Assessing the Fate of PAHs in the Boston Inner Harbor Using
Semipermeable Membrane Devices (SPMDs)
by
Amparo E. Flores
Environmental Engineering Science, B.S.
University of California at Berkeley, 1996
Submitted to the Departmentof Civil and EnvironmentalEngineering
In PartialFulfillment of the Requirements for the Degree of
MASTER OF ENGINEERING
IN CIVIL AND ENVIRONMENTAL ENGINEERING
at the
MASSACHUSETTS INSTITUTE OF TECHNOLOGY
June 1998
The author hereby grants to M.I.T permission to reproduce & distribute publicly
paper & electroniccopies of this thesis document in whole or in part.
Signature of the Author
r or_
Department of Civil and Environmental Engineering
May 15, 1998
Certified by__
i/
Philip Gschwend,Ph.D.
Professor of Civil and Environmental Engineering
Thesis Supervisor
Certified by
Professor Joseph Sussman
Chairman, Department Committee on Graduate Studies
© 1998 Amparo E. Flores.All rights reserved.
Assessing the Fate of PAHs in Boston Inner Harbor Using
Semipermeable Membrane Devices (SPMDs)
by
Amparo E. Flores
Submitted to the Department of Civil and Environmental Engineering
In Partial Fulfillment of the Requirements for the Degree of
Master of Engineering in Civil and Environmental Engineering
ABSTRACT
This study used a relatively new sampling tool called semipermeable membrane devices
(SPMDs) to quantify dissolved concentrations of the following polycyclic aromatic
hydrocarbons (PAHs) in three locations of the Boston Inner Harbor: phenanthrene, methylThe field
phenanthrene's, fluoranthene, pyrene, benz(a)anthracene, and chrysene.
measurements ranged from 0.61 to 90 ng/L for the various PAHs at the base of Tobin Bridge,
the mouth of Chelsea River, and near the Logan Airport. A vertical gradient in PAH
concentrations was observed at the base of Tobin Bridge, revealing a concentration ten times
greater in the upper layer (4 m from the surface) than the lower layer (8 m from the surface).
The concentrations increased with distance from the mouth of the Inner Harbor.
The ratios of methyphenanthrene's-to-phenanthrene and pyrene-to-fluoranthene were
calculated as possible indicators of source and transport behavior.
In all three sites,
methylated phenanthrene levels were found to be about twice that of phenanthrene levels,
indicating that one of the main sources of low molecular weight PAHs into these sections of
the harbor is of petrogenic origin. The abundance of small analytes in the samples suggested
a low-molecular weight petroleum source such as diesel, creosote, and/or coal tar. The ratios
of pyrene-to-fluoranthene suggested another type of origin. Based on the pyrene-tofluoranthene ratios obtained here, Boston air, street dust, and creosote are also potential
major primary sources of PAHs in the study area.
SPMDs appear to be a useful tool for quantifying dissolved PAHs in the field. The
measurements obtained in this study were in good agreement with the results of a box model
and 3-D hydrodynamic model of PAH concentrations in the Inner Harbor.
Thesis Supervisor: Philip Gschwend, Ph.D.
Title: Professor of Civil and Environmental Engineering
ACKNOWLEDGMENTS
I would like to thank the following people who have been instrumental in helping me
achieve this work
.......... my project group members, Sharon Ho, Peter Israelsson,and Ricardo Petroni,for
their insights and esprit de corps.
.......... John MacFarlane,for being an excellent and patient teacher who never tired of my
endless questions.
.......... Rachel Adams, for her assistance with the SPMD deployment and analysis.
.......... ProfessorPhilip Gschwend, for always pushing the boundaries of my knowledge and
inspiring me with his brilliance and energy.
.......... James Huckins of the US Geological Survey's Midwest Science Center, for taking the
time to guide me on the application of SPMDs.
.......... The US Coast Guard, for their help and cooperation in the deployment of the SPMDs.
They truly went beyond the call of duty.
On a more personal note, I would like to express my deepest gratitude to the following
people:
.......... Ana Pinheiro,without whom I would not have been able to stay awake during those
long nights. You were always there to provide support, friendship, and distractions.
.......... Charles Njendu, for the pleasure of discussing DNA sequences and Ayn Rand in the
wee hours of the night.
.......... Conny Mitterhofer, Ricardo Petroni, andPeter Israelsson,for sharing your friendship
and work experience with me. You taught me many things.
.......... My sister, Adeliza Flores, who has always provided unconditional support. You
inspired me with your patience and enthusiasm for your research work in
Chemistry. You deserve the best in life and I share all my accomplishments with you.
.......... a very special man in my life, Dr. DanielBarsky, full-time biophysicist, part-time
SPMD and C. parvum consultant. I am so lucky.
And, lastly, the people who showed me the beauty of science and inspired me with their
enthusiasm and encouragement, my teachers at Contra Costa College, Dr. Joseph Ledbetter,
Dr. James Conrad,and Mr. Don Wieber. Thank you.
This material is based upon work supported under a National Science Foundation
Graduate Fellowship. I am grateful to the NSF for providing me the incredible opportunity
to study here at MIT.
Any opinions,findings, conclusions or recommendationsexpressed in this publicationare those of the author
and do not necessarilyreflect the views of the NationalScience Foundation.
Acknowledgments
TABLE OF CONTENTS
LIST OF F IGU RE S .........................
6
..........................................................................................
L IST OF T A B LE S.............................................................................
............................................
7
8
......................................
1. IN TRO D U C TION ..................................................................
2. POLYCYCLIC AROMATIC HYDROCARBONS (PAHS)...................................................
13
2.1 Chemical Structure and Properties.....................................
........
....
2.2 Sources and Fate in the Environment .....................................
13
17
3. BOSTON HARBOR BACKGROUND AND HISTORY.................................
3.1 Physical and Hydrographic Characteristics .......................................
3.2 Sources of Contamination in Boston Harbor..................................
......
21
...... 21
....... 24
4. PROBLEM STATEMENT AND OBJECTIVES.................................
27
5. SEMIPERMEABLE MEMBRANE DEVICES........................................................................
31
5.1 H istorical D evelopm ent....................................................................................................
5.2 D esign of SP MD s........................................................ ................................................
.........................................
5.3 U ptake K inetics of SPM D s...............................................
31
35
39
..........................................
42
........................
6.1 Reagents and M aterials...................................................................
6.1.1 Reagents and solvents
6.1.2 Materials
...................................
6.2 M ethod s...................................................................................
6.2.1 General glassware cleaning procedure
6.2.2 Laboratory experiment
6.2.2.1 Preparationof the spikes
6.2.2.2 Analyte dischargefrom the SPMDs
6.2.3 Field Experiment
6.2.3.1 Construction of the SPMD cages
6.2.3.2 Sampling Sites
6.2.3.3 Deployment of the SPMDs: February13, 1998
6.2.3.4 SPMD collection: February24, 1998
6.2.4 Extraction and Analysis of the SPMDs
6.2.4.1 Dialysis
6.2.4.2 Kuderna-DanishConcentration
6.2.4.3 N2 blowdown and hexane exchange
6.2.4.4 SiO2 Gel Column Chromatography
43
6. EXPERIM ENTAL PROCEDURE...............................................
Table of Contents
45
6.2.4.5 Gas Chromatography
7. RESULTS AND DISCU SSION ...................................................
............................................ 56
7.1 Laboratory Experiment: Dissipation of Compounds from SPMDs........................
7.2 Field Measurem ents......................................................................................................
7.3 Comparison of Measurements to a Mass Balance (Box) Model and a 3-D
Hydrodynam ic Model................................................... .............................................
7.4 Comparison of Measured Water Column Concentrations to Sediment
C oncentrations............... ...........................................................................................
7.5 C om parison of Loadings................................................. ...........................................
8. C ON C LU SIO N ..................................................................................
73
76
78
....................................... 80
81
REFERENCES..........................................
APPENDIX A - Laboratory Experiment Data and Calculations............................
.....
APPENDIX B - Field Experiment Data and Calculations......................................99
Table of Contents
56
60
88
LIST OF FIGURES
Figure 1.1. Summary of estimated PAH loadings to Massachusetts Bay broken down by
source type (Menzie-Cura and Associates, 1995).............................................11
Figure 2.1 The structures of some polycyclic aromatic hydrocarbons commonly found in
the environm ent..................................................
Figure 3.1. B oston Harbor...................................................
........................................... 16
............................................ 22
Figure 3.2. Boston Inner Harbor.........................................................................................23
Figure 5.1. Correlation of log Ktw with log Kow for selected organic compounds............37
Figure 5.2. An exploded view of the triolein-membrane-water film model......................39
Figure 6.1. SPMD-loaded wire cage.....................................................48
Figure 6.2. Components of creosote.................................................50
Figure 7.1. Dissipation of pyrene from the SPMDs in the laboratory experiment...............57
Figure 7.2. PAH chromatogram of the Logan Airport - Buoy #12 (LA) sample after first
run through the silica column................................................61
Figure 7.3. (a) GC-FID chromatogram of the Tobin Bridge-surface PAH sample fraction.62
(b) High resolution gas chromatogram of PAHs in Charles River sediment......62
Figure 7.4. (a) Gas chromatogram of a PAH standard...............................
...... 64
(b) Gas chromatogram of the Tobin Bridge-bottom PAH fraction sample.........64
Figure 7.5. Summary of PAH concentrations at the different sampling sites...................70
Figure 7.6. Vertical profile of pyrene concentrations at the confluence of the Chelsea and
Mystic Rivers predicted by 3-D hydrodynamic model and data from SPMD
measurements at the Base of Tobin Bridge and box model
or mass balance predicted steady-state concentration.......................
.... 75
Figure 7.7. Sampling locations in the PAH sediment concentration study by Shiaris et al.
(19 86)...........................................................
List ofFigures
.................................................. 76
LIST OF TABLES
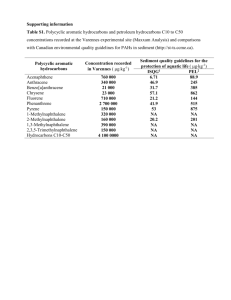
Table 1.1. Low molecular weight (LMW) PAHs and high molecular weight (HMW) PAHs
.... 10
as defined by Menzie-Cura and Associates (1995)...............................
