation as a method for producing
binders. It looks at the history and development behind this technology as well as a clarification
in terminology.
Subsequently, it examines the different reaction mechanisms and resulting
products that are formed. Next, the barriers that are limiting the implementation of alkali activation
technology will be examined, followed by a review of existing commercialized products. Finally,
the objectives of this work will be presented, highlighting the contributions that this thesis will
-21
-
make to the field of alkali activation technology.
M. LARACY 1 2015
2.1 Historical Background and Development
The inception of alkali activated materials in 1908 was a result of the work done by Hans Khl
who established a patent after he combined slag with an alkali source to form a hardened material
"fully equal to Portland cement". Major development of alkali activated binders continued in the
1940's when Purdon tested more than 30 combinations of slag and sodium hydroxide, comparing
their properties with Portland cement. He found that the alkali activated binders had comparable
compressive strength, but also increased tensile and flexural strength. Furthermore, he noted the
potential problems with commercializing these materials due to the difficulties in handling the
alkaline solution, an issue that is still relevant today (Provis and van Deventer 2014). In the 1960's,
Glukhovsky made a significant breakthrough in the development of binders which he called "soil
cements." These binders were made from an alkaline solution and aluminosilicate precursors
which were low in calcium or had no calcium (Shi et al. 2011). About 20 years later, Davidovits
revitalized the field of alkali activation after he developed and patented binders made from
metakaolin which he termed "geopolymers" (Pacheco-Torgal et al. 2008). A full list of important
contributions to the field of alkali activation between 1939 and 1985 can be seen in the following
review (Roy 1999).
Following the work of Davidovits, research on alkali activation and geopolymers through the 80's
and 90's was steady with no significant growth. It wasn't until the last decade that an exponential
growth in research was seen, ultimately as a result of the shift towards alternative binders that can
alleviate the carbon emissions associated with the cement and concrete industry (Bernal and Provis
2014). Alkali activated binders, including geopolymers, are at the forefront of this transition as
literature from life-cycle studies estimate a savings in CO 2 emissions between 30-80% in
comparison with OPC concretes (Van Deventer et al. 2010; Provis and van Deventer 2014). This
exponential growth in research can be seen in Figure 2.1 which shows the number of peer reviewed
papers published over the last 20 years under the keywords "alkali activation" or "geopolymers"
and "building materials". Close to 60% of the papers published during this time occurred during
the last 3 years alone.
22
-
-
CHAPTER 2: LITERATURE REVIEW
300
272
269
Engineering Village Keyword Search:
250
["Alkali Activation" OR "Geopolymers"]
AND "Building Materials"
205
200
-,
A150
131
9
90
C1
-
100
62
50
40 32 48
1 1
-
4 8
2 13
-
1 1 5
0
72
1994 1996 1998 2000 2002 2004 2006 2008 2010 2012 2014
Year
Figure 2.1: A histogram shows the increased interest in alkali activated materials over the last 20 years
2.2 Definition and Terminology
An issue surrounding the alkali activation research community is a lack of clear nomenclature for
the description of these materials. In addition to "alkali activated", these materials have also been
described in academic literature as 'geopolymers', 'mineral polymers', 'inorganic polymers',
'inorganic polymer glasses', alkali-bonded cements', 'alkali ash material', 'soil cements', 'soil
silicates', 'SKJ-binder', 'F-concrete', 'hydroceramics', zeocements', zeoceramics', and a number
of other names. This leads to confusion among researchers, particularly those who are not highly
knowledgeable in the field, and also makes researchers more susceptible to not finding important
papers when performing simple keyword searches (Van Deventer et al. 2010; Provis and van
Deventer 2014).
Two of the more popular names being used are inorganic polymer and
geopolymer. It should be clarified that inorganic polymers are a subset of alkali activated materials
and geopolymers are a smaller subset within inorganic polymers. The differences lie in the amount
of available reactive alumina and calcium, the amount of alkali content, and the resulting silicate
structure that is formed. A more clear distinction can be seen in Figure 2.2 which shows a
simplified view of the chemistry of these different binder systems. The classification of binders
as alkali activated materials, inorganic polymers, and geopolymers is described in more detail
below.
23
-
-
M. LARACY
| 2015
Portlandbased
cements
C
C
0
M
C
C
Increasing Al content
Figure 2.2: Simplified schematic of alkali activated binders and their subsets with comparison to OPC. Shading
corresponds to alkali content with darker shading being higher concentrations (Van Deventer et al. 2010).
Alkali activated materials
Alkali activated binders make up the broadest classification, and include any materials made
from the reaction between an alkaline salt and a solid silicate powder. The alkaline salt can be
a hydroxide, silicate, carbonate, sulfate, aluminate, or oxide just as long as it has the ability to
dissolve the solid and raise the pH of the reaction mixture. The solid precursor, which is often
an industrial by-product, can be an aluminosilicate such as fly ash, or a calcium silicate such
as blast furnace slag (Provis and van Deventer 2014; Provis 2013). The silicate structure that
is formed depends on the solid precursor, and will be chain like in the presence of calcium
silicates or network like in the presence of aluminosilicates.
Inorganic Polymers
As a subset of alkali activated materials, inorganic polymers generally have more alumina than
calcium and also a higher concentration of alkaline activator.
Dissolution of the solid
precursors can be done with a silicate, hydroxide, or carbonate, but typically not a sulfate.
Also, the silicate structure of inorganic polymers is more highly cross linked (Van Deventer et
al. 2010).
Geopolymers
24
-
-
CHAPTER 2: LITERATURE REVIEW
Geopolymers are a further subset of inorganic polymers in which the solid precursor is
predominantly aluminosilicate with little to no calcium. Activation of the precursors typically
requires a strong alkaline concentration generally in the form of a hydroxide and/or silicate.
Furthermore, the silicate structure in geopolymers is a very highly coordinated 3D network
(Van Deventer et al. 2010).
As can be seen above, the broad classification of alkali activated materials have very diverse
chemistry, which are primarily dependent on the amount of calcium content in the solid precursor
and greatly influence the structure of the product formed. Therefore, the reaction mechanisms and
products formed will be discussed in two categories based on calcium content. The categories are
low calcium alkali activated materials, such as geopolymers, and high calcium alkali activated
materials.
2.3 Reaction Mechanisms and Products Formed
2.3.1
Low calcium alkali activated materials (or 'geopolymers')
The reaction mechanism of alkali activated materials that primarily contain aluminosilicates and
have low amounts of calcium was first modeled in the 1950's by Glukhovsky. His process showed
concurrent reactions of destruction, coagulation, condensation, and crystallization (Provis 2013).
Since then, the model has been expanded and refined based on accumulated knowledge regarding
zeolite synthesis as a process of dissolution, rearrangement, condensation, and resolidification (Li
et al. 2010).
This transformation of a solid aluminosilicate precursor into a synthetic alkali
aluminosilicate is commonly referred to as geopolymerization and a simplified conceptual model
can be seen in Figure 2.3.
25
-
-
M. LARACY | 2015
AluminosiNcate Source
I
KewDhwssolon
I OeZ4--mHO
Aluminata & Silicate
Zqullbrlum
GOMM
Reorganization
G11
?*
Ge
and Hardening
Figure 2.3: Conceptual Model for alkali activation of aluminosilicate (Duxson et al. 2007)
Although Figure 2.3 shows geopolymerization as a linear process, a number of these steps occur
simultaneously. In the first step, the presence of a high pH from the alkaline source leads to the
dissolution of the solid aluminosilicate precursor by breaking down the covalent bonds Si-O-Si
and Al-O-Si into monomeric form. While in an aqueous phase, these monomeric precursors form
aluminosilicate oligomers. Then, through the process of condensation, water that was used for
dissolution of the solid precursor is released, allowing the aqueous oligomers to create large
networks in the form of a gel. Following gelation, the network is further developed as the system
continues rearranging and reorganizing.
In the end, a highly cross linked 3D network is
established. The product, or geopolymeric gel, that is formed is a sodium aluminosilicate hydrate
also known as NASH (Duxson et al. 2007).
26
-
-
CHAPTER 2: LITERATURE REVIEW
2.3.2
High calcium alkali activated materials
The conceptual geopolymerization model used to describe the reaction formation of low calcium
alkali activated materials is not applicable when a large amount of calcium content is present.
Although no detailed reaction path models have been published, the reaction products of blast
furnace slag (BFS) have been studied using thermodynamic (Lothenbach and Gruskovnjak 2007),
stoichiometric (Chen and Brouwers 2007), and gel nanostructural models (Myers et al. 2013). A
number of studies looking to identify the main reaction product of BFS have found that it is a
calcium aluminosilicate hydrate, known as CASH (Provis and van Deventer 2014), however other
studies suggest that two reaction products can coexist within the binder (Yip et al. 2005; Alonso
and Palomo 2001). They found that calcium silicate hydrate (CSH) gel, the main reaction product
in Portland cement, could coexist with NASH when the concentration of the alkaline source was
low.
2.4 Barriers to Implementation
While alkali activation has been recognized as the green technology for future building materials,
it still has a number of obstacles to overcome before its commercialization can be seen on a global
scale. The challenges alkali activation technology faces are listed below.
2.4.1
Fundamental Understanding
The exponential increase in research surrounding alkali activation has brought much recognition
to the field, however many of the publications do not present results that will drive commercial
uptake. These studies have proven particular mix designs work, but do not offer full understanding
of how the reaction mechanisms, gel chemistry, and binder microstructure relate to the
macrostructure and durability of the products formed (Provis 2013; Bernal and Provis 2014). The
issue with these results stems from the fact that they are often using a waste as the solid precursor
which comes from a specific source and will not be repeatable with other wastes in other locations.
While it is obvious that the last decade has seen a lot of progress in this area, further fundamental
understanding is a key priority in commercializing this technology.
27
-
-
M. LARACY 1 2015
2.4.2
Long Term Durability Results
A main limiting factor in the commercial adoption of alkali activated binders is the limited record
of in-service durability results. It is vital to know how these binders will behave over a long
period of time when exposed to problems like carbonation, acid resistance, sulfate attack, freezethaw, chemical stability, and other forms of degradation (Provis and van Deventer 2014).
Standardized accelerated aging tests have been utilized to try and determine these durability
results efficiently, however, they do not always represent true field conditions. For example,
specimens that are subjected to accelerated drying conditions will often suffer micro-cracking
which will in turn influence durability and strength parameters (Bernal and Provis 2014). Another
issue is that most of the accelerated aging methods are designed to assess the durability of OPC,
which has very different binder and pore solution chemistry, and is therefore not always useful in
accurately predicting long term results (Provis and van Deventer 2014).
Research and
development is needed to provide meaningful modifications to these methods which reflect the
properties of alkali activated binders.
2.4.3
Standards and Testing Procedures
For alkali activated materials to be a cost effective alternative, they typically require wastes to be
used as the solid precursors. However, there are a number of different wastes that can be used and
their varying chemical and physical properties influence the products that are formed.
Furthermore, even the same waste byproducts, whether it is fly ash or slag, can vary based on
location and day to day processing techniques. Therefore, coming up with a set of standards and
testing procedures for alkali activated materials is very challenging and needs to be tailored to the
different wastes, alkali activators, and applications for their use. Drafting such standards is not an
easy process and requires agreement amongst a majority of the committee which may be made up
of industrial manufacturers, trade associations, professional institutions, government, consumer
bodies, academia, education bodies, customers, and certification bodies (Provis and van Deventer
2014). While the primary goal is to ensure a quality product for the end user, many of these parties
are looking out for the best interest of their businesses and so commercial benefits must be present
in order to get such standards approved. Until such a set of standards is created, introducing alkali
activated materials to the market will be a challenge as it creates huge liabilities for engineers and
owners (Provis and van Deventer 2014).
-28-
CHAPTER 2: LITERATURE REVIEW
2.4.4
Development of Suitable Admixtures
The use of admixtures can greatly improve the wet and cured properties of binders, and has been
very effective in OPC concrete. Unfortunately, these same admixtures used in OPC concrete are
generally not effective when mixed with alkali activated binders. For example, superplasticizing
admixtures, which enhance flow behavior while allowing for a lower liquid to solid ratio (L/S),
were found to degrade under high alkaline conditions giving them no effect (Juenger et al. 2011).
This detrimental effect has been the case for the majority of commercially available admixtures,
making research on new admixtures tailored to enhancing the properties of alkali activated
materials a priority.
2.4.5
Raw Materials Sources
The raw materials for alkali activated binders are predominantly waste byproducts that are not
globally available and are constantly changing. This presents a large challenge to those who want
to produce alkali activated materials on a large scale as they cannot be sure of a consistent supply
chain over a long period of time, making investment in a production facility more risky (Provis
and van Deventer 2014). Further challenges come when producers are receiving raw materials
from multiple sources and need to tailor their design to the properties of the raw materials. These
issues may hinder the production of alkali activated materials at large scales.
2.4.6
Handling of Alkaline Source
Issues with handling alkaline solutions have been recognized for a long time, dating back to the
1940's when Purdon saw this as a challenge to implementation. These corrosive solutions continue
to make commercialization difficult, as handling of the solution requires careful training of
employees who may disregard its hazards anyway. A possible way around this problem is to
develop automated machinery that requires no handling of the alkaline solutions, although this
leads to increased costs. Some studies have tried to bypass an alkaline solution altogether by
developing one-part geopolymers which just require the addition of water, similar to OPC
(Hajimohammadi et al. 2008; Feng et al. 2012; Peng et al. 2014). While this may not be the most
pressing issue holding back the commercialization of alkali activated materials, it is certainly
something to consider.
29
-
-
M. LARACY 1 2015
2.4.7
Customer Acceptance
The search for building materials with reduced C02 emissions has raised awareness regarding the
impact alkali activated materials can have on the environment. However, these materials will not
be commercialized solely on their environmental benefits. They must gain acceptance by the end
user, and in order to do this they must offer other benefits whether they be economical or
performance related. This is particularly true in developing countries such as India where local
sources have stated that cost, performance and aesthetics come before environmental impact.
Until a number of these implementation barriers are overcome, global commercialization of alkali
activated materials is still a future thought. Nonetheless, isolated success stories of implementation
need to be recognized and the lessons learned must be shared with those doing cutting-edge
research. With effective communication and collaboration amongst researchers and producers of
alkali activated materials, a path will be carved towards large scale commercialization.
2.5 Existing Commercialized Products
The majority of commercialized products have been implemented in the developed areas of North
America, Europe, Asia, and Australia, where a conscious effort towards reducing greenhouse gas
emissions is in place. In general, it was found that projects utilizing alkali activated materials
sufficiently served their purpose and have not had issues with durability. These success stories are
very encouraging and provide evidence that alkali activation is indeed a viable solution as an
alternative binder.
In developing countries, such as India, there has been far less effort to introduce these
environmentally friendly binders into the market. CSIR- National Metallurgical Laboratory was
the first to implement a pilot plant in India which produced paving blocks from steel slag using
geopolymerisation (Kumar et al. 2012). They have been regularly evaluating the products for
strength and durability and now sell the technology to entrepreneurs in the region. Beyond this
venture, there exists no literature on the implementation of alkali activated materials in India,
making it a sensible market to explore.
-30-
CHAPTER 2: LITERATURE REVIEW
2.6 Research Objective
The objective of this thesis is to use alkali activation technology as a strategy for producing high
performance, low-cost, and environmentally friendly bricks that can be implemented in the Indian
market. The aim is to test the feasibility of using boiler ash as the solid precursor for alkali
activation. This will be a valuable contribution to the field of alkali activated materials, as a
thorough literature review revealed no papers that have been successful using boiler ash to produce
bricks. In order to accomplish this, the thesis has two specific research goals:
*
Characterize the physical, chemical, and mineralogical properties of the boiler ash to
determine if it exhibits desirable characteristics as a raw material for alkali activated
bricks. This work will be presented in Chapter 3.
* Valorize boiler ash as a raw material in alkali activated bricks by studying the mechanical
-31
-
properties of the products formed. Chapter 4 will cover this area of work.
1 2015
-
32
-
M. LARACY
CHAPTER 3
Boiler Ash Characterization
This chapter seeks to characterize boiler ash, which is an important first step in valorizing its use
in alkali activated masonry*. First, the desired properties of the boiler ash will be specified based
on what was discovered in previous literature on fly ash. Next, the materials procurement and
characterization techniques of the ash will be described.
Finally, the results of the physical,
chemical, and mineralogical characterization will be presented, followed by a discussion on the
findings and ways to improve its suitability for alkali activation.