Table 2.1. Physical/chemical constants of some PAHs commonly found in the
14
environm ent...........................................................................................................
Table 2.2. Typical concentration ranges of benzo(a)pyrene and total PAHs in various
aquatic system s...................................................
............................................. 19
Table 5.1. A comparison of log Kow values versus log Ktw values for a range of organic
compounds (Chiou, 1985)....................................................38
Table 6.1. Sampling depths at the different sites.............................................49
Table 7.1. Rs or sampling rates (relative standard deviation in % ), KSPMD, and ke or
dissipation rates at different temperatures....................................59
Table 7.2. Phenanthrene and Methyphenanthrene concentrations in ng/L and their ratios
at various sites....................................................
............................................. 68
Table 7.3. Fluoranthene and Pyrene concentrations in ng/L and their ratios at various
sites..................................................................................
................................ 68
Table 7.4. Benz(a)anthracene and chrysene concentrations in ng/L at various sites..........68
Table 7.5. Pyrene-to-fluoranthene concentration ratios of common sources of PAHs in the
environm ent......................................................
...............................................
71
Table 7.6. (a) Pyrene and fluoranthene loadings from rivers and CSOs and stormwater
drains into Boston Harbor..................................................
72
(b) Pyrene-to-fluoranthene ratios in rivers and CSOs and stormwater.............72
Table 7.7. Sources and sinks of PAHs in the Inner Harbor................................
..... 73
Table 7.8. SPMD-derived concentrations (accounting for + 42% error), predicted steady
-state concentration from mass balance or box model, and predicted
concentrations from 3-D hydrodynamic model................................
..... 74
Table 7.9. Weighted sediment pyrene concentrations....................................77
Table 7.10. Loadings of pyrene into the Inner Harbor.................................
List of Tables
...... 79
1. INTRODUCTION
For centuries, Boston Harbor has served as a receptacle for human waste (MWRA, 1996).
Over the past decades, the public has become increasingly concerned with the impact of
sewage disposal on the harbor. In the 1980's, litigation over the pollution of Boston Harbor
resulted in the development of a Federal court-ordered schedule to plan and build proper
sewage treatment facilities for the over five hundred million gallons of sewage generated by
2.5 million people and 5,500 businesses in Boston and surrounding areas (MWRA, 1991). In
1985, the Massachusetts Water Resources Authority (MWRA) was created for the purpose of
managing water and sewer services in the area. One of the MWRA's biggest projects is the
Boston Harbor Project, whose main goal is the improvement of water quality in the harbor.
Upon completion, the project is expected to cost an estimated 4 billion dollars (Sea Grant,
1996). Since the development of the MWRA, extensive work has been done to try to abate
the pollution in Boston Harbor.
Currently, metropolitan Boston is attempting to fix a different sort of problem in the harbor.
Much of the region's economy depends on the area's access and accessibility to the waters of
the Atlantic Ocean. As of 1996, harbor commerce was generating $8 billion annually in
revenue (MWRA, 1996).
However, as rivers and sewage outfalls empty into the harbor,
sediments carried by these sources settle to the harbor floor. Eventually, sediment build-up
raises the harbor floor to the point where the water column is too shallow for large ships to
navigate. To correct this problem, the state of Massachusetts has been allocating funds to
remove sediment from the bottom of the harbor floor. By dredging both accumulated and
native sediment, shipping lanes in the harbor will be deepened and better access will result
(USACOE, 1988).
Introduction
Fortunately, the dredging could also have a significant positive impact on the harbor's water
quality. It has been noted that the Boston Harbor sediments are extremely contaminated with
a multitude of toxic metals and organic compounds (MWRA,1990). Sediment concentrations
of certain metals and PAHs are so high that it is suspected that there is a chemical flux of
contaminants from the sediment to the harbor water column (Stolzenbach and Adams, 1998).
By removing this possible source of contaminants, the water column concentrations of these
chemicals may be decreased. If so, dredging could actually improve the water quality in the
harbor.
Because the sediments are extremely contaminated, they must be treated as hazardous waste
if they are removed from the harbor. The complete treatment and disposal of hazardous
waste can be extremely expensive, so an alternate plan was conceived. The United States
Army Corps of Engineers (USACOE) proposed to bury the contaminated sediments in the
harbor itself. Disposal cells will be dug in the northern area of the Inner Harbor and filled
with the dredged material. The cleaner sediments that are excavated to make the cells will be
dumped elsewhere. The cells will then be "capped" with approximately three feet of clean
sand, which is intended to eliminate (or at least drastically reduce) the chemical releases from
the dredged materials.
Both dredging and capping have been used in other areas as a means of remediation (Stivers
and Sullivan, 1994), but they have not been demonstrated to be effective in an area such as
Boston Harbor. In order to evaluate the impact of the dredging and capping processes on the
water quality of the harbor, it is necessary to first determine the pre-dredging and -capping
conditions. A particular group of compounds, polycyclic aromatic hydrocarbons (PAHs), is
taken here to represent the general state of the harbor's water quality. It has been recognized
for many years that some PAHs can cause cancer in laboratory mammals and possibly
humans, and occupational exposures to PAHs have been correlated with the incidence of
human cancer (International Agency for Research on Cancer, 1973; National Academy of
Sciences, 1972; Bridboard et al., 1976) so PAHs are of major concern to regulators and the
Introduction
general public. As a consequence, sixteen PAH compounds are on the EPA priority pollutant
list (MWRA, 1993).
Starting in 1991, a major study sponsored by the MWRA estimated PAH inputs into the near
shore regions of Massachusetts Bay, including Boston Harbor, using site-specific non-point
and point source PAH concentration data from waterborne sources (Alber and Chan, 1994).
A study by Golomb et al. (1996) looked into atmospheric PAH loadings into Massachusetts
Bay. The MWRA study found that the major waterborne sources for low molecular weight
(LMW) PAHs versus high molecular weight (HMW) PAHs varied. Low molecular weight
compounds ranged from naphthalene to dibenzothiophene, while high molecular weight
compounds ranged from fluoranthene to benzo(g,h,i)perylene (Table 1.1).
The greatest
loadings of LMW compounds were from sewage and sludge discharges from publiclyowned treatment works or POTWs, while the greatest source of HMW compounds were from
non-point sources including rivers (Cura and Studer, 1996) (Figure 1.1). The difference in
sources, and therefore discharge points, may have important implications for the distribution
of PAH compounds over Boston Harbor.
Since PAHs vary in their toxicity (National
Academy of Sciences, 1972), it is important to identify and quantify the individual PAHs
found in the water column of different regions of the harbor.
PAH COMPOUNDS
Low Molecular WeaghtPAEs
*Naphtabalee
C1-Naphthalen
C2-Naphtbhalen
C3-Naphthale=
C4-Naphrhalene
*Ac•phthylene
*Acenaphthene
Brphenyl
*Fluorenc
CI-Fluoree
C2-Fluorne
C3-Fluore•
*Pb t
*Anhrcen
C -Phen-nhrene/Anthracenc
C-PhenanthrneAnrhracccime
C3-Phcnaarm/ AathrneA
•hrcee
C4-PhbenanthreAnthracenc
Dibenzothiophene
CI-Dibentothiopbene
C2-Dibenzothopbene
C3-Dibenzothiopbnew
High MolecuLaWeight PABs
Fluormram=c
.Py* =
CI- Fluoranthen/Pyre
*Benzo(a)ahr;acene
*Chrysen
CI-Chrysene
C-Chzrysec
C3-Chrysc
C4schylsec
*Benzo(b)fluoranthezn
*Bcnzczk)fluorashcnr
Benzo(e)pyrne
°Bezo(:a)pyreW
Perylene
*Ideo(1 .2.3-cd)pyrene
'D*benz(a.h)anthracenc
*
o(g.h.i)perylene
SPrtorty PoUutant PAHs
Table 1.1. Low molecular weight (LMW) PAHs and high molecular weight (HMW) PAHs
as defined by Menzie-Cura and Associates (1995).
Introduction
Summary of Estimated HMW PAH Loadings to Massachusetts Bay by
Source Type
2600
2400
2242
2200
2242
2000
1800
1562
1600
1400
T
1200
i
1000
800
600
395
400
272
1,14
200
0
iH-t~-
Coastal
NPOES
Coastal
Runoff
Coastal
CSO
River
D•scharge
TOTAL
LOADING
of HMW
PAHs
1995). to Massachusetts Bay broken down by
PAH loadings
Figure1.1. Summary of estimated
and Associates,
Figure
1.1. (Menzie-Cura
Summary of estimated PAH loadings to Massachusetts Bay broken down by
source type
source type (Menzie-Cura and Associates, 1995).
Introduction
The quantification of PAH concentrations in the water column is not a trivial procedure.
Because of the low solubilities of hydrophobic PAHs, they tend to be found in very low
concentrations in the aquatic environment, often exceeding the detection capabilities of
current analytical techniques (MWRA, 1991).
quantify such minute concentrations.
One may wonder why there is a need to
The problem lies in the ability of certain PAH
compounds to elicit sublethal toxicological responses even at concentrations near or below
the current analytical limits of detection (Hofelt and Shea, 1997).
The conventional procedure for quantifying PAHs requires collecting large volumes of water
which are then extracted with an organic solvent. This procedure can be labor-intensive and
typically overestimates dissolved PAH concentrations because it includes PAHs that may be
bound to colloids and other fine particulate matter. To determine PAH concentrations for an
evaluation of the potential health hazards associated with them, one needs to look at the
freely-dissolved fraction at a given moment in time because only the freely-dissolved fraction
is instantaneously available for uptake by aquatic organisms (Lebo et al., 1992). Also, the
dose-response functions developed for compounds like benzo(a)pyrene were developed for
freely-dissolved concentrations so environmental data based on total concentrations, that is,
including bound PAHs, may not be comparable (Hofelt et al, 1997).
New devices, called semipermeable membrane devices or SPMDs, have recently been
developed for the purpose of quantifying PAHs without the problems described above.
These devices were used in this study to test their ability to determine accurately PAH
concentrations in the aquatic environment. The data was sought to reveal information about
the fate, including the distribution, of PAHs in the Boston Inner Harbor. The results from the
measurements will be compared to the results of the mass balance and 3-D models of PAH
concentrations for Boston Harbor developed by Ricardo Petroni and Peter Israelsson (1998).
Introduction
2. POLYCYCLIC AROMATIC HYDROCARBONS
2.1 Chemical Structure and Properties
Polycyclic aromatic hydrocarbons, commonly referred to as PAHs, are composed of two or
more aromatic rings. The term "aromatic" was originally used to describe these compounds
because of their fragrant odor. Over time, the term aromatic has evolved to mean the stable
nature of a particular group of organic compounds. Polycyclic aromatic hydrocarbons have
greater stability and lower reactivity than similar, acyclic conjugated compounds because of
resonance stabilization.
PAHs are planar hydrocarbons, that is, composed of carbon and hydrogen atoms only (Figure
oH), a PAH composed of two fused
2.1). The smallest and lightest PAH is naphthalene (C10
benzene rings. On the other extreme is graphite, a form of elemental carbon.
Physical and chemical characteristics of PAHs generally vary in a regular fashion with
molecular weight (Neff, 1979). For example, resistance to oxidation and reduction tends to
decrease with increasing molecular weight. Vapor pressure and aqueous solubility decrease
almost logarithmically with increasing molecular weight (Table 2.1). In general, PAHs have
very low solubilities in water because of their nonpolar, hydrophobic structures. There is a
wide range in the behavior, distribution in the environment, and the effects on biological
systems of individual compounds. Toxicities of individual PAHs vary widely and are of
concern because some are carcinogenic, tumorigenic and/or mutagenic compounds
(Crunkilton and De Vita, 1997).
Polycyclic Aromatic Hydrocarbons
0
*1"
0i
00
Table 2.1. Physical/chemical constants of some PAHs commonly found in the environment. Note the wide range in solubilities and
octanol-water partition coefficients. In the case of naphthalene and benzo(a)pyrene, for example, the solubilities differ by almost
four orders of magnitude (Schwarzenbach et al., 1993).
In general, polycyclic aromatic hydrocarbons undergo the following reactions to varying
degrees: electrophilic and nucleophilic substitutions, free radical reactions, addition
reactions, reduction and reductive alkylations, oxidation, rearrangements of the aromatic ring
system, and complex formations (Harvey, 1997). The transformation of PAHs through
oxidation is an important reaction of PAHs. The oxidative metabolism of PAHs (e.g.,
benzo(a)pyrene) by the cytochrome P-450 microsomal enzymes is responsible for their
carcinogenic potential in organisms (Harvey, 1997).
Polycyclic Aromatic Hydrocarbons
Naphthalene
Tar Camphor
While Tar
Moth Flakes
Benz a Janthracene
I 2 Benzanthracene
Tetraphene
2 3-Benrophenanthrene
Naph1thanthracene
2-Methylnaphthalene
P-Methylnaphthalene
Chrysene
1.2 Benrophenanthrene
oenzoc a]phenanthrene
Acenaphthene
N
N
Naphthyleneethylene
IN
CO
Phenanthrene
Pyrene
Benro(del]phenanthrene
o-Osphenylene ethyene
0,
Anthracene
NZ
4 -Methylphenanthrene
-
Benzof b fluoranthene
23- Genzofluoranthene
34- Senzofluoranthene
Benz(feacephenanthrylene
Benzo[ j] fluoranthene
78- Senzofluoranthene
/
10 11- Bentofluoranthene
oz&N
1-Methylphenanthrene
Benzo( k If
luoranthene
@9-Benzofluorantherfe
1117 Benzofluoranthene
3-Methylphenanthrene
Benzo e pyrene
.5- Benzpyrene
I2-Bentopyrene
2-Methylphenanthrene
&Qc"ý
Benzolol pyrene
1.2-8enzpyrene
3.L-Benzoyrene
Benzo(def chrysene
(X
9-Methylphenanthrene
N6
Perylene
per- Dinaphlhalene
Fluoranthene
Idryl
•2-Benzacenaphthene
Benzojk] fluorene
Coronene
lHeiabeniobenzene
Benz(a]ac enaphthylene
Figure 2.1. The structures of some polycyclic aromatic hydrocarbons commonly found in
the environment (Bjorseth, 1983).
Polycyclic Aromatic Hydrocarbons
2.2 Sources and Fate in the Environment
Polycyclic aromatic hydrocarbons are ubiquitous in the environment. Significant levels are
found in the atmosphere, in the soil, and in the aquatic environment (Neff, 1979). The PAHs
present in the atmosphere are primarily derived from the fossil fuels used in heat and power
generation, refuse burning, and coke ovens. These sources together contribute more than
50% of the nationwide emissions of benzo(a)pyrene, a hydrocarbon that is widely employed
as a standard for PAH emissions (Harvey, 1997). Vehicle emissions are another major
source of PAHs, particularly in the urban areas of industrialized countries, contributing as
much as 35% to the total PAH emissions in the USA (Bjorseth et al., 1985). Natural sources,
such as forest fires and volcanic activity, also contribute to the input of PAHs into the
atmosphere, but anthropogenic sources are considered to be the most significant sources of
PAHs in atmospheric pollution.
Because of their high melting points and low vapor
pressures, atmospheric PAHs are generally considered to be associated with particulate
matter (National Academy of Sciences, 1972).
The combinations of PAHs produced in pyrolytic reactions vary according to the temperature
of combustion.
High temperatures result in relatively simple mixtures of unsubstituted
PAHs. At intermediate temperatures, such as that of smoldering wood, complex mixtures of
alkyl-substituted and unsubstituted PAHs are formed (Harvey, 1997). Lower temperatures
lead to products predominantly composed of methyl- and other alkyl-substituted PAHs.
Crude oils formed from the fossilization of plants exhibit characteristic patterns of aromatic
hydrocarbon components in which alkyl-substituted PAHs are found in a much higher
percentage than unsubstituted ones (Harvey, 1997). The ratio of alkyl-substituted (e.g.,
methylnaphthalene) to unsubstituted compounds (e.g., naphthalene) present in a sample can
be used as an indicator of the source (Speers and Whitehead, 1969; Youngblood and Blumer,
1975).
Petrogenic (derived from petroleum) sources exhibit abundance patterns high in
alkylated forms, while pyrogenic (derived from combusted products, including petroleum)
sources are characterized by the dominance of the unsubstituted forms.
Polycyclic Aromatic Hydrocarbons
Besides being found in the atmosphere and in the soil, PAHs are widely distributed in the
aquatic environment because of various pathways of transport. PAHs, bound to particulate
matter carried through the air, can deposit onto aquatic surfaces; PAHs in water undergo
exchange with the air depending on the concentration gradient between the two media; runoff
of PAH-polluted ground sources drain into rivers and other water bodies; and municipal and
industrial effluents containing PAHs are discharged into receiving waters. Direct spillage of
petroleum into water also serves as a major source of PAHs (Neff, 1985). In the aquatic
environment, PAHs
can then enter the food chain by being absorbed or ingested by
plankton, mollusks, and fish which may eventually be consumed by human populations.
Because of their hydrophobicity and low solubility in water, PAHs tend to be associated with
the complex matrix of organic matter in particulate matter which eventually settle to the
bottom.
Thus, relative concentrations of PAHs are usually highest in the sediments,
intermediate in the aquatic biota, and lowest in the water column (Neff, 1985).
PAH concentrations in natural waters are a function of the sources and the sinks in the
system. In most cases, there is a direct correlation between PAH concentrations in rivers and
the degree of industrialization and other human activity along the banks and the rest of the
watershed.
Groundwater and well water usually contain PAH concentrations which are
lower than those in river water by a factor of ten or more (Neff, 1979). PAHs in groundwater
are thought to be derived from the leaching of surface waters through soils contaminated with
PAHs (Borneff and Kunte, 1969; Hellmann, 1974; Suess, 1976). Purified tap water and
reservoir water contain a PAH concentration similar to, or slightly higher than, that of
ground water (Table 2.2).
Polycyclic Aromatic Hydrocarbons
River Rhine at Mainz, Germany
River Rhine at Koblenz, Germany
River Aach at Stockach, Germany
Pskov Region, former USSR
(remote from human activity)
Sunzha River, former USSR
(below discharge of an oil refinery)
Thames River, England
50-110
10-60
4-43
0.01-0.1
730-1500
500-3000
1440-3100
no data
50-3500
no data
170-280
800-2350
Trent River, England
5.3-504
25-3790
Oyster River, Connecticut, USA
Ohio River at Huntington, West Virginia, USA
78-150
5.6
no data
57.9
Lake Constance, Germany
0.2-11.5
25-234
Lake Erie at Buffalo, NY, USA
0.3
4.7
Groundwater (Germany)
Goundwater (USA)
0.4-7.0
0.2
10.9-123.5
8.3
Well water (Germany)
Well water (England)
2-15
0.2-0.6
100-750
3.6-5.8
Tap water (Germany)
Tap water (9 US Cities)
0.5-9.0
0.2-1.6
29.2-125.5
0.9-14.9
Table 2.2. Typical concentration ranges of benzo(a)pyrene and total PAHs in various aquatic
systems. These values were taken from Tables 33 and 34, pp. 67-68 of Polycyclic Aromatic
Hydrocarbons in the Aquatic Environment (Neff, 1979). For exact references of individual
measurements, see Neff (1979).