* The work in this chapter was done in conjunction with Dr. Thomas Poinot
-
- 33
M. LARACY 1 2015
3.1
Desired Properties of Boiler Ash
A serious issue hindering the implementation of alkali activated materials is the inability to quickly
and reliably predict the mechanical performance of the products formed based on the properties of
the ash (Provis et al. 2012). A goal of this work is to contribute to this gap by performing a
literature review of the desired properties of the ash and then connecting the characteristics of the
boiler ash to the properties of the products formed. Since boiler ash has yet to be used in the
application of alkali activated materials, the desired properties of the boiler ash will be determined
based on literature of fly ash, which has been successfully used in the production of alkali activated
materials (Shi et al. 2011). It is important to characterize this ash to determine its suitability to be
used alone in alkali activated materials and to determine solutions to improve its suitability. It is
widely agreed upon that the reactivity of the ash will directly impact the final properties of the
product formed (Van Jaarsveld et al. 2003; Diaz et al. 2010). The overall reactivity of the ash is
dependent on a number of factors including the amount of reactive amorphous silica and alumina,
calcium and iron content, unburnt material, particle size, and morphology.
From a chemical standpoint, silica and alumina are the two key components necessary for alkali
activation. The percentages of these components can be determined through a bulk elemental
oxide analysis of the ash using X-ray fluorescence spectroscopy (XRF). However, this only
provides a preliminary indication of the suitability of the ash, as it does not quantify the amount
of reactive and nonreactive components (Rickard et al. 2011).
In order to get a better
understanding of the suitability of the ash, one needs to quantify the reactive amorphous (glassy)
phases and nonreactive crystalline phases in the ash. Amorphous phases are desirable because
they dissolve easier than crystalline phases during the early stages of alkali activation, releasing
more reactive silica and alumina (Diaz et al. 2010). The percentages of amorphous and crystalline
phases present in the ash is dependent on the cooling rate after the combustion process. If the ash
is cooled quickly it will not allow for a high degree of crystal formation, resulting in more
amorphous phases. On the other hand, if the ash is slowly cooled, the result will be a higher
amount of crystalline phases (Fernaindez-Jim6nez and Palomo 2003; Diaz et al. 2010). Typically,
amorphous phases account for 60-90% of the bulk fly ash composition (Chancey et al. 2010), thus
the goal in this work is to have at least 60%. Furthermore, Fernandez-Jimenez and Palomo (2003)
suggest at least 40% reactive silica be available. The presence of this amorphous material in fly
-34-
CHAPTER 3: BOILER ASH CHARACTERIZATION
ash was recognized in the 1950s and 1960s, although attempts to characterize these phases did not
occur until the 1980s (Chancey et al. 2010). Today, the most common technique for determining
the bulk amorphous content is X-ray diffraction (XRD) (Chancey et al. 2010). Through the use of
an internal standard mixed with the ash, a quantitative analysis of the crystalline phase is possible
using Rietveld Refinement. By combining the techniques of XRF and XRD, the percentage of
amorphous silica and alumina can be determined by subtracting the contribution of the crystalline
phases from the XRF data (Chen-Tan et al. 2009).
Additional studies for quantifying bulk
amorphous phases looked at using dissolution techniques that dissolve only the amorphous phases
in the ash (Fernitndez-Jimenez et al. 2006; Chen-Tan et al. 2009). The two key components, silica
and alumina, are often represented as a ratio of Si/Al, a term that is seen often in literature. While
some studies give this ratio based on the bulk composition of the aluminosilicate source, what
really matters is the Si/Al ratio of the reactive phases (Pacheco-Torgal et al. 2008b). FernandezJimenez et al. (2006) reported that ashes with Si/Al ratios of the reactive phases that are less than
2 perform the best.
In addition to silica and alumina, a number of other elemental oxides can be found in ash including
but not limited to calcium, iron, sodium, magnesium, potassium, titanium, and phosphorus.
Among these additional elemental oxides, calcium and iron have been found to have a significant
effect on the alkali activation process. The presence of calcium is beneficial as it leads to more
rapid strength development and also the formation of the reaction products CSH and/or CASH in
addition to NASH (Van Jaarsveld et al. 2003; Yip et al. 2005). Another benefit of calcium is it
allows for a lower concentration of the alkali source (Yip et al. 2005). On the contrary, a high
presence of iron can be detrimental as it has been observed to inhibit the dissolution of the
aluminosilicates during alkali activation (Chen-Tan et al. 2009), and should be lower than 10%
(Fernandez-Jimenez et al. 2006).
The presence of unburnt material in the ash is another critical factor when assessing the ashes
suitability for alkali activation. This can be determined with a simple loss on ignition (LOI) test.
The amount of unburnt material present in the ash is a function of the efficiency of the combustion
process (Diaz et al. 2010). When the boiler does not reach sufficiently high temperatures, complete
combustion of the raw materials does not occur, resulting in unburnt material often in the form of
carbon. This unburnt material is detrimental to the alkali activation process because it is not
35
-
-
M. LARACY
1 2015
reactive and it absorbs the activator solution, requiring the mix design to have a higher liquid to
solid ratio (Fernindez-Jim6nez and Palomo 2003). ASTM C618 requires that the LOI for fly ash
be less than 6%, thus the goal for this work will be to adhere to this standard.
The particle size distribution (PSD) of the boiler ash is generally the most important physical
characteristic influencing the reactivity of the ash (Fernindez-Jim6nez and Palomo 2003). It can
be determined using a simple sieve analysis or with more advanced techniques such as laser
scattering particle size distribution. The combustion process of the raw materials often governs
the PSD, as higher combustion temperatures lead to a finer grain distribution. The benefit of
smaller particles is the resultant higher total surface area, which increases ash reactivity as the
reaction occurs at the particle-liquid interface (Diaz et al. 2010). Furthermore, it is known that
very small particles (<20 micron) tend to have a more amorphous composition, as smaller particles
quench faster than larger particles (Rickard et al. 2011). Research by Fernindez-Jimenez and
Palomo suggested that 80-90% of the particles should be less than 45 micron. They found that
when particles greater than 45 micron were removed from an ash which initially had a high fraction
of coarse particles, the strength of the resultant product nearly doubled. In Australia, standards
require that 75% of the fly ash be less than 45 micron if it is to be used in cement (Rickard et al.
2011). Thus, particle sizes less than 45 micron will be considered the desirable size in this thesis.
The general morphology of the ash is also an important characteristic and can be observed using a
scanning electron microscope (SEM). It is ideal to have spherical shaped particles as it permits
for good workability of the binder at lower liquid to solid mix ratios (Rickard et al. 2011). A lower
liquid to solid ratio is desired because it reduces the amount of alkaline source required which is
generally a major cost in the production of alkali activated materials (Diaz et al. 2010).
Based on the above review of literature on fly ash in alkali activation, the desired characteristics
of the boiler ash are spherical shape (Rickard et al. 2011), more than 75% of the ash less than 45
pm (Rickard et al. 2011), LOI less than 6% (ASTM C 618), iron content less than 10% (FernandezJimenez et al. 2006), reactive silica greater than 40% (Fernandez-Jimenez et al. 2006), reactive
alumina greater than 15%, and total amorphous material greater than 60% (Chancey et al. 2010).
36
-
-
CHAPTER 3: BOILER ASH CHARACTERIZATION
3.2 Methodology
3.2.1
Materials Procurement
Three different boiler ash samples were used for the experiments in this paper. Each sample was
obtained from a different paper mill in the city of Muzaffarnagar, India. Muzaffarnagar is an
industrial city situated in the northwest corner of the state of Uttar Pradesh and is approximately
125 kilometers northeast of Delhi. The three paper mills, Bindlas, Silverton, and Siddhbadi, are
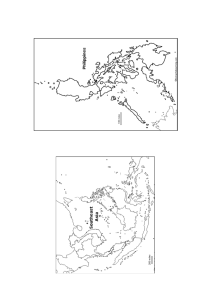
all located within 2 kilometers of each other and can be seen in Figure 3.1. The boiler ash will
often be referred throughout this paper by the name of the paper mill from which it was obtained.
The percentages by weight of raw materials combusted to produce the ashes were reported by the
owners of the paper mills and therefore may not be fully accurate. Bindlas boiler ash was reported
to be 63% bagasse pit, 27% rice husk, and 10% petroleum coke while the Silverton and Siddhbadi
ashes were byproducts of combustion of 100% rice husk.
3.2.2
Characterization Techniques
The work in this thesis utilizes a number of common techniques to characterize the boiler ash
including particle size distribution, X-ray fluorescence, carbon content, loss on ignition, X-ray
diffraction, scanning electron microscopy, and leaching testst. Particle size measurements on the
boiler ash were done in powder form using a Horiba LA920 laser scattering particle size
distribution analyzer.
Each sample was run 10 times to provide better accuracy.
A semi
quantitative elemental chemical analysis was performed according to ASTM D4326 on a dry,
ignited basis via XRF using a Bruker S4 Explorer. The loss on ignition (at 750'C) and density
were performed on a dry sample according to ASTM C3 11. A Leco SC632 Carbon Analyzer was
used to determine carbon content in the ash. XRD data was collected using high speed braggbrentano optics on the PANalytical X'Pert Pro MPD operated at 45 kV and 40 mA. Data was
obtained between 10' and 80' two theta using a step size of .0 1670 with each sample scan lasting
80 minutes. The diffractometer was configured with a 1/2' divergent slit, .04 rad soller slit, and
1 anti-scatter slit. The boiler ash was thoroughly ground by hand for 3 minutes and then packed
into a 27mm diameter sample holder. In order to quantify the crystalline peaks, a scan was run on
t XRF, PSD, LOI, and carbon content were outsourced to Headwaters Resources. Leaching tests were done by
TestAmerica.
37
-
-
M. LARACY
| 2015
the ash alone and on a sample with an aluminum oxide internal standard added at 10% the weight
of the sample plus standard.
Rietveld refinement was used for phase identification of the
crystalline materials using PANalytical HighScore Plus software version 4.1 and the ICDD PDF4+
database. SEM images were taken on a Philips XL30 FEG ESEM to observe the morphology of
the boiler ash particles. Samples were added to carbon tape and then compressed air was used to
remove any particles not firmly secured to the tape. The machine was operated on Hi-Vac at 5 kV
using a spot size of 1 and a secondary electron (SE) detector.
Muzaffarnagar
India
Figure 3.1: The upper three maps present the location of the city of Muzaffarnagar within India. The bottom figure is
a google earth image showing the location of the three paper mills where boiler ash samples were obtained.
-38-
CHAPTER 3: BOILER ASH CHARACTERIZATION
3.3 Results
3.3.1
Physical Properties
The size of the particles for all three ash samples ranged from 5 micron - 600 micron. The Bindlas
and Silverton ashes had nearly identical particle size distributions as seen in Figure 3.2, while
Siddhbadi was slightly larger. The mean particle size for the ashes were 112 pm (Bindlas), 105
gm (Silverton), and 118 pm (Siddhbadi). The goal of having the majority of the particles smaller
than 45 pm was not achieved for any of the boiler ashes as Bindlas and Silverton had 23% of the
particles smaller than 45 ptm and Siddhbadi had only 15% smaller than 45 pm. The shapes of the
particles were also highly variable for each ash as can be seen in the images of Figure 3.2. Hardly
any small spherical particles are visible, which is the desirable characteristic regarding shape.
What can be seen in all three samples is large angular pieces with a rough and bumpy surface.
These pieces are assumed to be rice husk based on their appearance and because they are especially
present in the Silverton and Siddhbadi ash, which is expected since these ashes are derived from
rice husk only (Chaudhary and Jollands 2004; He et al. 2013). In the image of the Bindlas ash one
can also see other types of particles which are likely unburnt carbon.
3.3.2
Chemical Properties
The results from the chemical analysis seen in Table 3.1 found that more than 80% of the ash is
made up of silica, one of the main elemental components of the solid source. The other main
component, alumina, is found in much lower quantities. There exists only 3.87%, 2.80%, and
2.61% of alumina for the Bindlas, Silverton, and Siddhbadi ashes, respectively. By combining
SEM with energy dispersive x-ray spectroscopy (EDS), elemental mapping of the Bindlas ash was
performed, further demonstrating the large presence of silica and lack of alumina (Figure 3.3).
Iron is present in sufficiently low quantities, less than 2% for all ashes. Calcium also has little
presence, representing only 1-3% of the elemental composition for the ashes. Other elements
found include iron (0-2%), sulfur (0-3%), sodium (0-1%), magnesium (1-2%), potassium (3-5%),
phosphorus (1-2%), and titanium (0-1%). The sum of these elements does not equal 100% as other
trace elements which were not tested for are likely present in the ash.
39
-
-
M. LARACY 1 2015
100
*
80
- - -Silverton
70
- - - Siddhbadi
-
-- Bindlas
90
60
C 50
40
C
30
20
W
.
10
0
10
1
100
Diameter (pm)
1000
100
90
=
-
--- Bindlas
80
-
70
-
Silverton
60
w
C
50
LL
40
C
4
I'-
30
A- 20
10
0
1
10
1000
100
Diameter (pm)
100
y
90
- - - Bindlas
80
- - - Silverton
70
-
Siddhbadi
60
50
/1-
u.; 40
C
3
30
0-
20
10
0
1
10
100
Diameter (pm)
1000
Figure 3.2: SEM images of the boiler ash along with their particle size distributions show the large variation in shape and the
range in size from 5 micron to 600 micron.
40
-
-
CHAPTER 3: BOILER ASH CHARACTERIZATION
The loss on ignition value for all three ashes is higher than the allowable limit of 6% for fly ash
prescribed by ASTM C 618. Siddhbadi and Silverton are closest to the target value at 8.75% and
13.1%, but Bindlas has an extremely high value of 34.9%. The results from the analysis of carbon
content show that the majority of this unburnt material is in fact carbon. For Bindlas, 86% of
unburnt material is carbon, followed by Silverton at 82%, and Siddhbadi at 68%. Values for the
density in units of grams per centimeter cubed are 2.23 (Bindlas), 2.49 (Silverton), and 2.58
(Siddhbadi), consistent with values seen in literature (Fermindez-Jimenez and Palomo 2003;
Chancey et al. 2010).
Table 3.1: The elemental composition shows that 80% of the boiler is silica while only 3% is alumina. A loss on ignition
test shows that between 8-35% of the ash was unburnt material, the majority being carbon.
~
AlkOs
Fe~oq
Bindlas
81.9
3.87
1.17
Silverton
82.7
2.80
Siddhbadi
85.2
2.61
_____
O&0
T1%T
N~
(%) (gcmA)
C-0
N*0
449P
Xg
2.84
1.55
0.49
1.24
3.55
1.04
0.20
34.9
30.0
2.23
1.90
0.97
1.45
0.45
1.35
4.68
1.52
0.16
13.1
10.8
2.49
0.79
0.49
2.24
0.59
1.03
3.98
1.06
0.14
8.75
5.97
2.58
A test for leaching of heavy metals on the Bindlas boiler ash was outsourced to Test America.
Testing for arsenic, barium, cadmium, chromium, lead, selenium, and silver were done according
to method 601 OC-Metals (ICP)-TCLP. Another test was done to detect any mercury using Method
7470A-TCLP Mercury. The results concluded there is no lead, silver, or mercury present. The
remaining elements are present with arsenic, barium, cadmium, and chromium being over the
acceptable limits. The presence of heavy metals over the acceptable limits in the Bindlas ash may
be a problem if they leach out as they can contaminate the ground water (Khale and Chaudhary
-41
-
2007).
100 Pm
| 2015
EHT
= 20.00 kV
WD = 10.1 mm
Signal
Mag =
A=
HE-SE2
36 X
TiltAe
0.0'
I Probe =
1 5nA
Date:
5 Dec
2014
Column Mode = Analytic
-
M. LARACY
Figure 3.3: Using EDS, an elemental mapping of the ash was performed which showed a large presence of silica (top
right) and lack of alumina (bottom right).
3.3.3
Mineralogical Properties
Looking at the diffractograms in Figure 3.4 generated through XRD analysis, it is evident that all
three boiler ash samples are similar and have both crystalline and amorphous material.
The
crystalline material is represented by the sharp peaks along the diffractogram and the amorphous
content is shown by the broad hump approximately located between 15 to 30 degrees two-theta.
The crystalline peaks were identified using the ICDD PDF4+ database. The majority of crystalline
materials were silica phases, primarily in the form of quartz, but also present as cristobalite and
tridymite, in agreement with literature (Pays et al. 2001).
Traces of other crystalline phases
included sodium aluminate silicate (albite, sodalite, anorthoclase), potassium sulfate (arcanite),
calcium carbonate (calcite), iron oxide (magnetite), and titanium dioxide (rutile). There were no
traces of crystalline alumina phases, suggesting that the bulk alumina content from the XRF
analysis is primarily amorphous material. Rigorous Rietveld Refinement is under progress to
quantify all the phases, both crystalline and amorphous. A first estimation based on the area of
the peaks in the diffractogram has found that the quantity of amorphous material is 62% for
Bindlas, 66% for Silverton, and 52% for Siddhbadi. From this value, the reactive silica content
can be estimated by subtracting the reactive alumina content from the total amorphous content.