In general, PAHs are quite stable and persistent, especially once they have become
incorporated into the anoxic environment of bottom sediments. Under certain conditions,
they can be subjected to various chemical transformations and degradative processes. In the
Polycyclic Aromatic Hydrocarbons
natural environment, the most important processes are photooxidation and biological
transformation (Neff, 1985). The delocalized pi-electron orbital system in PAHs makes them
susceptible to direct photolysis (the absorption of light energy directly) (Neff, 1985). PAHs
can also be transformed or metabolized by microorganisms such as bacteria, fungi, and algae
(Warshawsky et al., 1995). In aquatic systems, biodegradation occurs in oxidized, surficial
sediments (Shiaris, 1989). The rates of PAH degradation tend to decrease with increasing
molecular weight (Neff, 1985).
Polycyclic Aromatic Hydrocarbons
3. BOSTON HARBOR BACKGROUND AND HISTORY
3.1 Physical and Hydrographic Characteristics
Boston Harbor is located in the Northwest corner of Massachusetts Bay, which is itself a part
of the Gulf of Maine. Boston Harbor comprises an area of about 50 square miles, bounded by
180 miles of shoreline. The harbor can be divided into sub-regions which are easily defined
by topographical boundaries (Figure 3.1).
The regions north and south of the boundary
between Dorchester Bay and Quincy Bay are commonly referred to as the North Harbor and
South Harbor, respectively. The North Harbor can further be divided into the Inner Harbor
and the Northwest Harbor while the South Harbor can further be divided into the Central
Harbor and the Southeast Harbor.
The focus of this study is the Boston Inner Harbor region shown in Figure 3.2. The Inner
Harbor is bound by the confluence of the Mystic and Chelsea Rivers up North and the
entrance to Massachusetts Bay down South. The volume of the Inner Harbor varies from
approximately 7.8 x 107 m3 at high tide to 5.6 x 107 m3 at low tide. The Inner Harbor is
relatively shallow, with a nearly constant depth of 10 m under mean low water (MLW)
conditions (Bumpus et al., 1953; Alber and Chan, 1994). During winter, the residence time
of water in the Inner Harbor is approximately 2.7 days (Petroni and Israelsson., 1998).
Boston Harbor is only slightly stratified by salinity gradients because its freshwater input is
relatively small compared to tidal flushing. The Inner Harbor receives freshwater inputs from
the Charles River, Mystic River, and Chelsea River. The largest input is from the Charles
River with an average flow (1931-1992) of 8 x 106 m3/day (USGS, 1992). In the summer
Boston HarborBackgroundand History
months, Boston Harbor becomes thermally-stratified. The density currents that result from
the thermal stratification contribute to surface and bottom water exchange with
Massachusetts Bay; but, overall, this mechanism is minor relative to tidal flushing
(Stolzenbach and Adams, 1998).
P
MAMUUM
/
S=4
0\
X/C
iI·__~___~____e__l__~=_
~ ___
i_______
_ ____~
II__
--. __r -3i3i
C==iL~---ILii=~·~i--- ------iii
-_~i~=_
3
-------- ---
Figure3.1. Boston Harbor.
Boston HarborBackground and History
I
A
REVERE
EVERETT
CHELSEA
McArdle
EAST BOSTON
CHARLES
and Callahan Turnnels
Logan
Airport
SThird Harbor Tunnel (Proposed)
BOSTON
Deepen
Mystic River to 40'
ere
Inner Confluence to 40'
Reserved Channel to 40'
Chelsea River to 38'
US Army Corps of Engineers
New England Division
Scale in Feet
0
2000'
SOUTH BC)STON
4000'
Boston Harbor, Massachusetts
Navigation Improvement Project
July 1993
Figure3.2. Boston Inner Harbor (USACOE, 1988). The sampling locations of this study are
marked with a filled circle( * ).
Boston Harbor Background and History
3.2 Sources of Contamination in Boston Harbor
Contaminants have both point and non-point sources in Boston Harbor. These include
tributary rivers and groundwater flows, runoff from stormwater drains and combined sewer
overflows, industrial discharges, ship and boat traffic, sewage and sludge discharges from
treatment plants, and the atmosphere.
Each of the six major tributaries (Charles River, Neponset River, Weymouth Back River,
Mystic River, Weymouth Fore River, and Weir River) which empty into the harbor carry
with them varying levels of contaminants. The identities and the concentrations of these
contaminants vary according to the domestic and industrial activities within the
corresponding watersheds. Groundwater flowing into the harbor's shoreline also carries with
it contaminants resulting from current human activities and leachate from wastes in old
landfills (Alber et al., 1994).
In the 1800s, combined sewer-storm drains (commonly referred to as combined sewer
overflows or CSOs) were constructed by the surrounding towns of Boston, Cambridge,
Chelsea, and Somerville (MWRA, 1993) to handle both sewage and rainwater flows. During
heavy rain, some of these drains still empty directly into the harbor along the shoreline or
into tributaries which ultimately flow into Boston Harbor. Outflows from CSOs can carry
significant loads of contaminants into Boston Harbor. Stormwater runoffs carry contaminants
that have been washed away from the ground surface. Spilled motor oil on roadways and
driveways, for example, winds up in storm drains after being washed away by the rain. Of
the over 80 CSOs that discharge a combination of storm water and raw or partially-treated
sewage into Boston Harbor, 35 CSOs discharge directly into the Inner Harbor. As of 1992,
the average flow from these CSOs corresponded to about 1% of the average Charles River
input (Chan-Hilton et al., 1998).
Boston HarborBackgroundand History
Many industries developed along the harbor shoreline in the late 1800's. Historically, these
industries were able to discharge their raw wastes directly into the harbor. Even in this era of
heavy regulation, industries are still allowed to discharge controlled concentrations of
chemical wastes into the water.
Both industrial (commercial) and private boat and ship
traffic also contribute to contamination in the harbor through fuel leaks and motor emissions.
Sewage effluent and associated sludge discharges have, by far, been the largest sources of
organic contaminant input into Boston Harbor. Starting in 1878, sewage vats on Moon
Island with a capacity of 50 million gallons discharged raw waste into the harbor twice daily
with the outgoing tide (MWRA, 1996). Raw sewage pump stations were constructed in East
Boston in 1889 and on Deer Island in 1899. It was not until 1952 that the first primary
wastewater treatment plant was constructed on Nut Island. This facility provided screening
and sedimentation of solids and chlorination to reduce bacterial levels which had become a
recognized health risk within the harbor (Stolzenbach et al., 1998). Starting in 1968, primary
treatment was begun at Deer Island and the Moon Island outfall was reserved for wet weather
flows only (ceased in 1992). A new primary treatment plant was constructed in 1995 at Deer
Island and was upgraded to secondary treatment in 1997. A new outfall discharging 9.5
miles out into Massachusetts Bay is expected to be in operation at the end of 1998.
As of
1991, the Boston sewer system was transporting an average 500 million gallons of sewage
per day from Boston and surrounding communities to the Deer Island and Nut Island sewage
plants (MWRA, 1991).
According to Alber et al. (1994), the total (LMW+HMW) PAH input (Table 1.1) into Boston
Harbor was approximately 20,000 kg/yr.
Most of this total PAH input originated from
sewage effluent and sludge discharge. However, the major source of low molecular weight
PAHs like 2-methylnaphthalene (total loading of 1,800 kg/yr) was found to be sewage, while
that of high molecular weight compounds like benzo(a)pyrene (total loading of 22 kg/yr) was
found to be tributaries. Their study also estimated that 89% of the 2-methynaphthalene
Boston HarborBackground and History
discharged into the harbor was added to the North Harbor and 73% of the benzo(a)pyrene
was added to the South Harbor (Alber and Chan, 1994).
Boston HarborBackground and History
4. PROBLEM STATEMENT AND OBJECTIVES
Measurement of aqueous concentrations of organic contaminants such as polycyclic aromatic
hydrocarbons is problematic. Because of their nonpolar and highly hydrophobic nature, they
tend to be associated with dissolved organic matter such as humic acids and suspended
solids. As a consequence, their truly-dissolved concentrations in the water column are
typically at trace levels, on the order of parts per billion or less (Table 2.2). These aqueous
concentrations
are
frequently below conventional
concentration of the contaminants.
detection
limits, thus
requiring
A gas chromatograph with a flame ionization detector,
for example, typically has a detection limit in the order of a few nanograms per microliter or
a few parts per million.
It is important to quantify the truly-dissolved concentrations of chemical compounds in
surface waters for two main reasons. First, water quality criteria and aquatic toxicity data are
typically based on truly-dissolved concentrations.
Second, in order to completely
characterize the fate of a particular chemical of interest, one needs to look at its physicalchemical phase distribution because this plays a significant role in determining its chemical
behavior.
This applies to both inorganic and organic compounds of toxicological interest.
For example, in natural waters, nearly all of the mercury that accumulates in fish tissue is in a
dissolved methylated form, methylmercury (CH 3Hg) (CA EPA, 1994). This form of mercury
also happens to be the most toxic form of mercury, capable of causing serious damage to the
nervous system. The inorganic forms of mercury, such as the free metal, are less efficiently
absorbed and more readily eliminated from the body than methylmercury. They do not tend
to bioaccumulate and are, therefore, of less concern from a toxicological point of view.
Problem Statement and Objectives
PAHs bound to particulate matter have reduced bioavailability and toxicity (Crunkilton and
De Vita, 1997).
For example, only truly-dissolved contaminants are instantaneously
available for bioconcentration across biological membranes such as fish gills (Moring and
Rose, 1997). Direct measurement of PAHs in bulk (or unfiltered) water samples from natural
aquatic systems which may contain moderate to high concentrations of suspended solids can
therefore lead to a misleading estimate of the concentration immediately available for uptake
by biota. A large fraction of soot-bound PAHs, for example, is unavailable for equilibrium
partitioning with the water and may be irreversibly bound on time scales of 50-100 years
(McGroddy et al., 1996).
In an attempt to quantify only the dissolved fractions of organic compounds in water, large
quantities of water are filtered with filters with sub-micron pores and the "dissolved"
contaminants are then extracted into an immiscible organic solvent in which the compounds
have greater solubilities. The solvent typically has a low boiling temperature so that it can be
further concentrated through volatilization. The cost associated with this type of analytical
procedure makes it unacceptable for implementation in a routine contaminant monitoring
program. As a result, bulk or unfiltered water samples are often analyzed instead, leading to
a definite overestimation of the truly-dissolved concentration of contaminants.
Even the use of filters does not guarantee that only the truly-dissolved fraction will be
measured. Particles in a filtered solution considered to be dissolved may range in size up to
0.1 p~m which is about 100 times greater than the largest size hydrophobic molecules which
can cross biological membranes by passive processes (Crunkilton and De Vita, 1997). When
this "dissolved" fraction is analyzed, contaminants bound to these very small particles are
included, still leading to an overestimation of the total amount of bioavailable contaminants.
The collection and subsequent filtration of large samples for extraction is also a laborintensive process complicated by quality assurance and quality control (QA/QC) issues.
Volumes typically ranging from 4 to 10 L per sample need to be collected and transported.
Problem Statement and Objectives
These water samples then need to be stored in containers that would protect the PAHs from
photodegradative processes. The EPA requires the use of large amber bottles or foil-lined
containers for this purpose. It is also important that the method of collection and transport
minimize volatilization from the samples. Once the samples are collected, they have to be
extracted and analyzed in a timely fashion. For aqueous samples, the EPA recommends a
holding time of 7 days (EPA, 1998b).
Another problem with the conventional method is that grab samples will only reflect the
concentrations at the moment of sampling. Random events such as the passing of a leaky
motor boat at the sampling location may give an unreasonably high concentration at that
location that is not representative of typical conditions. In aquatic systems with varying
levels of flow and concentrations over a tidal cycle, for example, concentrations at a given
point in time may be deceptive.
An alternative, cost-effective, and convenient method has been sought after that can be used
to derive a more toxicologically-relevant measure of available PAHs in the field.
A
relatively new approach toward this end is the use of semipermeable membrane devices or
SPMDs. SPMDs were developed in the early 1990's by researchers at the Environmental
and Contaminants Research Center of the U.S. Geological Survey (Huckins et al., 1993).
SPMDs are designed to sample only the truly-dissolved fractions of organic compounds and
also provide a time-averaged concentration of compounds.
Because they are synthetic,
uniform physical devices, the development of a standardized protocol for the monitoring and
assessment of trace contaminants in diverse environmental settings should also be possible.
This is an advantage over the use of bivalves or fish, organisms typically used in monitoring
trace contaminant uptake, because organisms tend to have anatomical, physiological, and
behavioral differences which can affect their rate of contaminant uptake (Ellis et al., 1995).
The objective of this study was to examine the utility and applicability of SPMDs for the
assessment of the fate of PAHs in the Boston Inner Harbor. A distribution of sampling points
Problem Statement and Objectives
over the Inner Harbor was chosen to reveal possible variations in the sources and sinks of
PAHs in the Harbor and other relevant information about their fate. In order to evaluate their
effectiveness, the results from the SPMD measurements were compared to those predicted by
mass-balance and 3-D hydrodynamic modeling of PAH transport in the Inner Harbor.
Problem Statement and Objectives
5. SEMIPERMEABLE MEMBRANE DEVICES
5.1 Historical Development
The use of organic solvents to extract hydrophobic organic compounds from aqueous
solutions is a well-established procedure (Santodonato et al., 1981). Chemists often use socalled liquid-liquid extractions to transfer organic compounds in water to a water-immiscible
organic solvent for which the target compound has greater affinity. Typically, the water
sample is vigorously mixed with the solvent (e.g., methylene chloride) to allow the target
compounds to dissolve into the solvent. The water and the organic solvent are then allowed
to separate and the solvent is extracted. These procedures employ the organic solvent-water
partitioning properties of hydrophobic compounds which, at equilibrium, can be described by
their K,,sw:
C_[mol/Is1
Cw mol/ LwJ'
where Ksw = solvent-water partitioning coefficient,
Cs = concentration in the solvent,
Cw = concentration in the water.
As the equation demonstrates, the greater the Ksw for the analyte, the greater the
concentration in the solvent as compared to water.
One way of interpreting the physical
meaning of the Ksw is by thinking in terms of volumes; for a given number of moles of a
compound in one liter of a particular solvent, Ksw liters of water will be required to hold the
same number of moles. In the case of a highly hydrophobic compound like benzo(a)pyrene
which has a log Ksw of 6.50 in octanol at 250 C (Schwarzenbach et al., 1993), 1 liter of
octanol in contact with water will hold a mass of benzo(a)pyrene equivalent to that dissolved
in 3,200,000 liters of the water.
Semipermeable Membrane Devices
The low aqueous solubilities of many organic compounds, especially hydrocarbons, result in
low concentrations that are difficult to quantify.
For example, benzo(a)pyrene has an
aqueous solubility of only 10-8.22 mol/L or 1.52 ptg/L at 250C. The capacity of certain solvents
for dissolving organic compounds out of water can thus be used to concentrate them to levels
which can be more easily measured. This concept has been used extensively in laboratory
settings, but it has only been applied to in-situ field sampling recently. Field deployment
requires a convenient means of separating the solvent from the water so that the solvent can
later be collected and analyzed, while still allowing for the transfer of targeted compounds
from the water and into the solvent. In the last couple of decades, various groups have
developed the use of semipermeable membranes for this specific application.
Huckins et al. (1990) credit a group led by Miere (1977) as the first investigators of the use
of polyethylene film for dialysis of nonpolar organic contaminants from water into organic
solvents. Their work suggested that nonporous, synthetic polymeric films, including low
density polyethylene and polypropylene, could serve as semipermeable membranes allowing
for diffusion and concentration of organic molecules from water into relatively nonpolar
organic solvents. This process is governed by solvent-water partitioning coefficients which,
in the case of hydrophobic compounds, strongly favors concentration into the organic
solvents.
In 1980, a pair of investigators from the United Kingdom obtained a patent for a device
consisting of a nonpolar organic solvent contained in a semipermeable membrane such as
regenerated cellulose, vinyl chloride, polyvinylidene fluoride, or polytetrafluoroethylene. As
stated in the patent, the device was to be used as a concentrator for removing organic
contaminants from aqueous systems (Byrne and Aylott, 1980).
Their design represented a
new application of dialysis to liquid-liquid extraction of organic compounds from an aqueous
environment.
In 1987, Sodergren used solvent-filled dialysis membranes to simulate uptake of pollutants
by aquatic organisms (Sodergren, 1987). In his study, he used dialysis membranes to crudely
Semipermeable Membrane Devices
mimic biological cell membranes and 3 mL of n-hexane as the lipid pool capable of
collecting lipophilic organic compounds.
The solvent-filled bags were exposed to
organochlorine aqueous solutions (p,p'-DDE, p,p'-DDT, Clophen A50) in the lab for 8-10
days and various aquatic environments in the field (e.g., a 4-day exposure to a bleach pulp
plant effluent and a 2-week exposure to an activated sludge basin of a sewage treatment
plant) to examine uptake behavior.
The samples were analyzed by using a syringe to
penetrate the membrane and extract the solvent and shooting the extract directly into a gas
chromatograph (GC) without any clean-up procedure.
Johnson extended this study by using bigger volumes of n-hexane (40 mL) and performing a
32-day exposure of the bags to well water to examine the uptake kinetics of Arochlor 1248
into the bag (Johnson, 1991). As in Sodergren's study, the n-hexane was injected directly
into the GC after extraction from the bags. He also used fugacity-based bioconcentration
kinetics, interpreted with respect to Fickian diffusion across water and a lipid, to provide a
theoretical basis for the observed behavior of the solvent-filled bags.
In the early 1990's, researchers led by Huckins at the US Geological Survey's Midwest
Science Center developed a design based on the organic solvent-water partitioning concept
used in the previous studies (Sodergren, 1987; Johnson, 1991). Their design, which they
named Semipermeable Membrane Device (SPMD), consists of a 91-cm long strip of low
density polyethylene (LDPE) film as the membrane and the lipid, triolein, as the organic
solvent. Huckins and his group also developed a mathematical model of the uptake and
dissipation kinetics of the SPMDs (Huckins, 1993). While Johnson used his solvent-filled
bags for qualitative monitoring only, these SPMDs are designed to quantitatively determine
analyte concentrations in the field based on measured concentrations in the triolein after a
given exposure time.
The design and the kinetics model of these standardized and
commercially-available SPMDs are discussed in more detail in the following sections.
Huckins and his group also patented a new procedure for performing the analysis on these
devices (US Patents #5,098,573 and 5,395,426).
Unlike Sodergren's and Johnson's
procedure, where the lipid was shot directly into the GC, a dialysis is performed on SPMDs
Semipermeable Membrane Devices
to first extract the analytes from the triolein. The dialyzing solvent, e.g. cyclopentane, is
then concentrated using volume reduction techniques and fractionated into various groups
(e.g. PAHs, halogenated compounds, etc.) before analysis by a GC.
Since their development, these devices have been used and tested for a wide variety of
purposes in diverse environmental settings. In recent years, SPMDs have been used to
measure freely-dissolved concentrations of organic contaminants
in urban streams
(Crunkilton and De Vita, 1997), to determine contaminant residence times in an irrigation
water canal (Prest et al., 1997), to evaluate the bioavailability of contaminants associated
with sediments (Cleveland et al., 1997), to quantify organic contaminant concentrations in
compost (Strandberg et al., 1997), to simulate uptake by bivalves, the traditional biomonitors
(Hofelt and Shea, 1997), and to develop a spatial distribution of PAH concentrations in urban
streams (Moring and Rose, 1997).
SPMDs have been deployed in the waters of Dorchester and Duxbury Bays in Massachusetts
(Peven et al., 1996), the metropolitan areas of Texas (Moring et al., 1997), the Upper
Mississippi River (Ellis et al., 1995), the San Joaquin and Sacramento Rivers in California
(Prest et al., 1992), Corio Bay in Australia (Prest, Richardson et al., 1995), and Central
Finland (Herve et al., 1995), among others. They have also been used for toxicity testing of
sediments from Antarctica (Cleveland et al., 1997). Note that SPMDs are also capable of
extracting organic compounds from the air and have been used as passive air samplers (Petty
et al., 1993 and Prest et al., 1995) for organic contaminants.