42
-
-
CHAPTER 3: BOILER ASH CHARACTERIZATION
Q: quartz
C: cristobalite
T: tridymite
s: sodium aluminum silicate
c: calcite
a: arcanite
QC s
sTC s
caa
Q
QQsQ
Q
Q
A
20
30
50
40
Q
QC
Q
Q
L
Al AL
10
Q
Q Q
Bindlas
ASiddhbadi
60
70
80
28 ()
Figure 3.4: Diffractograms of the three boiler ash samples are all similar and show the presence of both crystalline and
amorphous phases.
3.4 Discussion
In the previous section, characterization was done on three different samples of boiler ash. A
summary of the results is presented in Table 3.2. In general, it was observed that the ashes do not
have many desirable characteristics to make the ash highly reactive including shape, size, LOI
value, and reactive alumina content. Although the ashes were observed to have similar properties
overall, there were a few key differences. First, the Bindlas ash had a significantly larger LOI than
Silverton and Siddhbadi. Second, the Siddhbadi ash had larger particle sizes than the other two
ashes. Third, the amount of amorphous content and reactive silica varied as Siddhbadi had far less
than Bindlas and Silverton. Apart from these differences, the ashes were found to be relatively
similar especially regarding their chemical analyses and mineralogy. Based on literature, a logical
explanation for this can be reached. It is expected that Silverton and Siddhbadi ashes would be
similar as they are both derived from the combustion of 100% rice husk, but Bindlas ash is derived
from 63% bagasse pit, 27% rice husk, and 10% petcoke. However, literature has shown that 23%
-43
-
of the rice husk that is burned is turned to ash (Della et al. 2002) whereas only 2% of bagasse pit
turns to ash (Montakarntiwong et al. 2013), and combustion of petcoke produces no ash.
M. LARACY | 2015
Therefore, this ash is actually closer to 83% rice husk ash and 17% bagasse pit ash, explaining its
similarities to the other ashes.
The differences observed are more likely due to the differences in the boilers where the raw
materials are combusted. The majority of the crystalline phases observed as quartz suggests that
the boiler was operated at a temperature between 500-900'C. Yet, the presence of cristobalite and
tridymite, which can be crystallized between 870-1470'C and 1470-1720'C (Shinohara and
Kohyama 2004), respectively, indicate that the temperature varies in different locations of the
boiler. Also, the fact that Bindlas has an LOI of 35% likely indicates that this boiler is very
inefficient and low temperatures in locations of the boiler are leading to a large amount of unburnt
material. Regarding the Siddhbadi boiler, the lower quantity of amorphous content may be due to
slower cooling rate of the ash particles which is known to produce more crystalline phases, as
described in Chapter 3.1.
Table 3.2: Results from the characterization of the boiler ash demonstrate its lack of desired qualities thus reducing the
overall reactivity of the ash.
Characteristic
Desired
Bindlas
Silverton
Siddhbadi
Shape
Spherical
Varies
Varies
Varies
Size
75% under 45 [tm
22% under 45 pn
23% under 45 prn
14% under 45 pmn
Loss on Ignition
Less than 6%
35%
13%
9%
Iron Content
Less than 10%
1.2%
1.9%
0.8%
Reactive Silica
More than 40%
~58%
~63%
Content
Reactive Alumina
Content
Total Amorphous
Content
~49%
_________
More than 15%
3.9%
2.8%
2.6%
-66%
~-52%
_________
More than 60%
-62%
I__________________
__________________
Another issue is the amount of reactive silica compared to reactive alumina. As was described in
section 3.1, the Si/Al ratio is of great important and has been shown to be most effective at a value
around 2.
In the case of these ashes, conservatively assuming all the alumina is reactive and the
least amount of silica is reactive, would still yield a Si/Al ratio around 20.
Due to the overall poor qualities of the ash, the use of boiler ash as the only solid source for alkali
activation is not expected to produce a brick with good final properties. Two approaches can be
taken to improve the reactivity of the ash. The first option is to process the ash by means of
44
-
-
CHAPTER 3: BOILER ASH CHARACTERIZATION
fractionation, mechanical activation, or combustion. The second option is to add supplementary
materials to the ash.
The use of fractionation to increase ash reactivity has been widely studied over the last few decades
(Slanieka 1991; Chindaprasirt et al. 2004; Chindaprasirt et al. 2005). This processing technique
can be performed by simply sieving the ash into different particle size ranges and testing for
compressive strength and/or durability. The general conclusion reached by all studies was that the
use of smaller particle sizes lead to an increase in strength and a reduction in the amount of liquid
phase necessary. For example, Chindaprasirt et al. (2004) found that the finest 10% of the fly ash
had a strength of 25 MPa after 3 days, the medium (25%) particles had a strength of 16.5 MPa,
and the coarsest particles (65%) had a strength of only 8.5 MPa. The reasoning behind the increase
in strength with finer particles is the belief that there is more amorphous content in finer particles
than in coarser particles, thus the amount of material readily available for dissolution is increased
(Chindaprasirt et al. 2004). The drawback of this processing technique is the residue of coarser
particles still needs to be disposed of (Kiattikomol et al. 2001) which takes away from the initial
goal of utilizing all of the boiler ash.
The use of mechanical activation to increase ash reactivity has been proven to be another viable
processing technique (Kumar et al. 2007; Fu et al. 2008; Temuujin et al. 2009; Kumar and Kumar
2011). Mechanical activation can be defined as an increase in ash reactivity from the combined
effects of increased surface area and physicochemical changes by means of high energy milling
using a vibratory mill, attrition mill, etc. (Kumar et al. 2007). All studies have shown an increase
in compressive strength with milling, however the explanations for this increase are not fully
agreed upon. Temuujin et al. (2009) determined the main influence of mechanical activation to be
a reduction in particle size and change in shape leading to increased strength, but found no change
in the mineralogical properties of the ash after mechanical activation. On the contrary, Fu et al.
(2008) and Kumar and Kumar (2011) found that the diffractogram of the mechanically activated
ash had lower intensity peaks and wider half peak widths, indicating a higher reactivity than the
original ash. A downside to mechanical activation is it is an extremely energy intensive process
as optimal grinding time has been found to be 45-50 minutes (Fu et al. 2008) which can lead to
increased production costs for the bricks.
45
-
-
M. LARACY | 2015
100
g0
425-600 pm
z80
150-180 pm
670
060
50
40
<63pm
S30
20
10
0
1
10
100
Diameter (<p)
1000
< 63 pm
LOI= 20%
150-180 Pm
LO = 29%
425-600
pm
LOI = 50%
Figure 3.5: Bindlas boiler ash was sieved into three sizes and tested for LOI. The images on the left were taken with
SEM and the images on the right are of the ash after combustion.
The third processing technique for the boiler ash, combustion, would require the ash to be fully
burnt to reduce the loss on ignition to zero. As was stated earlier, unburnt material is not reactive
and absorbs the alkaline source, so ideally by reducing the LOI one would be increasing the overall
reactivity of the ash. From the results on LOI, Bindlas boiler ash has the highest value at 34.9%
which is significantly higher than the other two ashes. Therefore, it was a goal to determine what
was causing there to be so much unburnt material. A first hypothesis was that the larger particles
may have a larger LOI than the smaller ones. This was tested by sieving the ash to different ranges
of size and then testing them for their LOL. Three ranges of particles were tested which included
less than 63 micron, 150-180 micron, and 425-600 micron. It was determined that the particle size
did have an influence on LOI as the particles less than 63 micron had an LOI of 20%, the particles
between 150-180 micron had an LOI of 29% and the particles between 425-600 micron had an
46
-
-
CHAPTER 3: BOILER ASH CHARACTERIZATION
LOI of 50%. Looking at Figure 3.5 of the SEM images (left) and post combustion (right), it is
clear that the different particles also produce very different color ashes after undergoing
combustion. While this technique is effective, it is also an energy intensive process that requires
temperatures around 750'C to fully combust the ash, therefore increasing production costs and
adversely impacting the environment.
Each of these processing techniques has been proven to increase the overall reactivity of the ash,
however they are all associated with environmental and/or economical drawbacks. Despite the
effectiveness of fractionation in increasing strength, it still requires that the coarser particles be
landfilled. The drawbacks of mechanical activation and combustion are the energy inputs required
to perform these techniques as well the costs associated with them. Most importantly, all of these
techniques cannot solve the problem related to the lack of reactive alumina, which needs to be
increased in order to lower the Si/Al ratio. This problem can only be solved with the addition of
supplementary materials. Therefore, this work focuses on the use of supplementary materials
rather than processing the ash.
The use of supplementary materials with an aluminosilicate waste source that is not sufficiently
reactive is known to increase the overall reactivity of the solid source (Provis and Bernal 2014).
The supplementary source can be an additional waste product, natural resource, or synthetically
produced material.
Additional sources of alumina in alkali activated materials often come as
metakaolin (calcined clay) or red mud (Provis and Bernal 2014). In this work, clay was used as
the additional alumina source in its original state, because calcining the clay would be an energy
intensive and costly process. Furthermore, it is locally available throughout India, whereas blast
furnace slag is available in isolated locations and is already heavily utilized in the cement industry
(Provis and Bernal 2014). Additional sources of calcium seen in literature include blast furnace
slag, cement, calcium hydroxide, calcium silicate, calcium carbonate, and class C fly ash (Provis
and van Deventer 2014).
The additional source chosen in this work was calcium hydroxide
(hydrated lime) as it is locally available throughout India and is relatively inexpensive. More
information regarding the selection of these supplementary materials can be found in Appendix A.
With the addition of these two supplementary sources, boiler ash can be used in alkali activation.
This is demonstrated in Chapter 4 where the mechanical properties of the products formed are
studied.
47
-
-
1 2015
- 48
-
M. LARACY
CHAPTER 4
Mechanical Properties of Products Formed
The work in this chapter seeks to understand the mechanical properties of the bricks that are
produce&. First, the methodology is described in relation to the materials procurement, sample
preparation, and sample testing. Next, results from some of the initial experiments performed
during the early part of this work that influenced the final brick formulation are summarized. This
formulation is then tested for its robustness using the three different samples of boiler ash from
Muzaffamagar. The parameters that the bricks are tested for include compressive strength and
durability in terms of water absorption, leaching, and strength loss. The chapter will conclude
with a discussion of the results.
The work in this chapter was done in conjunction with Dr. Thomas Poinot
49
-
-
M. LARACY 1 2015
4.1 Methodology
4.1.1
Materials Procurement
The materials used in these experiments include boiler ash, clay, hydrated lime, sodium hydroxide
pellets, and municipal water. As stated in Chapter 3, the three boiler ash samples were obtained
from the city of Muzaffarnagar, India. It should be noted that the Bindlas ash was shipped overseas
to MIT in a large quantity, whereas smaller quantities of Silverton and Siddhbadi were carried
back to MIT by aircraft. Therefore, all experiments summarized in section 4.2 were performed
using only Bindlas ash. The clay was also obtained from a location nearby the paper mills in
Muzaffarnagar.
Both the boiler ash and clay were placed in an oven at 105'C to remove any
moisture from the specimens. The clay was then ground with a blender and sieved to pass the #35
sieve (0.5 mm) to break up any large agglomerates. The hydrated lime, which contained 92-100%
calcium hydroxide by weight, was a Graymont product obtained from Madigan Lime Corporation
in Ayer, MA. The lime was also sieved to pass the #35 sieve to remove any large agglomerates.
These processing techniques were done in order to ensure better quality control and avoid any
inconsistencies between experiments. Laboratory grade >97% sodium hydroxide pellets were
acquired from Sigma Aldrich Company. Municipal water was used throughout to best represent
field conditions.
4.1.2
Sample Preparation
First, the sodium hydroxide pellets were dissolved in water by stirring until a homogenous solution
was formed. Due to the exothermic reaction, this was done a day in advance to allow the solution
to cool down to room temperature. Next, the dry materials (boiler ash, clay, and hydrated lime)
were weighed, added to a bowl, and mixed at low speed using a Kitchen Aid planetary mixer for
3 minutes to attain a homogenous composition of the solids phase. After, the NaOH solution was
weighed and added to the bowl. This was mixed with the solids phase at maximum speed until
the appropriate homogenous consistency was achieved. The mixture was then transferred into 2"
cubic molds where the samples were cast using tamping and vibration. Samples were cast in two
layers that were each hand tamped and vibrated for 1 minute to compact the mixture and remove
any air bubbles. Samples were then sealed using two layers of plastic wrap to minimize moisture
loss and placed in the oven to cure at 30'C. The temperature was chosen to mimic what the average
50
-
-
CHAPTER 4: MECHANICAL PROPERTIES OF PRODUCTS FORMED
temperature would be like during the summer in Northern India. Samples remained in the oven
up until their designated testing days. A more detailed account of sample preparation can be found
in Appendix D.
4.1.3
Sample Testing
Tests were performed to determine the mechanical characteristics of the bricks made using alkali
activation. Samples were tested for their unconfined compressive strength at 1, 3, 7 and 28 days
using a Baldwin Tate Emery Universal Testing Machine. Samples were prepared such that the
sides in contact with the testing plates were both smooth and parallel. A standard durability test
for water absorption was done according to ASTM C67-14 after 28 days of curing. It should be
noted that this is the test for water absorption of a clay fired brick which requires the sample to be
dried at 11
C for 24 hours before being placed in water. A test was performed to determine the
strength loss at 28 days after being immersed in water for 24 hours. For this test the sample was
removed from the oven after 28 days and placed in 200 mL of room temperature water for 24 hours
after which it was tested for its unconfined compressive strength. The strength of this sample was
compared to the normal sample tested for 28 day compressive strength. The pH of the water that
the brick was immersed in was taken after the 24 hours to determine if there was any leaching of
the lime or sodium hydroxide from the bricks. Three samples were used for all tests to ensure
accuracy.
4.2 Development of Brick Formulation
At the start of this project there were a number of variables to consider related to both the mix
design and the processing techniques. Individual studies were performed on a number of these
variables to determine which had the most influence over the final properties of the brick and
which could be made constants throughout the remainder of the research. A list of the different
variables, along with a brief description and their general result can be seen in Table 4.1. For a
more detailed description of all these experiments please reference Appendix A. It is realized that
these values are not fully optimized, and could be adjusted based on future experimentation.
-
- 51
M. LARACY
Table 4.1
Type
I
2015
A list of the variables studied that helped determine the final brick formulation.
Variable
Supemenary
Materials
Ash Content
Mix
NaOH Molarity
Liquid to Solids
Weight Ratio
Additional
Waste
Curing
BriefDescrption
ResW
Studying the effects of
Clay at 20% and lime at 10% of the
adding clay and lime to the
solids phase are necessary to
mix design
improve strength and durability
Determining maximum
70% ash is the maximum quantity
percentage of ash in the
of ash in solids phase without
solids phase of mix design
sacrificing strength
Finding appropriate molar
With addition of lime as a source of
concentration of the sodium
calcium, a low concentration (2M)
hydroxide
provides highest strengths
Weight of the liquids divided
Dependent on molding technique
(vibration=.45-.6 , pressing=.25-
by weight of solids
.45) and properties of ash
Addition of black liquor,
lime waste, and alum waste
Black liquor as alkali source may
give higher strength. Alum and
in the mix design
lime waste had negative effects
Determining the temperature
Lower temperatures (30'C) give
to cure the samples at
higher final strength than higher
Temperature
Process
Premixing
Dry vs. Wet
Clay
How long the material is
Increased mixing time leads to
mixed for and the change in
higher strength possible due to
consistency of the mix
enhanced dissolution
Looking at mixing particular
Premixing clay or ash may enhance
materials with NaOH first,
dissolution and lead to higher
then adding other materials
strength
Seeing effect of mixing the
Wet and dry clay had similar
raw materials with wet clay
strength results. Wet clay ideal for
versus dry clay
scaling-up, dry clay ideal for lab
-
52
-
Mixing Time
temperatures (1 00 0 C)
CHAPTER 4: MECHANICAL PROPERTIES OF PRODUCTS FORMED
The key findings from this early work that influenced section 4.3 should be highlighted. First, it
is necessary to have supplementary materials to make the boiler ash suitable for alkali activation
and this can be achieved by adding 20% clay as a source of alumina and 10% lime as a source of
calcium. Studies on ash content found the optimum to be 70% without taking away from the
strength of the products formed. Studies of the molarity of the NaOH showed that it is necessary
to have the alkali source as the strength of a sample made with 2M NaOH doubled that of the
sample made without any alkali. The liquid to solid ratio should be around 0.45 for samples made
using vibration. Finally, low curing temperatures (30'C) lead to greater final strength than higher
temperatures. These findings were essential in shaping the brick formulation.
4.3 Robustness of Brick Formulation
After determining the most critical variables influencing the final brick properties and developing
an initial brick formulation, the next step was to test its robustness using the Bindlas, Silverton,
and Siddhbadi boiler ash. For all three samples, the solids phase by weight was 70% boiler ash,
20% clay and 10% lime. The liquid to solid weight ratio was 0.45 for Bindlas ash and 0.46 for
Silverton and Siddhbadi ash. This slight increase was to allow for the mixtures to all have the
same consistency prior to vibration. The concentration of the NaOH was 2M for Bindlas ash and
1.95M for Silverton and Siddhbadi in order to account for the increased liquid to solid ratio while
keeping the alkali content constant.
The results for compressive strength and durability are
presented below, followed by a discussion of the findings.
4.3.1
Compressive Strength
As was stated previously in Section 2.4.3, no standards currently exist for alkali activated materials
making it necessary to use literature and standards related to different types of materials as a
guideline for targeted values. According to Indian Standard (IS): 1077, traditional clay fired bricks
are classified based on their average compressive strength with the minimum allowable strength
being 3.5 MPa. Despite the minimum, the goal of this research was to achieve at least 7.5 MPa, a
target suggested by multiple sources on the ground in India. The results in Figure 4.1 show that
the bricks made with Silverton and Siddhbadi ashes were able to achieve this goal after just 1 day
of curing at 30'C, while the Bindlas ash bricks fell just short at 6.8 MPa. Still, the early strength
development of these bricks was high, reaching 58% (Bindlas), 56% (Silverton), and 73%
-
- 53
M. LARACY
| 2015
(Siddhbadi) of the 28 day strength after 1 day curing. At the end of 3 days curing the average
strength of the bricks for each ash exceeded 9 MPa, with the lower bound of the standard deviations
being all greater than 7.5 MPa. By 28 days curing the bricks were found to be 57% (Bindlas), 94%
(Silverton), and 53% (Siddhbadi) stronger than the target goal of 7.5 MPa.
18
16
M Bindlas U Silverton U Siddhbadi
S14
12
5 10
8
t
....--..-
6
cL4
E
o
2
0
1
7
3
Curing Time (days)
28
Figure 4.1: The compressive strength results show the brick formulation is robust enough to account for all three
ashes, each achieving over 50% of the desired strength (7.5 MPa - shown by dashed line) and exhibiting signs of early
strength development.
4.3.2
Durability
The results from durability testing are summarized in Figure 4.2. According to IS: 1077, the
maximum water absorption for a traditional clay fired brick is 20%, thus the target value for this
thesis has been set to this value. The average water absorption results in this experiment were 33%
for Bindlas, 37% for Silverton, and 40% for Siddhbadi, all of which were above the targeted value.
Repeatability of the water absorption values were good for Bindlas and Silverton, with small
standard deviations of 1%, but were higher for Siddhbadi at 5%. The leachate pH values observed
were nearly the same for each ash, having values of 10.9 for Bindlas, 10.6 for Silverton, and 10.7
for Siddhbadi. Each of these was larger than the goal of 7, which is neutral pH. The strength loss
results at 28 days after 24 hour water immersion were highly variable. The values ranged from
7% reduction in strength for Bindlas to a 25% reduction in strength of the Silverton bricks.
Siddhbadi bricks fell closer to Bindlas losing 10% strength. There was very poor repeatability for
the strength loss of the Silverton and Siddhbadi bricks which had standard deviations of 8% and
16% respectively.
54
-
-
CHAPTER 4: MECHANICAL PROPERTIES OF PRODUCTS FORMED
12
0.9 *
140%
10.6
10
35%
'0. 30%
-5%
10.7
-co 8w
-
0
-10%
-
--
-15%
-
45%
0%
14
50%
-20%
-25%
O 25%
-30 %
-6
20%
-35%
4
15%
-40%
10%
2-45%
5%
-50%
0
0%
Bindlas
Silverton
Bindlas
Siddhbadi
Silverton
Siddhbadi
Figure 4.2: Durability parameters show water absorption values ranging from 33%-40%, pH leachate values close to
11, and a variation in strength loss from 7%-25% with large standard deviations.
4.4 Discussion
The compressive strength results for the bricks made using all three boiler ashes exceeded the
target goal of 7.5 MPa validating the robustness of the brick formulation. Moreover, the lower
bound of the standard deviation was at least 2 MPa higher than the target for all bricks, providing
a comfortable buffer zone to account for variations in the ashes properties. Silverton bricks had
the highest strength and also the best ash properties (highest amorphous content at 62%, low LOI
of 13%), demonstrating the correlation between the two. Bindlas and Siddhbadi bricks both had
slightly less strength than Silverton which can also be explained by their ash properties. While
Bindlas ash did have a high amorphous content of 58%, it's extremely high LOI of 35% likely is
the cause of the reduction in strength as the unburnt material is not reactive. Although Siddhbadi
ash had the lowest LOI at 8.75%, its low amorphous content of 52% and largest particle sizes
likely lead to its reduction in strength compared to Silverton.
The products formed that are giving these bricks their strength have yet to be confirmed but is a
good topic for future work. However, based on literature, reasonable hypotheses can be made
regarding the products that are formed. As was stated in section 3.1, the presence of calcium offers
the benefits of rapid strength development and the ability to form the products CSH and/or CASH.
With calcium added to the solids component, it was believed that the coexistence of reaction
products could be achieved which each would contribute to the performance of the brick. The two
products expected were NASH and either CASH or CSH.
55
-
-
This coexistence of NASH and
M. LARACY
1 2015
CSH/CASH is highly desirable as NASH gel provides the bricks with exceptional chemical and
thermal resistance while CSH/CASH offers chemical binding of the water in the system which
reduces permeability (J. Provis and Bernal 2014). Confirmation of the coexistence was done using
fourier transform infrared spectroscopy (FTIR) by comparing two samples that had equivalent
strengths, one of which had lime and one which did not. The results from the FTIR analysis can
,
be seen in Figure 4.3. It was observed that both samples had bands located close to 1060 cm-1
indicative of the formation of NASH gel (Garcia-Lodeiro et al. 2011). However, the sample that
contained lime also showed a band at 960 cm-1 that was not present in the other sample. This
additional band at 960 cm- associated with asymmetrical stretching vibrations of Si-O bonds is
typical for the presence of CASH or CSH (Garcia-Lodeiro et al. 2011).
NASH
CSH/CASH
AA
-Without Lime
Lime
-With
-Without Lime
-With Lime
4000 3600 3200 2800 2400 2000 1600 1200
Wavenumber (cm-1)
800
400
1400
1200
1000
800
Wavenumber (cm-1)
600
Figure 4.3: FTIR analysis of a brick made with and without lime shows that an additional band indicative of CSH or
CASH is present in the sample made with lime giving evidence to the coexistence of gels.
With calcium present in the solids component, the molarity of the alkali source will also play a
significant role in the products that are formed. In these experiments, a low concentration of
sodium hydroxide (2M) was used as literature has shown that high calcium alkali activated
materials achieve higher strength with lower alkali concentrations (Yip et al. 2005). If too high of
a concentration was used, then the high pH of the alkali source would cause the calcium to
precipitate as portlandite, rather than forming CSH or CASH (Alonso and Palomo 2001).
However, if the concentration is too low it will not allow for the dissolution of the alumina and
silica, which will prevent NASH from forming.
56
-
-
CHAPTER 4: MECHANICAL PROPERTIES OF PRODUCTS FORMED
Durability is critical to the performance of the brick and was found to be problematic for all three
ash samples. The biggest issue was the high water absorption values which are tied directly to the
pore system of the product formed. The volume of pores and their distribution in size is of extreme
importance as they are known to control both durability and strength (Lloyd et al. 2009). Having
a dense network within the structure with little pores is critical in preventing the ingress of things
like chlorides and carbonates (Lloyd et al. 2010; Provis et al. 2012). Furthermore, bricks with high
water absorption tend to have problems when mortar is bonded to them, because they absorb much
of the water out of the mortar, reducing the cohesion between the two materials (Riza et al. 2010).
One hypothesis for the high water absorption in these bricks is they have too great a quantity of
the liquid phase. Literature has found that excess water can lead to an increased number of pores
and can also induce micro-cracking in the structure which will decrease the compressive strength
(Yip et al. 2005). Another hypothesis is that unreacted clay may be leading to these high water
absorption values, as this was found to be a problem in literature (Van Jaarsveld et al. 2002).
Finally, the large water absorption may be due to the drying of the sample at 11 0C which is
required in ASTM C67-14. Water is chemically bonded in CSH and CASH, thus drying can
deteriorate the gel and damage the microstructure of the product.
In an attempt to test these hypotheses, images of the bricks were taken via SEM and can be seen
in Figure 4.4. The bricks imaged were those tested for water absorption which were exposed to
the drying temperatures of 1 10 C. Images were taken at a magnification of 65 to get an overall
view of the surface of the brick and then at a magnification of 1000 to look deeper into the
microstructure. It was observed that at a magnification of 65 the binders all appeared to be dense,
but when zooming in to a magnification of 1000 there was micro-cracking present which may be
related to the drying of the samples for the water absorption test. Also, it is believed that the flat,
smooth platelets (circled in Figure 4.4) are unreacted clay particles. This is evidence for the
hypothesis that unreacted clay may be causing high water absorption. Since no particles of ash
can be visibly seen in the images, it is assumed that the ash is being dissolved by the alkali source
and therefore reacting.
57
-
-
M. LARACY
1 2015
A
100Ox
65x
B
t5x
A
1
100.0x,00
C
Figure 4.4: SEM images of A) Bindlas B) Silverton and C) Siddhbadi bricks at 65 and 1000 magnification show the
presence of what is believed to be clay (circled) and evidence of microcracking
58
-
-
CHAPTER 4: MECHANICAL PROPERTIES OF PRODUCTS FORMED
In order to test the hypothesis that higher liquid to solid ratios lead to high water absorption, an
experiment was performed that used hydraulic pressure to mold the bricks rather than vibration.
Based on experimentation in the field in India, it was learned that molding with hydraulic pressure
allowed for a reduced quantity of the liquids phase. The hypothesis was that by reducing the
liquids phase, one would reduce the water absorption. Bricks were made with a L/S ratio of 0.325
(compared to 0.45 with vibration) and the mixture was compressed to pressures of 5, 10, 15, 25,
and 35 MPa. It was observed that increased forming pressures did decrease water absorption,
however, the lowest value at a forming pressure of 35 MPa was 35% which is still significantly
higher than the goal of less than 20%. Therefore, it was concluded that forming pressure alone
will not be enough to reduce the water absorption to the target value.
A second experiment was performed that looked at the influence of particle size on water
absorption. In this experiment the ash was sieved into three ranges (< 75 micron, 75-150 micron,
and >150 micron). The results from this experiment found that the water absorption (and
compressive strength) values for each particle range all had similar values to the ash in its original
form. Although all values fell within 3% of each other it was interesting to see that the largest
particle range (>150 micron) had the lowest water absorption of the group, as literature has shown
smaller particles to be advantageous for reducing water absorption, as discussed in Chapter 3.4.
More information on these two experiments can be seen in Figure B. 1 and Figure B.2 which are
located in Appendix B.
Leaching of the sodium hydroxide and/or lime was also found to be a problem. After the brick
was placed in water for 24 hours the pH rose to almost 11. It is believed that this leaching is due
to unreacted sodium hydroxide and/or lime. If this hypothesis is true, then it means that the
quantity of sodium hydroxide and/or lime can be reduced which will also reduce the production
cost of the brick. It is also important to improve this problem because leaching of these materials
can run off into the soil or nearby bodies of water causing environmental issues. It must be noted
that the pH values are dependent on the volume of water that the brick was immersed in, and a
larger volume of water will lead to a lower pH. However, finding a precise value was not the goal
of this experiment, it was solely meant to determine if any leaching was occurring.
The final test for durability, strength loss after 24 hour water immersion, is not a standardized test,
although it is common to see with compressed stabilized earth bricks (CSEB) (Riza et al. 2010).
59
-
-
M. LARACY
1 2015
In CSEB the average strength loss is close to 35% (Riza et al. 2010), however, these bricks are
made from a mix of soil, sand, cement, and water, and do not have any alkaline source. Thus, a
direct comparison between the values found in this experiment is difficult to make. The cause for
the large standard deviation of the strength loss in these bricks is unknown and needs to be
researched further. Since some samples actually gained strength after 24 hour water immersion,
it is obvious that the bricks can handle being immersed in water and it is difficult to conclude that
this a good test for durability.
Based on the results for compressive strength and durability it is clear that the formulation is robust
enough to account for the variability of the ash properties. However, issues with durability related
to water absorption and leaching still need to be improved before the product can be implemented
in the Indian market.
60
-
-
CHAPTER 5
Conclusions and Future Work
A number of important findings will be summarized in this chapter. However, the work presented
in this thesis is merely the beginning of a longer project. There is still a tremendous amount of
work to be done and a number of suggestions have been stated in the Future Work section of this
chapter.
61
-
-
M. LARACY | 2015
5.1 Conclusions
India's growth in industry is leading to the production of massive quantities of wastes that are
often being landfilled or dumped into bodies of water.
These wastes can be converted into
resources by using them as a raw material in the production of building materials. The waste being
studied in this thesis, boiler ash, is a byproduct with variable physical and chemical properties that
have prevented it from being utilized thus far.
The goal of the work is to produce a high
performance, low-cost, and environmentally friendly brick that can be implemented in the Indian
market. This has the potential to be a twofold solution that eliminates the landfilling of boiler ash
and provides an alternative to the clay fired brick and its associated environmental impacts.
It was determined that alkali activation technology is the best strategy for accomplishing the goal
of this research. Alkali activation relies on a chemical reaction to give the bricks their strength,
allowing them to be cured at ambient temperature, thus reducing the overall environmental impact.
It is a field that is rapidly growing, and upon overcoming some of the barriers to implementation,
is expected to be a green building solution in the future.
Two major contributions are presented in this work.
First, characterization of the physical,
chemical, and mineralogical properties of three samples of boiler ash to assess their suitability as
a raw material in alkali activation.
This work was presented in Chapter 3.
Second, the
development of a brick formulation followed by a study of the mechanical properties of the
products formed. This work was presented in Chapter 4.
Results of the characterization of the boiler ash samples show they have a number of undesirable
characteristics for alkali activation. These negative characteristics include varying particle shape,
large particles sizes from 5 to 600 micron, high loss on ignition ranging from 8-35%, a lack of
alumina (< 4%), and leaching of heavy metals. However, with the addition of supplementary
materials, in the form of clay as a source of alumina, and lime as a source of calcium, boiler ash
can be used to produce bricks using alkali activation.
A brick formulation was developed and tested for its robustness using the three boiler ashes to
determine if it could account for variability in the boiler ash properties. The formulation created
is 70% boiler, 20% clay, and 10% lime by weight of the solids phase. The alkali source is sodium
62
-
-
CHAPTER 5: CONCLUSIONS AND FUTURE WORK
hydroxide at a concentration of 2M. The liquid to solid weight ratio is 0.45 and the temperature
the samples are cured at is 30'C.
The alkali activated bricks tested for their compressive strength at 1, 3, 7, and 28 day were found
to have high 28 day strength between 11-15 MPa, sufficiently above the target goal of 7.5 MPa.
Furthermore, early strength development was observed, as more than 50% of the 28 day strength
was achieved after 1 day curing.
The durability of the bricks is still a work in progress. They were tested for water absorption,
leaching, and strength loss after 24 hour water immersion. Results found water absorption values
to be in the range of 33-40%, significantly higher than the target value of 20%. Also, pH values
over 7 indicated leaching of lime or sodium hydroxide. Finally, strength loss values ranged from
7-25% with large standard deviations, indicating the need for further testing of this parameter.
While the brick formulation is not fully optimized, it provides a solid benchmark for future work.
It successfully produced bricks with high compressive strength for all three ashes demonstrating
its robustness. Issues related to the durability need to be further investigated and are a main topic
of future work.
5.2 Future Work
There is a significant amount of future work to be done on this project, both technical and business
related. An initial brick formulation has been established that appears to be robust enough to
account for the variability in the boiler ash, but questions pertaining to the specific products formed
remain unanswered, requiring future work.