Semipermeable Membrane Devices
5.2. Design of SPMDs
A semipermeable membrane device consists of two components: the membrane and the
solvent or sequestration phase.
micrometers)
nonporous
polymer
The membrane is typically a thin-walled (50-250
like
LDPE,
plasma-treated
silicone
or silastic,
polypropylene, polyvinylchloride, or other similar materials (Huckins' tutorial, 1997).
Although the membranes used in SPMDs are typically referred to as "nonporous", they
actually have pores up to approximately 10 A in diameter. The pores are transitory and are
formed by the random thermal motions of the polymer chains. For the membrane to serve its
purpose well, it needs to retain the sequestering phase within the membrane while allowing
for the diffusion of target compounds. The lower limit of the molecular size of the
sequestration phase or solvent is such that the solvent molecules are not able to cross the
membrane and escape to the surrounding water to a significant extent.
Besides containing the solvent within the device, the small diameters of these cavities dictate
an upper limit for the sizes of the compounds that can penetrate the membrane and reach the
solvent. Huckins pointed out that the diameters of many environmental contaminants of
interest approach the maximum size of the pores in nonporous membranes; therefore it is
likely that analytes associated with aqueous particulate matter and dissolved organic carbon,
such as humic acids, cannot penetrate the membrane (Huckins, 1993). The use of nonpolar
membranes will also impede the passage of ions into the membrane.
SPMDs can therefore
be expected to sequester only freely-dissolved, nonionic compounds, an advantage over
conventional PAH sampling procedures.
Studies have shown that polar nonporous membranes such as cellulose can reduce or
eliminate solvent losses to the surrounding environment, but a corresponding reduction in the
uptake rates of nonpolar analytes was also observed (Huckins, 1993). A study by Gray et al.
(1991) compared the concentration potential of lipid-containing polyethylene membranes to
hexane-filled cellulose dialysis bags (Sodergren, 1987) and found that lindane and trifluralin
were sequestered to a much a greater extent in the former.
Semipermeable Membrane Devices
35
The sequestration phase is typically a large molecular weight (2 600 Daltons) nonpolar
liquid such as a neutral lipid, silicone fluid, or other lipid-like organic fluid (Huckins'
tutorial, 1997). Various investigators have used relatively low molecular weight, nonpolar
compounds like hexane, but this resulted in losses to the surrounding water because of their
membrane solubility and permeability. Huckins et al. (1993) noted that the diffusion of the
sequestration phase out of the membrane may also impede analyte uptake because the analyte
will have to diffuse against an outward solvent flux leading to concentration polarization at
or near the membrane exterior surface.
Obviously, this problem is eliminated when a
solvent is chosen such that the molecules are too large to significantly diffuse through the
membrane.
Huckins' commercially-available standardized SPMDs consists of LDPE layflat tubing and
high-purity synthetic triolein (> 95%). The SPMDs are 2.5 cm wide by 91.4 cm long flat
tubes which contain 1 mL (0.915 g) of triolein as a thin film spread over the entire tube. The
LDPE tubing is 75-90 micrometers thick. The SPMDs are heat-sealed at both ends.
Triolein or glyceryl trioleate is 9-octadecenoic acid, 1,2,3-propanetriyl ester. It has a
molecular weight of 885.45 g and consists of 57 C's, 104 H's, and 6 O's (Budavari (ed.) et
al., 1996). Triolein is approximately 27 angstroms in length and approximately 28 angstroms
in breadth so it should not be able to diffuse through the 10-angstrom cavities in the
membrane to a significant extent.
Triolein is the major neutral lipid in many aquatic
organisms and was chosen as the sequestration phase because SPMDs are designed to
simulate bioaccumulation in aquatic organisms. Chiou's work (1985) demonstrated that
when the published bioconcentration factors (BCF) of organic compounds in water, that is,
the ratio of the steady-state concentration of a compound in the organism (or a part of it)
compared to that in water, are based on lipid content rather than on total mass, they are
approximately equal to the equilibrium triolein-water partition coefficients,
Kt.
This
suggests that partitioning of organic compounds between fish and the surrounding water are
determined by the near equilibrium partitioning between triolein and water. The uptake of
Semipermeable Membrane Devices
organic contaminants by SPMDs can therefore simulate passive, that is, non-metabolic,
uptake of organic contaminants by fish and, perhaps, other aquatic organisms.
Besides minimizing solvent loss and simulating bioconcentration, the use of pure triolein as
the sequestering media for SPMDs has other major advantages. Chiou demonstrated that
there is a close correlation between Ktw's and the corresponding octanol-water partition
coefficients, Kow's, for many organic compounds (Chiou, 1985). Figure 5.1 shows a plot of
the log Ktw's versus the log Kow's of a wide range of organic compounds. Table 5.1 lists
some of the actual values for log Kt that were measured by Chiou and the corresponding
published log Kow's.
As demonstrated by Figure 5.1, a compound's Ktw can be closely approximated by its Kow, a
value that is well documented by the pharmaceutical industry because of its significance in
toxicity studies. In addition, since Kow's are large for hydrophobic organic contaminants (see
Table 5.1), the concentration capacity of triolein-containing SPMDs for these contaminants is
also large.
0
36, P378
38~
5
04
0
(4
34
29/
r,
3
~2
22
9
20ý
17 10
C
2
16
LI
0C
2
96
U
4
201
0
Ip
0
Figure5.1. Correlati
1985).
on
1
5
6
4
3
2
Octanol-Water Partition Coefficient. Log Ko
7
of log Ktw with log Kow for selected organic compounds (Chiou,
Semipermeable Membrane Devices
aniline
benzene
hexachloroethane
1,2,4,5-tetrachlorobenzene
2-PCB
2,5,2',5'-PCB
0.90
2.13
4.14
4.70
4.51
5.81
p,p'-DDT
6.36
0.91
2.25
4.21
4.70
4.77
5.62
5.90
Table 5.1. A comparison of log Kow values versus log Kt, values for a range of organic
compounds (Chiou, 1985).
Semipermeable Membrane Devices
5.3. Uptake Kinetics of SPMDs
In modeling the uptake kinetics of compounds from water into the SPMDs, Huckins et al.
(1993) made the following assumptions: a) the chemical concentration in the water is
constant and there is no significant resistance to diffusion in the lipid (i.e., rapid mixing
occurs), b) the steady-state flux, F, of an analyte into the device is controlled by the sum of
the resistances to mass transfer in the membrane and a water boundary layer, and c) the
capacity of the membrane to dissolve chemicals is negligible.
The diagram below shows an exploded view of a membrane bounded by water on one side
and solvent on the other. In the case of Huckins' SPMD, the membrane is polyethylene and
the solvent is triolein.
[1
UI
Cm
KC
-Ml
, mb
CW
,
membrane
water film
Cvl
triolein
0ir
S
= concentration in the triolein
Figure 5.2. An exploded view of the triolein-membrane-water film model. The diagram
shows the tortuous pathways created by the membrane polymer which allow for retention of
the triolein and transport of smaller compounds through the membrane. (Drawingcourtesy
ofAna Pinheiro.)
Semipermeable Membrane Devices
At constant temperature, the flux F (g/h) is given by the mass-transfer equation:
dCs
D
(1) F = Vs dCs = A(CMo - Cm) = kpA(CMo - Cw) = kwA(Cw - Cwi)
dt
Y
where D = diffusivity or permeability of the analyte in the membrane (m2/h)
A = membrane surface area (m2)
Y = membrane thickness (m)
k,= membrane mass-transfer coefficient (MTC) = D/Y (m/h)
kw = water boundary layer MTC (m/h)
Vs = volume of the lipid or solvent used for the sequestering media (m3)
t = time of exposure (h)
CMO = analyte concentration at the outer surface of the membrane (g/m 3 membrane)
CMI = analyte concentration at the inner surface of the membrane (g/m 3 membrane)
Cw = analyte concentration in the bulk water (g/m 3 water)
Cwi = analyte concentration at the interface or the water boundary layer (g/m 3 water)
Cs = analyte concentration in the solvent (g/m 3 solvent)
= Ct in Figure 5.2, where t refers to triolein.
Defining equilibrium partition coefficients for the inner membrane and the solvent as
KMs = CMI/Cs, for the outer membrane and water as KMW = CMo/CwI, and for the solvent and
water as Ksw = Cs/Cw, the interfacial concentrations can be eliminated and equation (1) can
be simplified to
(2) F = Vs
dCs
dt
= koA(CwKfw - CsKMs)
where ko is the overall MTC and is a measure of the combined resistances of the waterboundary layer and the membrane to molecular transport:
(3)
1
-
1
Kmw
+ k
Implicit in this equation is the assumption that the resistances for the membrane, 1/kp, and the
water layer, KMw/kw, are additive and independent of each other. It has been suggested that
in the case of the solvent, triolein, and the membrane, polyethylene, the membrane resistance
Semipermeable Membrane Devices
is typically the dominant term so ko is approximately equal to kp. In the case of a very large
value for KMw, the second term can become significant and the resistance may become
dominated by the water boundary layer. There is currently insufficient data on the transition
from membrane-controlled diffusion to aqueous film-controlled diffusion for SPMDs.
Assuming that Cw is constant, integrating equation (2) yields
(4) Cs = Cw
KMw
KIS
(1- exp(-koAKMst / Vs)) = CwKsw(1 - exp(-kut))
where ku = overall uptake rate constant (hr l )
KMs
s
(5) k = koA
Vs
and the response time, zr, for the analyte in the SPMD is given by the reciprocal of ku.
Huckins' model is similar to those used by other investigators who have modeled other
membrane systems (Johnson, 1991; Hassett et al., 1989; Yasuda, 1967).
The value of ku can be determined by measuring the loss or dissipation of a compound from
the device into pure water over time. This assumes that the uptake behavior of the system is
identical to its dissipation behavior. The loss from the SPMD can then be described by the
following equation where Cso is the initial concentration of the compound:
(6) Cs = Csoexp(-kut).