Understanding the Chemistry
It is known that the amount of reactive silica, alumina, and calcium will strongly govern the final
properties of the brick, but these values have yet to be fully quantified. The approximate amount
of reactive silica in the boiler ash has been determined, but no analysis has been done on the
amount of reactive alumina the clay provides or the amount of reactive calcium that the lime
provides. SEM images have shown that unreacted clay is present, thus it is crucial to determine
the contribution this clay has to the strength and durability of the brick. A place to start may be
using induced coupled plasma atomic emission spectroscopy (ICP-AES), which would allow
63
-
-
M. LARACY
1 2015
quantification of the dissolved species. If it is determined that the clay is playing little to no role,
then it will be necessary to identify an alternative source of alumina. Also, establishing quantities
of reactive silica, alumina, and calcium, will help in determining what products are being formed
in the brick whether it's NASH, CASH, CSH, or a coexistence of gels.
Fundamentally
understanding the reactivity of the materials and the products formed is instrumental for the next
section of future work, which is improving the durability.
Improving Durability
Initial durability studies have been performed in this thesis, but more long term studies are
necessary before the product can be ready for commercialization.
A first priority should be
reducing water absorption in the brick, which is directly linked to its porosity. Measurements of
the porosity of the bricks can be done using mercury intrusion porosimetry (MIP). It has been
determined thus far that hydraulic pressing or the use of smaller particles will not be sufficient in
lowering water absorption. A key place to start may be understanding the particle packing theory
and seeing if this can be optimized with the materials in the mix design. Upon improving water
absorption, further durability studies must be done on leaching of lime and NaOH, freeze-thaw,
strength loss, etc. Furthermore, tests need to be done to be sure that the heavy metals present in
the boiler ash are not leaching out from the brick. Once the issues with durability are solved, a
secondary step will be to optimize the formulation, and suggestions for doing so can be found in
the next section of future work.
Optimizing the Formulation
The focus thus far has been creating a robust brick formulation, making optimization a second
thought. However, future work should consider identifying ways of improving the brick through
the use of alternative alkali sources, additional waste products, or the use of admixtures.
The use of alternative alkali sources has the potential to be a good strategy for improving the
performance of the bricks and/or lowering the production cost. The only alkaline solution that
has been used thus far is sodium hydroxide. The use of sodium silicate used alone or mixed with
sodium hydroxide should be tested to see if the performance of the bricks is enhanced. Also,
alkali sulfates may be a good source as their moderate pH allows for gel coexistence. Some initial
64
-
-
CHAPTER 5: CONCLUSIONS AND FUTURE WORK
tests were done using sodium aluminate (solid form) to produce "one-part geopolymers", but
despite positive results, this method was not commercially feasible as the price would be too high.
The use of additional waste byproducts in the brick design would be effective in making the bricks
more environmentally friendly and further reducing the cost. In Muzaffarnagar, a number of
waste products exist in close proximity to where the boiler ash is being produced. These wastes
include black liquor, aluminum sulfate (alum) waste, and lime waste. It should be expected that
other industrial cities would have a similar situation, although the waste streams will likely vary.
Some initial experiments were made with black liquor, alum waste, and lime waste and the results
can be found in Appendix A.
Research on the effect of admixtures within the binder could be very useful in enhancing the
properties of the products formed. Initial tests could be done with commercial admixtures for
portland cement, although literature has suggested these are not effective with alkali activated
materials. The one exception to this, is naphthalene-based superplasticizers, which do not lose
their fluidity based properties in the presence of NaOH (Sathonsaowaphak et al. 2009).
Therefore, these admixtures would likely need to be engineered for this specific application. In
particular, the design of a superplasticizer would be useful to decrease the liquid to solid ratio,
which would ideally help decrease the water absorption.
Acceptance among Customers and Masons
The product that is created needs to be accepted by the customers, as well as the masons who
handle the bricks each day. Therefore, it is necessary that these bricks are functionally equivalent
in all aspects to the bricks being used currently. Up until this point the focus has been on the
strength and durability of the brick and has been reluctant in considering its behavior once
implemented. To determine the desired qualities of the brick, it is suggested that masons and
customers be interviewed. A few initial tests to look at include bonding between the brick and
mortar, ability to install masonry anchors, and ease of breaking the bricks to the desired shape.
Additional studies could look into the thermal properties of the bricks as ash can be a good
insulator.
65
-
-
| 2015
-
66
-
M. LARACY
APPENDIX A
Additional Experimental Results
A number of experiments were performed over the past two years that are not included in the body
of thesis. These results, although not presented above, have been instrumental in allowing the
research to progress to its current state. They include Supplementary Materials, Ash Content,
Molar Concentration, Curing Temperature, Liquid to Solid Weight Ratio, Additional Waste
Products, Mixing Time and Consistency of Mix, Premixing, and Dry versus Wet Clay. The finding
.
from each of these experiments is presented below
The work in this appendix was done in conjunction with Dr. Thomas Poinot
67
-
-
M. LARACY
1 2015
Supplementary Materials
Despite the undesirable characteristics of the boiler ash, the starting point for experimental work
was to test the hypothesis that it could not be used alone in alkali activation. The boiler ash was
mixed with sodium hydroxide solution and the bricks were found to have both low compressive
strength and poor resistance to water. These results which were expected, supported the need to
add supplementary materials to the mix design. The first step was to find an additional source of
alumina as the boiler ash had only 2-4%. The solution was to add clay as it is locally available
throughout India, and is a known source of alumina. A chemical analysis was done on the clay
via XRF and the results which can be seen in Table A. 1 found that it had 13.84% alumina. Bricks
that were made using boiler ash, clay, and a high concentration (5M- 1 OM) of sodium hydroxide
solution were found to have compressive strengths that were much higher than when boiler ash
was used alone. Despite the sufficiently high compressive strength, durability was still an issue as
the bricks were dissolving in water (Figure A. 1 A) and/or sodium hydroxide was leaching out
(Figure A.1B), particularly when the bricks were cured at lower temperatures (30-50'C). Work
done by Duxson et al. (2007) provided reasoning for this as they found that alkali activated binders
with excessive sodium to alumina ratios tend to dissolve in water. Therefore, it was necessary to
find a solution to either reduce the sodium content or increase the alumina content in the
formulation. Referring back to the desired properties of the ash in section 3.1, the presence of
calcium in the ash allows for a lower concentration of the alkaline source, which would help to
reduce the sodium content. However, since the boiler ash has only 1-2% calcium, it would need
to come from an alternative source. Therefore, hydrated lime was added to the mix design as a
source of calcium. It was found that with the addition of just 10% lime in the solids phase, good
strength could be achieved and the bricks were not dissolving in water even at low curing
temperatures. The addition of lime also allowed for the concentration of the sodium hydroxide to
be significantly reduced to under 2M therefore accomplishing the goal of reducing the sodium to
alumina ratio. The findings from these experiments established boiler ash, clay, and lime as the
three components of the solids phase.
Table A.1: A chemical analysis of the clay from Muzaffarnagar shows 14% is alumina.
Element
Es Oxid
as Oxide
SiO2
A1203
CaO
Fe2O3
MgO
SO3
7101
1
Weight %
67.2
13.84
0.74
5.09
2
0.07
0.72
2.9
-68-
a O
StO2
L01[
0.42
0.09
6.93
APPENDIX A: ADDITIONAL EXPERIMENTAL RESULTS
Figure A.1: Samples made with boiler ash, clay, and highly concentrated NaOH were dissolving in water (A) and
leaching out excess NaOH (B).
Ash Content
One of the objectives of these bricks is to use the maximum amount of waste that is feasible without
taking away from the performance of the brick. In this experiment, the ash to clay solids weight
ratio was the variable and the four ratios tested were 60/30, 70/20, 80/10, and 90/0.
In all
experiments the lime weight percentage was held constant at 10%. The only other variable was
the liquid to solid weight ratio which had to be changed according to the mixture in order to achieve
the same workability for vibrocasting. For the 60/30 and 70/20 mixes the liquid to solid ratio was
0.45, and for the 80/10 and 90/0 mixes it was 0.50 and 0.55 respectively. The molar concentration
of the sodium hydroxide was 2M for all samples and each was placed in the oven at 1000 C and
tested for compressive strength after 3 days curing. Only one sample was used for each mixture.
The results which can be seen in Figure A.2 show that the compressive strength was approximately
the same for the 60/30 and 70/30 mixes at 11.5 and 11.6 MPa respectively. Once the ash content
was increased to 80% the strength almost halved at 6.4 MPa. At 90% ash content the strength
greatly decreased to 2.2 MPa. The results of these experiments influenced future research by
turning this variable into a constant, establishing boiler ash at 70% of the solids phase by weight.
69
-
-
M. LARACY
I
2015
14
--
S10
--
8
11.5
Curing Time: 3 days
11.6
Curing Temp: 1000 C
[NaOHJ: 2M
6.4
-
12
V)4
c>
4-
2.2
C-
E
0
90/0/10
%
60/30/10
70/20/10
80/10/10
Ash/Clay/Lime Solids Phase wt.
Figure A.2: The maximum amount of ash that can be used while maintaining high brick strength is 70%.
Molar Concentration and Curing Temperature
Both the concentration of the alkaline source and the curing temperature play a vital role in the
strength and durability of the brick. In this experiment the concentration of the sodium hydroxide
solution was varied to test OM (just water), 2M, and 5M. Also, the effect of curing temperature
was tested by curing one set of samples at 30'C and the other at 100'C. For these experiments the
liquid to solid ratio was held constant at 0.5 and all samples were cast using vibration. The solids
content was held constant at a ratio of ash/clay/lime = 70/20/10. Measurements of strength were
taken at 1, 3, 7, and 28 days and three samples were used for each measurement.
Looking to the
results in Figure A.3, it can be seen that for all three molarities the 1 day strength was higher for
the samples cured at 1000 C, but by 28 day strength testing the 30'C samples had surpassed the
1000 C samples and become stronger. Also, the strength of the 100'C samples hardly increased
after 1 day, and in some cases decreased.
These results are in agreement with literature which
shows that increased temperature enhances the reaction kinetics giving the brick its high strength
after one day of curing (Bakharev et al. 1999). The lack of development in strength thereafter is
likely a result of too much water being evaporated from the brick, as water is vital in keeping the
reaction going (Pacheco-Torgal et al. 2008b).
70
-
-
APPENDIX A: ADDITIONAL EXPERIMENTAL RESULTS
101
8
0F10
0
-
-.- 100CC
1-100C
30-C
-
--
U'n4
8
8
e
LA 4
2
2
2M2
1
Cuig
U
0
00
10
ie
dys4)in
OL
M
20
im
30
U
0
Curing Time (days)
-30C
-30-C
10
dasIurn
20
2M IPL5
30
Curing Time (days)
0
U
Tm
0
10
(as
20
30
Curing Time (days)
Figure A.3: This figure shows that both a lower concentration of alkali and a lower curing temperature lead to higher
compressive strength.
In terms of molar concentration, 2M was found to have the highest strength, followed by 5M, and
finally OM. This was true at both 30 C and 100 C. The fact that the OM samples did have some
strength is evidence that the lime is reacting and contributing to the strength of the brick. However,
the significantly higher strength of the 2M sample demonstrates the importance of the alkali
source. The lower strength of the 5M samples compared to the 2M samples is expected as a lower
concentration of the alkali source in the presence of calcium will lead to higher strength. Based
on the results of this experiment, 30'C will be designated as the curing temperature and 2M will
be the concentration of the alkali source.
Liquid to Solid Weight Ratio
An experiment was done that studied the liquid to solid weight ratio, another critical variable that
influences the compressive strength and durability of the brick.
The liquid to solid ratio is
important because it determines the workability and consistency of the mixture and will be varied
significantly based on the technique chosen for molding the brick. For example, if we want to use
a hydraulic press to manufacture the bricks, a lower liquid to solid ratio will be needed, somewhere
in the range of 0.25 to 0.40. The reasoning behind this is that under high forming pressures and
high liquid to solids ratios, the excess liquid will be squeezed out, decreasing the amount of
available alkali, thus decreasing strength (Ahmari and Zhang 2012). However, if the binder will
be molded using vibration, then a higher liquid to solid ratio will be necessary, between 0.45 and
0.60, to achieve the consistency of a paste which can settle on its own under vibration. The large
range for these values is due to the variations in the raw materials, particularly the boiler ash. From
a more technical side, achieving the optimum liquid to solid ratio is critical because a shortage of
liquid will lead to poor cohesion between the solid particles, and too much liquid will lead to
71
-
-
M. LARACY
1 2015
greater pore sizes in your brick at the hardened state. These large poor sizes can be detrimental to
the brick as they will lead to higher water absorption values.
16
>
14
. . . . . . . I',
100%
..
-e-2M-.45
- -OM-.45
-*-0M-.5
-o-2M-.5
0
12
10
-Z
-
c
0
~ 10
60%
90%
80%
70%
* L/S =0.5 E L/S =0.45
640%
20%
Curing Temp: 30C
Ash/Clay/Lime: 70/20/10
0.2
U
0
' ' '
0
' '
5
40%
-W30%
AIR
10%
'
10
15
0%
20
25
30
Curing Time (days)
OM
2M
NaOH Molar Concentration
Figure A.4: A lower liquid to solid ratio is shown to significantly increase strength and reduce water absorption, likely
due to the reduction in pore size of the hardened state.
Building off the previous results on molar concentration and curing time, the experiments were
further developed to see if the liquid to solid ratio could be decreased to increase the compressive
strength of the bricks. In this experiment the curing temperature was held constant at 30'C and
only OM and 2M concentrations were used. The two liquid to solid ratios studied were 0.50, from
the previous study, and 0.45 which was the lowest L/S ratio possible to achieve the appropriate
consistency for vibration. The results in Figure A.4 show that for both OM and 2M the 1, 3, 7, and
28 day strength was higher at a liquid to solid ratio of 0.45. Of particular interest is the 2M samples
as this is the concentration that will be used to test the robustness of the formulation in the next
section. Here it can be seen that the sample with a L/S ratio of 0.45 had nearly a 50% increase in
strength at 28 days compared to the sample at 0.50.
Looking at durability results, the water
absorption for both the OM and 2M samples was reduced by 18% when the L/S ratio was decreased
from 0.50 to 0.45. Also, the 2M samples had a lower water absorption value than the OM samples.
From this experiment it was determined that the lowest liquid to solid ratio that can attain an
appropriate consistency for vibration should be chosen, with the baseline set at 0.45 and
adjustments made thereafter.
72
-
-
APPENDIX A: ADDITIONAL EXPERIMENTAL RESULTS
Additional Waste Products
The three additional waste streams that were incorporated in the bricks and can be seen in Figure
A.5 were black liquor, alum waste, and lime waste. Each of these wastes were being produced in
close proximity to the paper mills in Muzaffarnagar where the boiler ash was being produced. The
black liquor and lime waste are actually produced on site at the paper mills while the alum waste
comes from a factory nearby.
Basic characterization was done on these different wastes to
determine if they may be of use as a raw material for the bricks and some experiments were also
made.
Black Liquor
Alum Waste
Lime Waste
Figure A.5: Additional waste streams including black liquor, alum waste, and lime waste may be incorporated into the
mix design to reduce cost and increase the positive environmental impact.
Black liquor is a waste product from the kraft paper process. Another student at the MIT Tata
Center, Charlene Ren, is working on treating this waste using membrane filtration. However, it
may have some ideal properties to be used in bricks prior to treatment. The pH of the black liquor
is high (11-12) when it is freshly produced, therefore it has the potential to be used as a partial
replacement of the alkali source. Over a short period of time (a few days), this pH drops and is
believed to be ineffective in the bricks. Black liquor was shipped overseas to MIT, but by the time
it had arrived the pH was close to neutral and organic matter had begun to develop, rendering it
useless. Therefore, the only experimentation that could be done with black liquor occurred during
a trip to India. In this experiment, sodium hydroxide was replaced by black liquor at 0%, 25%,
50%, and 100%. Silverton boiler ash was used throughout, and the ash/clay/lime content was
70/20/10. The concentration of the sodium hydroxide was 2M, the liquid to solid weight ratio was
0.35, and the samples were cured at 30'C for 28 days. As can be seen in Figure A.6, it was found
that 0% black liquor and 50% black liquor had nearly the same strength, while the replacement of
sodium hydroxide with 25% black liquor saw an increase in strength of over 2 MPa. The sample
-
- 73
M. LARACY
1 2015
with 100% black liquor had no strength and failed to get hard. These positive results provide
evidence that the addition of black liquor as a substitute for the alkali source is feasible and an
excellent area for further research.
10
8
Curing Temp: 30*C
Curing Time = 28 days
[NaOH] = 2M
6
CL
E.
0
Ash/Clay/Lime: 70/20/10
L/S wt. ratio = 0.35
4
bD
2
0.
U
0
0
25%
0%
100%
50%
Black Liquor % of liquid phase
Figure A.6: The replacement of sodium hydroxide with 25% black liquor saw an increase in strength and is a good
area for future work.
Alum waste, a byproduct of aluminum sulfate, was hypothesized to be a good supplementary
source of alumina. An XRF analysis revealed that 14 %of the waste is made up alumina, however,
it also had some negative qualities including 10% Sulfur, 6% titanium, and an LOI of 32% as
shown in Table A.2. An experiment that used 20% of alum waste in the solids phase revealed that
the strength decreased when compared to the sample that only used clay as its alumina source. A
bigger issue regarding the use of alum waste is the presence of efflorescence on the bricks that
appeared in large quantities just 1 day after being exposed to the air.
Table A.2: A chemical analysis of the alum waste from Muzaffarnagar.
Element
SiO2
A1201
CaO
FeOs
Mg
Weight %
33.34
14.06
2.99
1.29
0.12
SOs
10.02
T
5.88
0.27
0.42
0.02
31.59
Lime waste is a byproduct of paper mills produced during the process of bleaching paper with
lime. This waste is being produced in small quantities in comparison with the other wastes,
therefore it is not of high priority. No characterization was done on this waste, but a brick sample
was made using 20% lime waste in the solids phase. The result showed lower compressive strength
-
74
-
3, M0ZO .t 0KI
APPENDIX A: ADDITIONAL EXPERIMENTAL RESULTS
in comparison to the samples made with no lime waste, thus working with this waste became of
low importance.
The results from this section are meant to make the reader aware that there exists opportunities for
using additional waste streams in the bricks. Boiler ash is still the priority when it comes to
utilizing waste in the bricks, however, future work may consider experimenting with these
byproducts.
Mixing Time and Consistency of Mixture
The amount of time that a binder is mixed for strongly influences its consistency. It was observed
that the same mixture will become increasingly wet as the amount of time it is mixed increases.
As shown in Figure A.7, the transition goes from a dry mix (1), to small agglomerates (2), to larger
agglomerates (3 and 4), and sometimes to a very wet and sticky mix (5).
1
3
2
4
5
Figure A.7: The amount of time the binder is mixed influences the consistency. Longer mixing times have been found
to produce wetter mixes.
This evolution of consistency is not the same for all mixes and is strongly correlated with the liquid
to solid ratio and the amount of mixing time. For example, a mixture with a high liquid to solid
ratio may reach stage 4 or 5 in just a few minutes. However, the same mix with a lower liquid to
solid may take 30 minutes to reach that consistency. The consistency also becomes important
when choosing the technique for molding the bricks. If vibration is going to be used to mold the
bricks the consistency must reach stage 3 or 4 so that it can become homogenous during vibration.
A consistency of 1 or 2 with vibration will not allow for cohesion between the particles and a
consistency of 5, generally means too much liquid is being used. On the other hand, if hydraulic
pressure is used to mold the bricks a consistency of 1 will be necessary as anything higher will
cause the liquid to squeeze out during pressing, leading to a loss in alkaline solution.
75
-
-
M. LARACY
1 2015
An experiment was done that looked at how mixing time and the change in consistency influenced
the compressive strength of the bricks.
At the time of this experiment, the current brick
formulation was not yet developed, thus a number of the values for the variables will not be
consistent with most experiments throughout the thesis. Nonetheless, the results offer insight into
the effect of mixing time. In this experiment the variables were ash/clay/lime = 70/30/0, L/S ratio
= 0.6, curing temperature = 80'C, [NaOH] = 6M, and the bricks were tested after 7 days curing.
The mixing of the binder was stopped at 5, 10, 20, and 30 minutes and all samples were molded
with a combination of hand tamping and vibration. Looking at the results in Figure A.8 it is clear
that longer mixing time leads to an increase in strength with a nearly linear trend. It is unclear if
the increased mixing time allows for further dissolution of the aluminosilicates leading to higher
strength of the brick or if the increase in strength is related to the consistency of the mixture. Either
way it is an important variable to consider when designing an experiment as choosing a constant
mixing time or constant consistency can influence the results for strength.
20
Ash/Clay/Lime: 70/30/0
L/S wt. ratio =0.6
Curing Temp: 80*C
15
Curing Time = 7 days
c
[NaOH]=6M
- 10
U)
V)
C.
E
0
0
0
10
20
30
Mixing Time (min)
40
Figure A.8: Longer mixing time leads to a more wet consistency and an increase in compressive strength.
Premixing
The order that materials are mixed may influence the strength of the brick. It has been observed
in literature that the rate of release of aluminum affects the final strength of the brick (Li et al.
2010) and that the alumina bonds are more readily broken than silica bonds during dissolution
(Femndez-Jim6nez et al. 2006b). Therefore, experiments were performed that tested premixing
the clay with the goal of enhancing the dissolution of alumina. Premixing, simply means mixing
76
-
-
APPENDIX A: ADDITIONAL EXPERIMENTAL RESULTS
a select solid source with the alkaline solution first, and then adding the additional solid sources
for longer mixing. Premixing was also done with 30% of the ash to try and enhance the dissolution
of the silica.
These experiments were performed before lime was added to the mix design, therefore the only
materials used were rice husk ash, clay, and NaOH. The ash/clay weight ratio = 70/30, L/S wt.
ratio = 0.6, NaOH concentration = 5M, mixing time = 25 minutes, and the premixing time = 10
minutes. Samples were cured at 50'C, 75'C, and 100'C. Compressive strength testing was done
at 1 and 7 days and only one sample was made for each test. The results can be seen in Figure
A.9. The notation Reg. stands for the experiment that did not have any premixing. It was observed
that the premixing had no influence on 1 day strength, but after 7 days curing the compressive
strength was higher for the premixing of both the clay and ash. While this result may indicate that
premixing is effective in increasing strength, it could also be due to the overall longer mixing time
(premixing 10' + mixing 25' versus mixing 25'), which was shown to increase strength in the
previous section. A more accurate test would compare the "Reg." sample mixed for 35 minutes
to the premixed samples. This experiment is only meant to make the reader aware of premixing's
potential to give the brick better strength and should be further studied.
25
220
1i
El day
N 7 day
Ash/Clay/Lime: 70/30/0
L/S wt. ratio = 0.6
[NaOH] =5M
Mixing Time = 25 min
Premixing Time =10 min
4-J
>
10
E
0
Reg. Clay Ash Reg. Clay Ash Reg. Clay Ash
750C
50*C
1000C
Figure A.9: Premixing the clay or 30% ash shows an increase in 7 day strength but no increase at 1 day strength
77
-
-
M. LARACY 1 2015
Dry versus Wet Clay
From an implementation standpoint it is essential to reduce the number of energy consuming
processes required to produce bricks. Therefore, it is ideal to use clay in its original wet form. On
the other hand, if laboratory experiments are being performed it is better to use the clay after
removing all moisture, because moisture content may vary over time, leading to inconsistencies
across experiments. To be sure that the form (wet or dry) of the clay does not influence strength,
an experiment was designed to compare wet clay, dry grinded clay, and recently rehydrated dry
grinded clay. The mix design was the same for all experiments, and the water content in the wet
clay was taken into account when calculating the amount of solids. It was found that all three
samples had sufficient strength after 3 days curing. The sample that used wet clay had a strength
of 10.2 MPa. The sample with dry grinded clay had a strength of 14.0 MPa. Finally, the sample
which used dry grinded clay that was rehydrated right before the other materials were added had
a strength of 15.4 MPa. In general, it was concluded that the clay could be used in any form and
provide acceptable results.
The increased strength for the dry clay and rehydrated clay may be
due to the fact that the clay was grinded thus increasing the total surface area available for
dissolution.
The hypothesis that wet clay can be used for industrial production was tested during a trip to India.
Industrial equipment was used to produce full scale bricks and wet clay was used. While the bricks
were found to have sufficient strength, there was an issue with homogenizing the mix using the
industrial sized pan mixer. The wet clay was forming large agglomerates that could be seen in the
bricks when broken. In the future it may be necessary to use an alternative mixer or to process the
clay in order to avoid this problem.
-78-
APPENDIX B
Improving Water Absorption
As was discussed in chapter 4, the high water absorption is an important issue related to the pore
structure of the bricks. It is crucial that this parameter be reduced, thus studies are being performed
to identify possible strategies for achieving this**.
** The work in this appendix was done in conjunction with Cecilio Aponte. More information is availablein his senior
thesis.
79
-
-
M. LARACY 1 2015
In these experiments rice husk ash obtained from Texas was used throughout in order to conserve
the limited supply of boiler ash from India. The parameters being studied will not be affected by
the change in ash. The mix design chosen was slightly different from the one used in chapter 4's
experiments. A solids phase of ash/clay/lime = 70/15/15 was chosen with a sodium hydroxide
concentration of 2, curing temperature of 50'C, and liquid to solid ratio equal to 0.325. The binder
was mixed for 10 minutes and a dry consistency similar to that in Figure A.7 - 1 was observed.
The bricks were tested for strength and water absorption after 3 days and three samples were used
per test to ensure repeatability.
The first experiment studied the influence of forming pressure on strength and water absorption.
The hypothesis was that using hydraulic pressure rather than vibration to mold the bricks would
allow for a decrease in the liquid to solid ratio which would in turn decrease the water absorption.
Five different forming pressures were used: 5, 10, 15, 25, and 35 MPa. For all forming pressures,
200 grams of material was used. Steel molds have been designed specifically for producing bricks
using this method and more information on these can be found in Appendix D.
35
70%
S30
60%
-c 25
'50%
4-,
0
20
20%
lu 15
30%
-
0
0
Compressive Strength
---- Water Absorption
CL.-
03
0
10%0
II0%
0
10
20
30
40
Forming Pressure (MPa)
Figure B.1: It was determined that water absorption decrease as forming pressure increases, however the water
absorption values still exceeded the goal of under 20%.
The results from this experiment (Figure B. 1) show that increasing forming pressure leads to
increasing strength and decreasing water absorption. While the trends being seen are desirable,
the initial goal of decreasing water absorption to below the target goal of 20% failed, as the highest
forming pressure only reduced the water absorption to 35%. However, it is important to note that
80
-
-
APPENDIX B: IMPROVING WATER ABSORPTION
these experiments verified using hydraulic pressure for molding is better than vibration, as higher
strengths can be achieved at lower liquid to solid ratios.
A second experiment was performed which assessed the influence that particle size had on water
absorption. In this experiment the ash was sieved into three ranges of particles, < 75 micron, 75150 micron, and >150 micron. The same mix design was used as in the first experiment and a
constant forming pressure of 15 MPa was used. The results in Figure B.2 show that the different
particle size ranges have little influence on water absorption, as they are all close to the value for
the standard which was the ash in its original state. What is interesting is that the largest particle
size range had the lowest water absorption and the smallest particles had the highest water
absorption. Although their values did not differ by a significant amount, it is worth noting as
literature suggests smaller particles lead to increased durability performance.
25 ,50%
2'
2Compressive Strength 0 Water Absorption
100
10
~20
-> 150 _Am
-15
7
-C
--
>410
45%
40%
35%
%
80
70
>60
50
iz 40
30
2...4.
""3%
25% 0
"20%<
415%
20
10
0
2
0
1
10
100
10%
5%
5
E
1000
0%
"
<75 pm
:
75-150 pm
>150
gm
Standard
Diameter (pm)
Figure B.2: The influence of particle size on water absorption was tested by sieving the ash into three particle size
ranges. It was determined that each range had relatively similar water absorption values, although the largest
particles had the lowest value.
The results from these two experiments provide evidence that continuing research is necessary
regarding water absorption. It should be clearly stated that the test being done for water absorption
is a standard for fired bricks and therefore may not be the most appropriate test. Sintered bricks
that undergo this test have already been exposed to high firing temperatures and therefore their
microstructure is unaffected by the 11 0C drying temperature required in the standard.
It is
possible that this accelerated drying is damaging the microstructure of the alkali activated bricks
leading to increased water absorption (Bernal and Provis 2014). Therefore, a more appropriate
test may look at capillary rise which is a test done on cementitious products, and only requires
81
-
-
M. LARACY | 2015
drying of the sample at 55 0C. It is clear that further research is needed in relation to a test for
water absorption in alkali activated bricks.
82
-
-
APPENDIX C
Environmental and Economic Impact
In addition to developing a brick with high technical performance, it was also a goal to create a
brick which was environmentally friendly and could be produced at a low cost allowing it to be
implemented in the Indian market. An environmental and economic comparisontt to the clay fired
brick was done in order to demonstrate the impact of the alkali activated brick. A life-cycle
assessment was used to compare the environmental tradeoffs of the two bricks and a simplified
economic comparison was done looking at the production costs of the two bricks with relation to
the materials, labor, and energy. Further discussion surrounding implementation will relay what
has been learned thus far and what needs to be done in the future.
1t
Many of the findings regardingimplementation were thanks to the India Labs team ofAriel Chua, Rafael Secundo,
PatrickSwanson, and Liz Voeller. LCA studies were done by Cecilio Aponte.
-
- 83
M. LARACY
1 2015
Environmental Impact
A common way to assess the environmental impact of a product is by doing a life cycle assessment
(LCA). The goal of this LCA is to compare the alkali activated brick to the traditional clay fired
brick. According to literature, LCA's of alkali activated materials have produced varying results
due to the dramatically different raw materials and mix designs across studies (Provis and van
Deventer 2014). Therefore, it is necessary to produce an LCA for this particular waste and mix
design in order to validate the previous claim that the alkali activated bricks are more
environmentally friendly than the clay fired bricks.
The functional unit in this study will be one brick that is 9" x 4" x 2.5" and the densities of the
bricks compared in the analysis will be the same. The value chosen for the density of clay fired
bricks is 1.36 g/cm 3 based on the average from a set of bricks in India. The same density was
obtained for the alkali activated bricks by using a forming pressure of 25 MPa. The mix design
chosen for the alkali activated brick was based off the experiments from chapter 4 in combination
with the mix design used in Appendix B. Thus, the solids phase chosen was ash/clay/lime =
70/20/10, the L/S ratio= 0.325, and the concentration of NaOH = 2M. The system boundary only
includes the resources and processes involved in manufacturing the brick, and does not include
any processes regarding the bricks actual use or disposal. The analysis was done with SimaPro 8
LCA software with data extracted from the Ecoinvent3 library and the method used being
IMPACT2002+. The values in the LCA are reported as a factored score.
15
-
10
-
20
-
25
5
0
Human Health
Ecosystem
Climate Change
Resources
Quality
U Alkali
Activated - 25Mpa 70/20/10 2M
N Clay Brick - SEC=1.22
Figure C.1: LCA results show the alkali activated brick performs better than the clay fired brick in the three major
categories: human health, climate change, and resources.
84
-
-
APPENDIX C: ENVIRONMENTAL AND ECONOMIC IMPACT
The four categories being compared were human health, ecosystem quality, climate change, and
resources. Looking at Figure C. 1, it can be seen that the alkali activated brick performed better in
the three largest categories, human health (24% better), climate change (15% better), and resources
(33% better). The human health category was primarily driven by the production of respiratory
inorganics, the climate change by C02, and the resources category by the depletion of
nonrenewable resources. The alkali activated brick did perform marginally worse than the clay
fired brick in ecosystem quality, likely due to the leaching of heavy metals in the boiler ash.
The total environmental impact from the sum of the four categories in Figure C. 1 was then broken
down to see what processes had the largest impacts, as shown in Figure C.2. Results yielded that
the largest driver for environmental impact of the alkali activated bricks was the lime (51%) and
sodium hydroxide (3 9%). All other processes accounted for only 10% of the total environmental
impact. Regarding the clay fired brick, 26% of the environmental impact came from mining coal
and another 61% from its combustion. The use of clay accounted for 8% of the total impact, while
the remaining processes accounted for 5%.
40
40
35
30
25
-20
15
10
5
-
-
Alkali Activated Brick
-
25
-20
15
10
5
0
-
-
35
30
Clay Fired Brick
0
\b
12
IV (-P
,cz lo
Figure C.2: The total environmental impact was broken down to determine which inputs have the largest effect. For
the alkali activated brick it is the lime and NaOH. For the clay brick it is the mining and burning of the coal.
Since the main contributors to environmental impact for the alkali activated brick were lime and
sodium hydroxide, a sensitivity analysis was done around these two inputs. The lime content was
increased to 15% and also decreased to 5%. The molar concentration was also lowered to look at
1.5M and IM. Finally, a lower forming pressure of 15 MPa was examined. The results in Figure
-
- 85
M. LARACY
1 2015
C.3 show that all versions of the alkali activated brick perform better than the clay brick, except
for the one that uses 15% lime, which has an equal environmental impact to the clay fired brick.