Equation (4) can be modified to account for the lag time, to, required for the analyte to first
penetrate the membrane resulting in a delay in the concentration increase in the solvent. In
the early stages of uptake, to is the positive intercept of the model on the time axis.
(7) Cs=I- exp
koAKs (t -t°)
Vs
Semipermeable Membrane Devices
jKswCw
, t > to.
Three Regions of the SPMD uptake curve:
The SPMD analyte uptake curve (Cs vs t) described by equation (4) or (7) can be divided into
three regions: linear, curvilinear, and asymptotic. These scenarios differ depending upon the
physicochemical properties of the analyte and the duration of the exposure.
Linear uptake region:
When the term koAKMs(t-to)/Vs is small (i.e., kut<<l) or when Cs/Cw << Ksw, equation (4)
reduces to
(8) Cs =
KMwkoAt
Cw = RwsCw,
Vs
a linear equation with a slope of Rws (m3 water/m 3 triolein), which is a function of time. In
the linear uptake kinetics region, Cs is controlled by the amount of chemical encountered by
the device in relation to the volume of the solvent. The term KMwkoAt (m3) represents the
volume of water from which the chemical has been extracted, while KMwkoA can be thought
of as the SPMD sampling rate (m3/h). Rws, the ratio of the sampling rate to the volume of
solvent, Vs, thus determines the accumulation of the chemical in the solvent. As long as
Cs/Cw << Ksw or Ksw>>Rws, the value of Ksw is irrelevant.
Controlled laboratory
experiments can be used to measure Rws for a particular compound (fixed partitioning
coefficient) and a particular SPMD configuration (fixed Vs, A, and membrane properties)
then Cs can be used to quantify Cw. According to Huckins et al. (1993), SPMD exposure
times of < 0.5 r or 0.5/ku can be expected to be in the linear uptake region.
Curvilinear uptake region:
In the curvilinear uptake region, the sampling rate and, equivalently, the slope of the curve
decreases as the solvent approaches saturation or equilibration with the water. Because Ksw
and Rws are now similar in magnitude, neither term can be ignored and both would have to
be known to relate the value of Cs to Cw at a given point in time. Given a known Ksw, Cw
Semipermeable Membrane Devices
can be derived by fitting equation (4) or (7) to measured values of Cs over time. Huckins et
al. (1993) suggest that in using this method, the number of estimated parameters (e.g., Cw
and ku) be no more than half the number of Cs values.
Asymptotic uptake region:
When the group koAKMs(t-to)/Vs in equation (4) is large or Rws/Ksw is very close to 1 or
t>> r, the exponential term becomes negligible and equation (4) reduces to the familiar
equation
(9) Cs = KswCw
In this region, equilibrium has been reached between the solvent and the water and Rsw is
simply the Ksw. A compound's water concentration, Cw, can then be determined from Cs in
a straightforward manner given the compound's Ksw.
In summary, the relationship of Cs to Cw for a given exposure time is determined by two
terms, Rws and Ksw.
Rws is design-specific, that is, it is determined by the type of
sequestering media used, the membrane surface area, the membrane resistance, and the
volume of the solvent. Ksw is purely a function of the solvent and the compound of interest.
For large values of Ksw, the uptake rate constant ku decreases, resulting in a longer
equilibration time.
This phenomenon has been observed in polymeric membrane
permeability and bioconcentration studies and may be due to increased resistances (1/ko) to
diffusion in both the water layer and the membrane.
In general, Cs can be expected to respond proportionally to Cw regardless of the region; but
in order to interpret the data properly and obtain an accurate value for Cw based on a
measured value of Cs, it is necessary to determine the applicable uptake kinetics region for
the specific compound and exposure time.
Semipermeable Membrane Devices
6. EXPERIMENTAL PROCEDURE
In the experiment, known amounts of 2-methylnaphthalene, pyrene, and benzo(a)pyrene
were spiked into the SPMDs and allowed to dissipate over 4-, 8-, 12-, and 16-day intervals in
order to see the dissipation behavior of the SPMDs for each of these compounds. The
compounds were chosen to represent a range of molecular weights and properties of PAHs.
Three recovery standards, d8-naphthalene, p-terphenyl, and dz2-perylene, were spiked into the
SPMDs prior to analysis to simulate recoveries of the respective PAH compounds.
It has been shown by Huckins et al. (SPMD tutorial, 1997) that the dissipation behavior is
representative of the uptake behavior in SPMDs. The results of the laboratory experiment
can therefore provide some important information for deducing water concentrations from
SPMD concentrations in the case of field measurements. In the field sampling performed
here, five SPMDs were deployed in various sites in the Boston Inner Harbor over 14 days.
Experimental Procedure
6.1 Reagents and Materials
6.1.1 Reagents and solvents
All solvents (methylene chloride (DCM), hexane, and methanol) used were JT Baker Ultra
resi-analyzed grade. 2-Methylnaphthalene, pyrene, and benzo(a)pyrene dissolved in DCM
were ordered from Supelco, Bellafonte, PA in 200 ptg/mL concentrations. The solutions
came in sealed amber ampules.
The recovery standards used were d8-naphthalene, dl2 -
perylene, and p-terphenyl. The ds-naphthalene and d12-perylene dissolved in DCM were in
2000 jpg/mL concentrations and were kept in sealed amber ampules. The p-terphenyl came
in solid form and was dissolved in DCM per the procedure described below. n-C 24 was used
as the internal standard and was used for sample volume calculations. djo-acenaphthene (800
ng) and dio-phenanthrene (400 ng) were spiked into all the SPMDs before they were heatsealed by Environmental Sampling Technologies.
6.2 Materials
Ten SPMDs were purchased from Environmental Sampling Technologies (EST), a division
of CIA Labs, St. Joseph, Missouri. The tubes were 32-36" x 1" and contained 1 mL high
purity triolein. Upon request, each SPMD was spiked with 800 ng of dlo-acenaphthene and
400 ng of dlo-phenanthrene. EST is currently the only licensee of the government-owned
SPMD patents (US Patents #5,098,573 and 5,395,426). The SPMDs came individually
stored in sealed, argon-filled cans. Upon arrival at the lab, the cans were stored in a freezer
at -750 C.
The metal cages for the SPMDs were constructed using a metal wire screen with 1 cm x 1 cm
grid holes (61 cm wide), nylon cords, metal snap hooks, copper wire, and zinc alloy wire.
For the deployment, cinder block and bricks were used as weights. The SPMD deployment
also required the use of floats, nylon rope, and duct tape for attachments.
Experimental Procedure
Kudema-Danish concentrators (reservoirs, receiving flasks, and condensers with 50 mL and
500 mL capacities) were used to reduce sample volumes.
SiO 2 gel columns were prepared by making a slurry of SiO 2 and hexane: 3.8 grams of fullyactivated SiO 2 (4500C, overnight) in a small beaker were mixed with hexane until the SiO 2
was saturated. A small plug of glass wool was added to the bottom of each column. The
slurry was poured into the column slowly using hexane to wash the solids out of the beaker
and into the column. Approximately 0.5 grams of Na2 SO 4 was added to the top of each
column to absorb any water in the sample. The column was then conditioned with 50 mL of
hexane.
Experimental Procedure
6.2 Methods
6.2.1 General glassware cleaning procedure
All glassware used was soaked for at least two days in a NaOH/trisodium nitrilotriacetate
cleaning solution prior to use. They were then rinsed with water then air-dried and stored in
a laminar-flow clean hood. All water used in the laboratory was reverse osmosis pre-treated,
cycled through ion-exchange resins and activated carbon filters until a resistance of 18-MQ
was achieved, then passed through a 0.2 jtm filter pack just prior to dispensing. Immediately
before use, glassware was rinsed three times each with DCM followed by hexane.
If
glassware was washed recently, it was rinsed first with methanol then followed by DCM and
hexane, in order of decreasing polarity. All other materials (pipette, spatula, scissors, etc.)
that were placed in direct or indirect contact with the samples were rinsed with DCM and/or
hexane prior to use.
6.2.2 Laboratory experiment
6.2.2.1 Preparationof the spikes
The
1 mL
2-methylnaphthalene-,
pyrene-,
and benzo(a)pyrene-solutions
were
quantitatively transferred from the amber ampules into individual 10-mL volumetric
flasks. Hexane was added to the 10 mL mark, then the solutions were transferred to 10
mL amber vials to protect them from photodegradation. The final spike solutions had
concentrations of 20 ptg/mL.
6.2.2.2 Analyte dischargefrom the SPMDs
An 8-L cylinder (approximately 15 centimeters in diameter and 46 centimeters in height)
was filled to within 1 cm from the top with water. A pair of wooden sticks were crossed
over, taped together, and placed on top of the cylinder.
Experimental Procedure
Four cans containing SPMDs were removed from the freezer. Each SPMD was removed
from the can and a pair of scissors was used to cut a 2-4 mm slit at one end of the
polyethylene film. 25 p[L of each spike solution (500 ng of analyte) were injected into
these slits using a syringe. The film surface area exposed to the air was minimized as
much as possible. Inevitably, some air (approximately 1 mL or less) was introduced into
the SPMD during this procedure.
After spiking, the film was folded transversely so that the two ends met. One of the ends
was then rotated 1800, so that the loop resembled a Mobius strip. This was done, as
suggested by Lebo et al. (1992), to prevent the two sides of the SPMD from clinging
together, thereby assuring maximum surface area. The two ends were folded over twice,
and then they were secured with a metal clip to ensure that the hole would not allow
leakage of the triolein and the spike. A second metal clip was fastened to the bottom of
the loop to act as a weight when the SPMD was suspended in water.
The four SPMDs were then lowered into the water-filled cylinder and were kept
suspended from the sticks. The SPMDs were separated from each other as far as possible
without allowing them to stick to the sides of the glass cylinder. The top and sides of the
cylinder were kept covered with aluminum foil to prevent photodegradation of lightsensitive PAHs.
The temperature in the laboratory was about 180 C + 30C over the
duration of the experiment.