The results from the LCA validated the claim that the alkali activated bricks have less of an
environmental impact than the clay fired bricks. Although they only perform 22% better from an
environmental perspective, they perform much better in terms of compressive strength which is
nearly three times the value of the clay fired brick. Furthermore, there is opportunity to lower the
environmental impact by reducing the lime and NaOH quantities and by using additional wastes
in the mix design.
70
60
50
- Human health IIII Ecosystem quality 0 Climate change N Resources
I-
40
30
20
10
0
-.
~I0
41
I'
It54111;
Figure C.3: A sensitivity analysis was performed to see how the total environmental impact was influenced by
changing the forming pressure, the lime content, and NaOH concentration.
86
-
-
APPENDIX C: ENVIRONMENTAL AND ECONOMIC IMPACT
Economic Impact and Implementation Strategies
While the environmental benefit of the alkali activated brick is pivotal in creating a more
sustainable world, it will not be a large factor in making the brick more marketable in developing
countries such as India. In these settings, the main driver of a products success is its price. Hence,
developing a product with equivalent or lower production costs than the competition is essential
to enter the market. There are a number of other factors that influence a new products adoption
including performance characteristics, aesthetics, and aspirations, but none triumph the price of
the brick.
In this analysis, the production costs will be compared for a clay fired brick in the city of
Muzaffamagar and an alkali activated brick molded with hydraulic pressure. According to local
sources on the ground in India, the production cost of a clay fired brick in the city of Muzaffarnagar
is 3 INR, establishing the maximum production cost for the alkali activate bricks to that value. It
is realized that brick prices vary throughout India, but this value has been taken as a baseline since
much of work towards implementing the technology has been done in Muzaffarnagar.
The alkali activated brick formulation used in this analysis is the same one used above for the
environmental analysis. The formulation was ash/clay/lime = 70/20/10, L/S = 0.325, and [NaOH]
= 2M. The costs taken into consideration included the materials, the labor, and the energy required
to produce the brick. Costs for the materials were based off local prices in Muzaffarnagar. The
required amount of labor and their wages were determined based on similar production scenarios,
which use automated machinery as would be used in this scenario. The energy demands were
based off similar machinery to what would be required and the energy prices were based on
Muzaffarnagar's rates. The analysis was based off the production of 10,000 bricks per day, a
reasonable capacity for an automated machine, and under the assumption that the plant would
operate for 26 days of the month (Sundays off) with one eight hour shift per day. Figure C.4 show
tables with the materials costs and also a breakdown of how the production cost for one alkali
activated brick was determined.
-
- 87
M. LARACY
| 2015
Paterial cost/unit
skied Labor Rate
abor cost/month540
abor cost/brick
N
0.208
(lunit=1 kWh)
J sift35
nis /8 h.
number of shifts/day
peration cost/month
perational cost/brick
cost/month
cost/brick
INR/month
6000
1
6
0.0245
INR
8878
2.8
Figure C.4: Tables showing the materials costs in Muzaffarnagar and the breakdown
alkali activated brick.
of the production costs for one
It was found that the production cost for this brick formulation was 2.88 1NR, not taking into
consideration any overhead costs. These costs were then broken down and are displayed in Figure
C.5 using a pie chart with comparison to the clay fired bricks costs. It can be observed that 92%
of the costs associated with the alkali activated brick come from the materials, whereas only 7%
is labor, and 1% is energy. The materials costs are further broken down such that 51% is NaOH,
35% is lime, and 14% is clay. Ash is considered to be of no cost, but could be considered a
negative cost as it is currently costs money to landfill.
These values will of course vary with
changes in the mix design, but it is apparent that NaOH and lime are the key contributors to the
production cost of these bricks. These numbers can be compared to the cost breakdown of the clay
fired brick which is 58% energy, 40% labor, and 2% materials (Schuchman 2014). A significant
difference is the cost of energy, which for alkali activated bricks is a small amount of electricity
to power a machine whereas the clay fired brick requires huge amounts of coal to fire the bricks.
88
-
-
APPENDIX C: ENVIRONMENTAL AND ECONOMIC IMPACT
Alkali Activated Brick
Clay Fired Brick
2%
7%
1
1%
40%6
92%
0 Raw Material IM Labor M Energy
Figure C.5: A breakdown of the costs associated with the production of clay fired bricks and alkali activated bricks.
The numbers that have been presented do not necessarily represent the true production cost of the
alkali activated brick, but are meant as a proof of concept that these bricks can be produced for
similar costs as the clay fired brick, making them a viable alternative in the market. Given this
information, careful consideration must be taken in choosing where to implement the technology
and the appropriate business model to create a profitable venture.
In choosing a location to implement the technology, a number of questions need to be answered.
The first question one may ask is what is the existing brick market? It is crucial to avoid regions
where bricks are highly accessible, affordable, and of good quality. Figure C.6 displays a map of
the average clay fired brick strengths in different regions of India. It is clear that regions like
Punjab, Delhi, Uttar Pradesh, and West Bengal should be avoided as these areas produce high
quality bricks. Furthermore, these regions are located on the fertile alluvial regions of the IndoGangetic plain, where over 65% of the country's brick production occurs (Yadav 2015).
Therefore, the availability of bricks in these regions is also high, which reduces prices as the cost
of transportation is not needed. Although current work to open a pilot plant has been focused on
the city of Muzaffamagar, Uttar Pradesh, future work should avoid this area as penetrating the
market will be difficult.
Some pictures of the work done in Muzaffarnagar using industrial
equipment is presented in Figure C.7.
89
-
-
M. LARACY
1 2015
3 MPa
3.5 - 5 MPa
U
U
U
U
U
5 MPa
3 - 7 MPa
3 - 10 MPa
7- 15 MPa
10 - 25 MPa
I
Figure C.6: A map of average clay fired brick strengths throughout a number of states in India.
(adopted from Guidelinesfor Structural Use of Reinforced Masonry 2005)
Another question one needs to ask is what are the availability of the raw materials? It is important
to produce bricks at a location that is close to sources of raw materials in order to avoid
transportation costs. Therefore, it is crucial to determine where boiler ash is being produced, its
quantity, and its availability throughout the year.
A third question one may ask is who is the customer? In tier 1 cities, such as Mumbai, the main
customer would be large scale residential and commercial developers. In tier 2 and 3 cities, there
would be a variety of customers from contractors to developers to individual home owners. It is
important to understand what these different customers desire in their bricks, so a location can be
chosen where the demand for the bricks will be high.
90
-
-
APPENDIX C: ENVIRONMENTAL AND ECONOMIC IMPACT
Once a location is chosen, one needs to determine the best strategy for selling the bricks. A few
different business models have been considered, weighing out the pros and cons of each, but one
key question is determining how to protect the intellectual property (IP) of our technology, to
prevent copycats. The different business models considered were proprietorship, join venture, and
selling the technology as a package deal.
Proprietorship would involve setting up a factory nearby a boiler ash source and producing the
bricks in house and directly selling to customers. The primary benefit of this business model is it
best protects the IP. Some cons are that it requires a large initial capital, it requires the factories
producing boiler ash to honor any deal made, and doing business in India without a local partner
is challenging.
The second business model, joint venture (JV), could be established with multiple small producers
of boiler ash or a few large producers and would require that the profits be shared. Some pros to
this model include a reduced capital and participation in the operations. Cons include the ease of
IP being leaked and the challenges in managing multiple JV's.
The third business model is selling the technology as a package to an entrepreneur or producer of
boiler ash. This package would include equipment, initial mix design formulation, and training on
the equipment and would be a one-time cost for the investor.
Additional revenues could be
collected on this business model through annual fees for maintenance, upgrades, or changes in the
mix design. The benefit of this model is there is very low capital and risk. However, there will be
no participation in the operations which may lead to poor brick quality and the IP has a high chance
of being leaked.
These business models that have been considered are suggestions and a more extensive comparison
is necessary once the technical work has progressed more. However, it is critical to understand
the needs of the customer and the potential business models as they will inform technical decisions
along the way. Future work should focus on identifying sources of boiler ash throughout the
country, in particular some of the larger producers. Also, it would be useful to get an initial cost
estimate of the equipment required to produce the bricks as well as a list of the pros and cons of
outsourcing its manufacturing or producing in house.
91
-
-
M. LARACY
1 2015
Figure C.7: Laboratory experiments were scaled up using industrial equipment in the city of Muzaffarnagar. The above
picture show: 1) Materials being weighed out 2) Materials being added to the pan mixer 3) A manual press (yellow) was
used to produce low pressure bricks 4) A hydraulic press was used to produce high pressure bricks 5) Measurements of the
bricks being taken 6) Testing the bricks for compressive strength.
92
-
-
APPENDIX D
Detailed Procedure for Sample Preparation
Preparation of the samples is extremely important and must be consistent across experiments in
order for results to be comparable. The goal of this appendix is to give step by step instructions
of the procedure used so others can replicate experiments in the future.
93
-
-
M. LARACY
| 2015
The first step in making the samples is weighing out the solid materials. After weighing, these
materials should be mixed in the kitchen aid on the lowest speed for 3 minutes or until the mixture
looks thoroughly homogenized. It has been observed that adding the boiler ash to the bowl first is
most effective, as clay and lime are more dense and do not mix as well when starting at the bottom
of the bowl. Low speed is used to prevent the material from flying into the air. The next step is
to remove the bowl from the mixer and add the designated amount of alkali source. The alkali
source should be prepared at least a day in advance to allow it to cool as the reaction is exothermic.
Once the alkali source has been added, the bowl should be placed back into the kitchen aid and the
speed should be slowly increased over a period of about 30 seconds until maximum speed is
reached. The material should continue to be mixed at maximum speed until its designated mixing
time is reached or it has achieved a particular consistency (see Appendix A for more information).
It is good to periodically stop the mixture (every 10 minutes for a longer mix) and homogenize by
hand to be sure no material is stuck at the bottom. Figure D.I spells out the steps related to mixing
the materials.
STEP 3
STEP 2
STEP 1
STEP 4
HIGH SPEED
LOW SPEED - 3 MINUTES
LIME33
Figure D.1: The four steps required for mixing of the materials during sample preparation include weighing out the
solids, mixing at low speed, adding the alkali source, and mixing at high speed.
Once mixing of the materials has finished, the binder is ready to be molded. There are a number
of different molds that can be used for this step pending the molding method selected (vibration
or hydraulic pressure). The mold options shown in Figure D.2 include 3 cube steel molds, 3 cube
plastic molds, single plastic cube molds, and the mold designed for hydraulic pressure. It has been
found that the most effective and efficient way to mold for vibration is by placing the single plastic
cube molds into the 3 cube plastic molds. The single plastic mold allows no liquid to escape and
the stiff walls of the 3 cube plastic mold prevent the sides of the single mold from expanding. For
molding with hydraulic pressure it is necessary to use the mold that was designed at MIT.
94
-
-
APPENDIX D: DETAILED PROCEDURE FOR SAMPLE PREPARATION
3 Cube Steel Mold
3 Cube Plastic Mold
Single Plastic Mold
Combination
Hydraulic Mold
Figure D.2: Various molds are available, however it has been determined that the combination mold is best for
vibration and the hydraulic mold is best when molding with hydraulic pressure.
Molding with vibration requires the sample to be cast in two layers. First the mold is loosely filled
and tamped by hand so that it is approximately half full. The mold is then transferred to the
vibration table where it is vibrated for 1 minute. Next, the mold is filled to the top and tamped
again before it goes back to the vibration table for another 1-2 minutes of vibration. Any material
that is overflowing the top of the mold is scraped away. The top of the mold is then sealed with
plastic wrap and duct tape to minimize the amount of moisture that escapes. Samples are then
labeled and weighed before being placed in the oven at their specified temperature.
B
A
Figure D.3: A steel mold was designed for molding bricks using hydraulic pressure via the Baldwin Tate Emery.
When samples are molded using hydraulic pressure, the molds designed at MIT to withstand high
pressure must be used. They essentially are made up of a square tube with two plungers. In
preparing the samples for molding, the tube is first temporarily supported using clamps as shown
in Figure D.3A. Next, a plunger is placed in the bottom, the tube is filled with mix, and a plunger
is inserted on top. The entire assembly is then transferred to the center of the Baldwin Tate Emery
95
-
-
M. LARACY
| 2015
machine for pressing using a load controlled program. After approximately 1000 N of pressure,
the temporary clamps are removed and the mold is able to "float" due to the pressure exerted on
the sides of the mold as seen in Figure D.3B. The plungers are now free to slide through the tube
ensuring the mixture is compressed from both directions. At the conclusion of pressing, the molds
are disassembled, the brick is removed, and they are wrapped, weighed, and placed in the oven.
At the designated compressive strength testing time, the samples should be removed and allowed
to cool down to room temperature before testing. Photos of some of the bricks made in the lab
and in the field can be seen in Figure D.4.
A
B
C
D
E
F
Figure D.4: Photos of bricks made in the lab and in the field. A) 2" cube made using vibration. B) 2" cube made using
hydraulic pressure. C) Full sized bricks made in the field in India using hydraulic pressure. D) Comparison of full
sized brick to lab scale brick. E&F) Dimensions of full sized brick.
96
-
-
References
Ahmari, Saeed, and Lianyang Zhang. 2012. "Production of Eco-Friendly Bricks from Copper Mine
Tailings through Geopolymerization." Construction and Building Materials29: 323-31.
doi:10.1016/j.conbuildmat.2011.10.048.
Alonso, S., and A. Palomo. 2001. "Alkaline Activation of Metakaolin and Calcium Hydroxide Mixtures:
Influence of Temperature, Activator Concentration and Solids Ratio." MaterialsLetters 47: 55-62.
doi: 10.1016/S0167-577X(00)00212-3.
"Alternative Building Materials." 2015. In Anil Agarwal Dialogue. Development Alternatives.
ASTM Standard C67-14. 2014. "Standard Test Methods for Sampling and Testing Brick and Structural
Clay Tile". ASTM International. West Conshohocken, PA.
ASTM Standard C311-13. 2013. "Standard Test Methods for Sampling and Testing Fly Ash or Natural
Pozzolans for Use in Portland-Cement Concrete". ASTM International. West Conshohocken, PA.
ASTM Standard C618-12a. 2013. "Standard Specification for Coal Fly Ash and Raw or Calcined Natural
Pozzolan for Use in Concrete". ASTM International. West Conshohocken, PA.
ASTM Standard D4328-13. 2013. "Standard Test Method for Major and Minor Elements in Coal and
Coke Ash By X-Ray Fluorescence". ASTM International. West Conshohocken, PA.
Bakharev, Tatiana, Jay Gnananandan Sanjayan, and Yi Bing Cheng. 1999. "Alkali Activation of
Australian Slag Cements." Cement and Concrete Research 29 (1): 113-20. doi:10.1016/S00088846(98)00170-7.
Bernal, S, and J Provis. 2014. "Durability of Alkali-Activated Materials: Progress and Perspectives."
Journalof the American Ceramic Society 97: 997-1008. doi:10. 1111/jace.12831.
Bhattacharyya, S.K., L.P. Lingh, and A.K.. Agarwal. 2012. "Fly Ash: An Overview." The Built
Environ(CSIR-CentralBuilding Research Institute (CBRI)) 8 (2). Roorkee.
http://cbrienvis.nic.in/newsletter/NL(2) 2012/ENVIS2.pdf.
Black, George. "India's Struggle to Get Reliable Power to Hundreds of Millions of People. " OCtober 31,
2014. Accessed May 13, 2015.
Chancey, Ryan T., Paul Stutzman, M. C G Juenger, and David W. Fowler. 2010. "Comprehensive Phase
Characterization of Crystalline and Amorphous Phases of a Class F Fly Ash." Cement and Concrete
Research 40: 146-56. doi:10.1016/j.cemconres.2009.08.029.
Chandramouli, C. 2011. ProvisionalPopulation Totals. Census of India 2011.
http://www.censusindia.gov.in/20 11 -prov-results/datafiles/india/FinalPPT_201
_chapter3.pdf.
Chaudhary, D. S., and M. C. Jollands. 2004. "Characterization of Rice Hull Ash." JournalofApplied
Polymer Science 93 (1): 1-8. doi: 10.1002/app.20217.
-
97
Chen, W., and H. J H Brouwers. 2007. "The Hydration of Slag, Part 1: Reaction Models for AlkaliActivated Slag." JournalofMaterials Science 42: 428-43. doi: 10.1007/si0853-006-0873-2.
Chen-Tan, Nigel W., Arie Van Riessen, Chi V. Ly, and Daniel C. Southam. 2009. "Determining the
Reactivity of a Fly Ash for Production of Geopolymer." Journalof the American Ceramic Society
92 (4): 881-87. doi:10.111l/j.1551-2916.2009.02948.x.
Chindaprasirt, P, C Jaturapitakkul, and T Sinsiri. 2005. "Effect of Fly Ash Fineness on Compressive
Strength and Pore Size of Blended Cement Paste." Cement and Concrete Composites 27 (4): 425-
28. doi:10.1016/j.cemconcomp.2004.07.003.
Chindaprasirt, P., S. Homwuttiwong, and V. Sirivivatnanon. 2004. "Influence of Fly Ash Fineness on
Strength, Drying Shrinkage and Sulfate Resistance of Blended Cement Mortar." Cement and
Concrete Research 34 (7): 1087-92. doi:10.1016/j.cemconres.2003.11.021.
Della, V. P., I. KUhn, and D. Hotza. 2002. "Rice Husk Ash as an Alternate Source for Active Silica
Production." Materials Letters 57 (4): 818-21. doi: 10.10 16/SO 167-577X(02)00879-0.
Diaz, E. I., E. N. Allouche, and S. Eklund. 2010. "Factors Affecting the Suitability of Fly Ash as Source
Material for Geopolymers." Fuel 89: 992-96. doi: 10.101 6/j.fuel.2009.09.012.
Duxson, P, John L. Provis, Grant C. Lukey, and J. S J van Deventer. 2007. "The Role of Inorganic
Polymer Technology in the Development of 'Green Concrete."' Cement and Concrete Research 37:
1590-97. doi:10.1016/j.cemconres.2007.08.018.
Duxson, P., A. Fernindez-Jimenez, J. L. Provis, G. C. Lukey, A. Palomo, and J. S J Van Deventer. 2007.
"Geopolymer Technology: The Current State of the Art." JournalofMaterials Science 42: 2917-33.
doi: 10.1007/s10853-006-0637-z.
"Eco Brick." 2012. http://ecobrick.in/Default.aspx.
Feng, Dingwu, John L. Provis, and Jannie S J Van Deventer. 2012. "Thermal Activation of Albite for the
Synthesis of One-Part Mix Geopolymers." Journalof the American Ceramic Society 95 (29905):
565-72. doi:10.1111/j.1551-2916.2011.04925.x.
Fernidndez-Jim6nez, A., and A. Palomo. 2003. "Characterisation of Fly Ashes. Potential Reactivity as
Alkaline Cements." Fuel 82: 2259-65. doi:10.1016/S0016-2361(03)00194-7.
Fermindez-Jim6nez, A., A. G. De La Torre, A. Palomo, G. L6pez-Olmo, M. M. Alonso, and M. A G
Aranda. 2006a. "Quantitative Determination of Phases in the Alkali Activation of Fly Ash. Part I.
Potential Ash Reactivity." Fuel 85: 625-34. doi:10.1016/j.fuel.2005.08.014.
Ferindez-Jimenez, A., A. Palomo, I. Sobrados, and J. Sanz. 2006b. "The Role Played by the Reactive
Alumina Content in the Alkaline Activation of Fly Ashes." Microporous and Mesoporous Materials
91 (1-3): 111-19. doi:10.1016/j.micromeso.2005.11.015.
Fu, Xiaoru, Qin Li, Jianping Zhai, Guanghong Sheng, and Feihu Li. 2008. "The Physical-Chemical
Characterization of Mechanically-Treated CFBC Fly Ash." Cement and Concrete Composites 30
(3): 220-26. doi:10.1016/j.cemconcomp.2007.08.006.
-
98
Garcia-Lodeiro, I., A. Palomo, A. Ferndndez-Jim6nez, and D. E. MacPhee. 2011. "Compatibility Studies
between N-A-S-H and C-A-S-H Gels. Study in the Ternary Diagram Na20-CaO-AI203-SiO 2H20." Cement and Concrete Research 41 (9): 923-31. doi: 10.101 6/j.cemconres.2011.05.006.
Guidelinesfor Structural Use of Reinforced Masonry. 2005. Kanpur.
file:///C:/Users/Michael/Downloads/GSDMA_2005.pdf.
Hajimohammadi, Ailar, John L. Provis, and J. S J Van Deventer. 2008. "One-Part Geopolymer Mixes
from Geothermal Silica and Sodium Aluminate." Industrialand EngineeringChemistry Research
47: 9396-9405. doi:10.1021/ie8006825.
He, Jian, Yuxin Jie, Jianhong Zhang, Yuzhen Yu, and Guoping Zhang. 2013. "Synthesis and
Characterization of Red Mud and Rice Husk Ash-Based Geopolymer Composites." Cement and
Concrete Composites 37 (1): 108-18. doi: 10. 1016/j.cemconcomp.2012.1 1.010.
"India I Data." 2014. The World Bank. http://data.worldbank.org/country/india.
"Industrial Waste Generation and Management in India." 2012.
http://www.eai.in/ref/ae/wte/typ/clas/indiaindustrialwastes.html.
James, K S. 2011. "India's Demographic Change: Opportunities and Challenges." Science (New York,
N.Y) 333: 576-80. doi:10.1126/science.1207969.
Jitendra, C. 2015. "Brick Kiln Workers in India Underpaid and Exploited." Anil Agarwal Dialogue,
March 12. http://www.downtoearth.org.in/content/brick-kiln-workers-india-underpaid-andexploited.
Juenger, M. C G, F. Winnefeld, J. L. Provis, and J. H. Ideker. 2011. "Advances in Alternative
Cementitious Binders." Cement and Concrete Research 41: 1232-43.
doi:10.1016/j.cemconres.2010.11.012.
Khale, D, and R Chaudhary. 2007. "Mechanism of Geopolymerization and Factors Influencing Its
Development: A Review." Journal of MaterialsScience 42 (3): 729-46. doi:10.1007/s10853-006-
0401-4.
Kiattikomol, K., C. Jaturapitakkul, S. Songpiriyakij, and S. Chutubtim. 2001. "A Study of Ground Coarse
Fly Ashes with Different Finenesses from Various Sources as Pozzolanic Materials." Cement and
Concrete Composites 23 (4-5): 335-43. doi: 10.10 16/SO958-9465(01)00016-6.
Komnitsas, Konstantinos A. 2011. "Potential of Geopolymer Technology towards Green Buildings and
Sustainable Cities." In ProcediaEngineering, 21:1023-32. doi: 10.101 6/j.proeng.2011.11.2108.
Kumar, Rakesh, Sanjay Kumar, and S. P. Mehrotra. 2007. "Towards Sustainable Solutions for Fly Ash
through Mechanical Activation." Resources, Conservationand Recycling.
doi: 10.101 6/j.resconrec.2007.06.007.
Kumar, S, D.P. Sahoo, S.K. Nath, T.C. Alex, and R Kumar. 2012. "From Grey Waste to Green
Geopolymer." Science and Culture 78 (11-12): 511-16.
file:///C:/Users/Michael/Downloads/Sciculture (1).pdf.
-
99
Kumar, Sanjay, and Rakesh Kumar. 2011. "Mechanical Activation of Fly Ash: Effect on Reaction,
Structure and Properties of Resulting Geopolymer." Ceramics International37 (2): 533-41.
doi: 10.1016/j.ceramint.2010.09.038.
Li, Chao, Henghu Sun, and Longtu Li. 2010. "A Review: The Comparison between Alkali-Activated Slag
(Si + Ca) and Metakaolin (Si + Al) Cements." Cement and ConcreteResearch 40: 1341-49.
doi:10. 1016/j.cemconres.2010.03.020.
Lloyd, R, J Provis, K Smeaton, and J van Deventer. 2009. "Spatial Distribution of Pores in Fly Ash-Based
Inorganic Polymer Gels Visualised by Wood's Metal Intrusion." Microporous and Mesoporous
Materials 126: 32-39. http://ac.els-cdn.com/S1387181109002595/1-s2.0-S1387181109002595main.pdf?_tid=0e8f60ee-e830-1 1e4-941600000aacb35d&acdnat=1429625563_8bd38472ebf4e680742f6dce3cf4e644.
Lloyd, Redmond R., John L. Provis, and J. S J Van Deventer. 2010. "Pore Solution Composition and
Alkali Diffusion in Inorganic Polymer Cement." Cement and Concrete Research 40 (9): 1386-92.
doi: 10. 1016/j.cemconres.2010.04.008.
Lothenbach, B., and A. Gruskovnjak. 2007. "Hydration of Alkali-Activated Slag: Thermodynamic
Modelling." Advances in Cement Research. doi: 10.1 680/adcr.2007.19.2.81.
Maithel, Sameer, and R. Uma. 2012. Brick Kilns Performance Assessment: A Roadmapfor Cleaner Brick
Productionin India.
http://www.catf.us/resources/publications/files/BrickKilnsPerformanceAssessment.pdf.
Montakarntiwong, Kawee, Nuntachai Chusilp, Weerachart Tangchirapat, and Chai Jaturapitakkul. 2013.
"Strength and Heat Evolution of Concretes Containing Bagasse Ash from Thermal Power Plants in
Sugar Industry." Materialsand Design 49: 414-20. doi:10.1016/j.matdes.2013.01.03 1.
Mufioz Velasco, P., M. P. Morales Ortiz, M. A. Mendivil Gir6, and L. Muhoz Velasco. 2014. "Fired Clay
Bricks Manufactured by Adding Wastes as Sustainable Construction Material - A Review."
Constructionand Building Materials. doi: 10.101 6/j.conbuildmat.2014.03.045.
Myers, Rupert J., Susan A. Bernal, Rackel San Nicolas, and John L. Provis. 2013. "Generalized Structural
Description of Calcium-Sodium Aluminosilicate Hydrate Gels: The Cross-Linked Substituted
Tobermorite Model." Langmuir 29: 5294-5306. doi:10.1021/la4000473.
Pacheco-Torgal, Fernando, Jodo Castro-Gomes, and Said Jalali. 2008a. "Alkali-Activated Binders: A
Review. Part 1. Historical Background, Terminology, Reaction Mechanisms and Hydration
Products." Construction and Building Materials. doi: 10.101 6/j.conbuildmat.2007.10.015.
Pacheco-Torgal, Fernando, Jodo Castro-Gomes, and Said Jalali. 2008b. "Alkali-Activated Binders: A
Review. Part 2. About Materials and Binders Manufacture." Constructionand Building Materials.
doi: 10.101 6/j.conbuildmat.2007.03.019.
Pappu, Asokan, Mohini Saxena, and Shyam R. Asolekar. 2007. "Solid Wastes Generation in India and
Their Recycling Potential in Building Materials." Building and Environment 42: 2311-20.
doi: 10. 1016/j.buildenv.2006.04.015.
100
-
-
Payi, J, J Monz6, M.V Borrachero, A Mellado, and L.M Ordofiez. 2001. "Determination of Amorphous
Silica in Rice Husk Ash by a Rapid Analytical Method." Cement and Concrete Research.
doi: 10.101 6/S0008-8846(00)00466-X.
Peng, Mei Xun, Zheng Hong Wang, Shao Hua Shen, and Qiu Guo Xiao. 2014. "Synthesis,
Characterization and Mechanisms of One-Part Geopolymeric Cement by Calcining Low-Quality
Kaolin with Alkali." Materials and Structures 48: 699-708. doi: 10.1617/s11527-014-0350-3.
Provis, J. 2013. "Geopolymers and Other Alkali Activated Materials: Why, How, and What?" Materials
and Structures 47 (1-2): 11-25. doi:10.1617/s11527-013-0211-5.
Provis, J, and S Bernal. 2014. "Geopolymers and Related Alkali-Activated Materials." Annual Review of
MaterialsResearch, in press, DOI 10.1 146/annurev - matsci - 070813-515.
doi:doi:10.1 146/annurev-matsci-070813-113515.
Provis, J, and J van Deventer. 2014. Alkali Activated Materials: State-of-the-Art Report, RILEM TC 224AAM. 1st ed. Springer Netherlands. doi:10.1007/978-94-007-7672-2.
Provis, John L., Rachel M. Harrex, Susan A. Bernal, Peter Duxson, and Jannie S J Van Deventer. 2012.
"Dilatometry of Geopolymers as a Means of Selecting Desirable Fly Ash Sources." JournalofNonCrystalline Solids 358 (16): 1930-37. doi:10.1016/j.jnoncrysol.2012.06.001.
Provis, John L., Rupert J. Myers, Claire E. White, Volker Rose, and Jannie S J Van Deventer. 2012. "XRay Microtomography Shows Pore Structure and Tortuosity in Alkali-Activated Binders." Cement
and Concrete Research 42 (6): 855-64. doi: 10.1016/j.cemconres.2012.03.004.
Rickard, William D A, Ross Williams, Jadambaa Temuujin, and Arie van Riessen. 2011. "Assessing the
Suitability of Three Australian Fly Ashes as an Aluminosilicate Source for Geopolymers in High
Temperature Applications." Materials Science and EngineeringA 528 (9): 3390-97.
doi:10.1016/j.msea.2011.01.005.
Riza, Fetra Venny, Ismail Abdul Rahman, Ahmad Mujahid, and Ahmad Zaidi. 2010. "A Brief Review of
Compressed Stabilized Earth Brick (CSEB)." In CSSR 2010 - 2010 InternationalConference on
Science and Social Research, 999-1004. doi: 10.1 109/CSSR.2010.5773936.
Roy, Della M. 1999. "Alkali-Activated Cements: Opportunities and Challenges." Cement and Concrete
Research 29: 249-54. doi:10.1016/S0008-8846(98)00093-3.
Sathonsaowaphak, Apha, Prinya Chindaprasirt, and Kedsarin Pimraksa. 2009. "Workability and Strength
of Lignite Bottom Ash Geopolymer Mortar." Journalof HazardousMaterials 168 (1): 44-50.
doi: 10.1016/j.jhazmat.2009.01.120.
Schuchman, N. 2014. "Environmental and Economic Tradeoffs in Building Materials Production in
India." Massachusetts Institute of Technology.
Shi, Caijun, A. Fernandez Jim6nez, and Angel Palomo. 2011. "New Cements for the 21st Century: The
Pursuit of an Alternative to Portland Cement." Cement and Concrete Research 41: 750-63.
doi:10.1016/j.cemconres.2011.03.016.
-
- 101
Shinohara, Yasushi, and Norihiko Kohyama. 2004. "Quantitative Analysis of Tridymite and Cristobalite
Crystallized in Rice Husk Ash by Heating." IndustrialHealth 42 (2): 277-85.
doi: 10.2486/indhealth.42.277.
Slanioka, S. 1991. "The Influence of Fly Ash Fineness on the Strength of Concrete." Cement and
Concrete Research. doi: 10.1016/0008-8846(91)90010-F.
Temuuj in, J., R. P. Williams, and A. van Riessen. 2009. "Effect of Mechanical Activation of Fly Ash on
the Properties of Geopolymer Cured at Ambient Temperature." JournalofMaterialsProcessing
Technology 209 (12-13): 5276-80. doi:10.1016/j.jmatprotec.2009.03.016.
Van Deventer, Jannie S J, John L. Provis, Peter Duxson, and David G. Brice. 2010. "Chemical Research
and Climate Change as Drivers in the Commercial Adoption of Alkali Activated Materials." Waste
and Biomass Valorization 1: 145-55. doi:10.1007/s12649-010-9015-9.
Van Jaarsveld, J. G S, J. S J Van Deventer, and G. C. Lukey. 2002. "The Effect of Composition and
Temperature on the Properties of Fly Ash- and Kaolinite-Based Geopolymers." Chemical
EngineeringJournal89 (1-3): 63-73. doi:10.1016/Si385-8947(02)00025-6.
Van Jaarsveld, J. G S, J. S J Van Deventer, and G. C. Lukey. 2003. "The Characterisation of Source
Materials in Fly Ash-Based Geopolymers." MaterialsLetters 57: 1272-80. doi:10.1016/S0167577X(02)00971-0.
"World Bank to Revolutionise Brick Making in India." 2006. The Hindu News.
http://www.stoves.bioenergylists.org/cdmbrickindia.
Yadav, N. 2015. "Can Polluting Brick Kilns Be Cleaned up? Down To Earth," March 12.
http://www.downtoearth.org.in/content/can-polluting-brick-kilns-be-cleaned.
Yip, C. K., G. C. Lukey, and J. S J Van Deventer. 2005. "The Coexistence of Geopolymeric Gel and
Calcium Silicate Hydrate at the Early Stage of Alkaline Activation." Cement and Concrete Research
35: 1688-97. doi:10.1016/j.cemconres.2004.10.042.
Zhang, Lianyang. 2013. "Production of Bricks from Waste Materials - A Review." Construction and
Building Materials. doi: 10.101 6/j.conbuildmat.2013.05.043.
102
-
-