To purge the water in the cylinder of any PAHs and other chemicals that diffused out of
the SPMDs, water was flowed through the cylinder daily for a minimum of 30 minutes at
a rate of 1 L/min. For the first two days, water was introduced at the top of the cylinder
and the water was stirred with a spatula to induce mixing and water volume exchange.
Realizing the inefficiency of this process, a 50-cm teflon tube was attached to the water
nozzle and was then placed in the bottom of the cylinder to ensure better water exchange
in the cylinder for the remaining fourteen days.
Experimental Procedure
SPMDs were removed at 4-day intervals resulting in 4, 8, 12, 16-day exposures. The
storage cans were prepared by blowing N2 gas through them to purge them of any
possibly-contaminated air. The SPMDs were then placed inside the can, and N2 gas was
blown through them again to induce partial drying.
The SPMDs were not dried
completely before the cans were resealed and replaced in the freezer at -100 C for later
analysis.
6.2.3. Field Experiment
6.2.3.1 Constructionof the SPMD cages
Wire cages were constructed to hold one SPMD each. A 46 cm x 61 cm piece of the wire
screen was rolled into a 61 cm long cylinder with a radius of 7 cm. The two lengthwise
ends were sealed together, and three 7-cm cuts were made at the tops and bottoms of the
cylinders with approximately equal spacing between them.
The three sections were
folded over later to close the tops and bottoms of the cages. Copper wire was wrapped
around the cylinder in an attempt to minimize biofouling. Two snap metal hooks were
connected to the side of each SPMD using nylon cord woven through and around the
wire screen. The SPMD was held in place by a wire running diametrically across the top
and two pieces of wire on the bottom sides. See Figure 6.1 for an illustration of an
SPMD-loaded wire cage.
ExperimentalProcedure
~·-
I~
-·
i
·-
·..
~c. ;·~
`·
;·
"
i"
"~.
·:,
j~,
`·
X
'~·.
j
-·
· I~
i'·~
~~\
_I
··S
~·~:
.
~"- `·;~-"·,
i~=·l:i··, i":·
I·
:·· i
·-. i
i:
-"
ii
Figure 6.1. SPMD-loaded wire cage. (Drawingcourtesy of Vittorio Agnesi).
6.2.3.2 Sampling Sites
The SPMDs were placed in four sites in the Boston Inner Harbor. Figure 3.2 shows a
map of the Boston Inner Harbor indicating the location of the sampling sites.
One SPMD each was placed in 3 of the 4 sites: (1) mouth of Chelsea River, (2) across
from the mouth of the Charles River, and (3) Logan Airport and two were placed at the
ExperimentalProcedure
base of Tobin Bridge.
The sites were chosen for two main reasons.
First, a good
distribution of the sampling sites over the Boston Inner Harbor was necessary in order to
see any spatial variation of the PAH concentrations.
Besides revealing important
information about their sources and fate, the results were also intended for comparison
with a 3-D model of the PAH concentrations in the Boston Inner Harbor. Second, the site
had to have a convenient and accessible point of attachment for the SPMDs. The initial
plan included a sampling site at the confluence of the Mystic and Chelsea Rivers to
incorporate PAH contributions from both sources, but obtaining a site there turned out to
be difficult because of navigational reasons. It was also difficult to obtain permission
from the private owner of the pilings in that area of the Harbor. As a compromise,
sampling sites were placed near the mouths of the Mystic and Chelsea Rivers. Two
SPMDS at different depths were placed at the Base of Tobin Bridge to check for a
vertical variation in PAH concentrations. This sampling site is located in one of the areas
of the Inner Harbor that will be dredged so it is of particular interest. See Table 6.1 for
the sampling depths at each site.
Bottom (BTB-b)
W 0710 02.85 min
Base of Tobin Bridge -
N 42' 23.122 min
4m
Surface (BTB-s)
Mouth of Chelsea River
W 0710 02.85 min
N 42°23.050 min
4.3 m
(MCR)
W 071002.534 min
Across from the Mouth of the
N 42' 23.139 min
Charles River -
W 071 0 02.816 min
Buoy #16 (AMCR)
Logan Airport -
N 420 20.955 min
Buoy # 16 (LA)
W 0710 01.124 min
4.6m
4.6 m
Table 6.1 Sampling depths (measured from the top) at the different sites. Note that the
average depth of the harbor is approximately 10 m.
Experimental Procedure
The SPMD cages were attached vertically to nylon ropes using the metal hooks. The
ropes were marked to allow for measurement of the depths of the SPMD cages once they
were lowered into the water. For the Base of Tobin Bridge, a buoy had to be constructed
so that the SPMD cages could be placed farther away from the shore and some wood
pilings in the area. There was some concern that the wood pilings could serve as a local
source of PAHs because some pilings in the harbor are known to be coated with creosote
Table 6.2 shows the chemical composition of creosote
to retard their decomposition.
(Supelco, 1996).
11
1. Naphthalene
2. 2-Methylnaphthalene
3. 1-Methylnaphthalene
4. Biphenyl
5. 2,6-Dimethylnaphthalene
6. 2,3-Dimethylnaphthalene
7. Acenaphthalene
8. Dibenzofuran
ene
^-A-
I
I I
0
4
I I I I
8
12
I I I I
16
20
Min
I
I I I I I I I
24
28
32
36
40
Figure6.2. Components of creosote (Supelco, 1996).
6.2.3.3 Deployment of the SPMDs. February1 3 th
The SPMDs were stored in a -75 0 C freezer until the day of deployment. They were then
transported to the sites in a cooler with dry ice. The SPMDs were kept sealed in the cans
to prevent contamination from the ambient air. The sites were accessed from a motor
boat. During assembly of the SPMD apparatus, the motor of the boat was kept in a
Experimental Procedure
downwind position relative to the SPMDs to minimize their exposure to the exhaust from
the motor. The motor could not be turned off because this would have made control of
the boat difficult.
The SPMDs were quickly but carefully taken out of the cans, folded transversely and
placed in the cages. The two free ends were attached to opposite sides of the cages with
wire. The tops and bottoms of the cages were closed by folding over the three sections of
the wire screen and were lowered into the water as quickly as possible.
In addition to the five SPMDs deployed, a blank was also brought to the field.
At the
same time the fifth SPMD was removed from its can, the blank can was also opened to
expose the SPMD inside to the ambient air for the duration of the assembly. After the
fifth SPMD was deployed into the water, the blank can was resealed and replaced in the
cooler then stored back in the freezer.
6.2.3.4 SPMD collection: February2 7 th
The SPMD cages were retrieved after 14 days. The average temperature of the water
over the exposure time was approximately 40 C. The SPMDs were removed from the
cages as quickly as possible and directly returned to the cans. The resealed cans were
kept in a cooler with dry ice until they could be placed in the freezer again. The blank
SPMD was re-exposed to account for exposure during a collection procedure. As before,
the boat motor was kept downwind. It should be noted that there was more exhaust from
the motor of the boat used during the collection than the boat used during deployment.
Very little biofouling had occurred on the SPMDs, most likely due to the low temperature
in the water. The cans were stored in the freezer at -75 0 C until later extraction and
analysis.
ExperimentalProcedure
6.2.4 Extraction and Analysis of the SPMDs
6.2.4.1 Dialysis
* Lab samples
The four SPMDs from the lab experiment were removed from the freezer and allowed to
warm up to room temperature. Each SPMD was then spiked with 100 ýtL of a combined
recovery standard solution (2.9 ug/mL p-terphenyl, 3.0 ptg/mL ds-naphthalene, 3.0 pýg/mL
d 12-perylene) through the original slits using micropipets. 600 mL beakers which served
as the dialysis chambers were each filled with 485 mL DCM and 25 mL CH 3 0H. The
SPMDs were kept suspended in individual beakers with the clipped slits out of the
solution to prevent leakage. An extra beaker containing only the solvent was included to
serve as a blank.
All beakers were covered with aluminum foil to minimize
photodegradation and evaporation. All five beakers were placed in a N2-filled glove bag
to prevent possible contamination from the ambient air. The blank, day-4, day-8, day-12,
and day-16 samples were dialyzed for 40, 46, 48, 69, and 71 hours, respectively.
*
Field samples
In order to optimize the dialysis process and reproduce the methods utilized in previous
studies by other investigators (Meadows et al., 1993), a solution of 450 mL hexane and
50 mL DCM was used for the dialysis of the field samples. This was carried out in
stoppered flasks that were used as dialysis chambers in order to minimize solvent
evaporation and exposure to contaminants. The flasks were kept in a laminar flow-clean
hood over the duration of the dialysis. The use of a N2 -filled glove bag was deemed
unnecessary in light of the above-mentioned method improvements.
The SPMDs were allowed to warm up to room temperature before they were wiped down
quickly with lint-free wipes and spiked with 100 ptL of the combined recovery standard
ExperimentalProcedure
solution. As previously mentioned, the SPMDs experienced very little biofouling and it
was decided that attempting to clean the SPMDs (e.g., soaking in a KOH solution then
isopropyl alcohol) might actually lead to more contamination, thus negating its potential
benefit. After spiking, the SPMDs were lowered into the flasks and as much of the film
as possible was exposed to the solvent (in some cases, adding approximately 50 mL
solvent (9:1 hexane/DCM) was necessary). Again, a flask containing only solvent was
included in the dialysis procedure. The field SPMDs were dialyzed for 80 hours.
6.2.4.2 Kuderna-DanishConcentration
A Kuderna-Danish (KD) concentrator was used to reduce the volume of the dialysates. It
was noted that in the case of the field samples, some solvent had penetrated the SPMDs
but their volumes were deemed insignificant relative to the total volume of the dialysates.
The dialysates were transferred to the reservoir quantitatively. Kuderna-Danish
concentration was also used after the silica gel column chromatography procedure
described below. Concentrating proceeded until the sample volumes were reduced to
approximately 4-8 mL.
6.2.4.3 N 2 blowdown and hexane exchange
A stream of dry nitrogen gas was used to further reduce volumes of samples. This step
was performed to produce sample volumes of 1 mL for silica gel column chromatography
and a few hundred microliters for the gas chromatographic analysis. The extracts were
exchanged into hexane by reducing the volume to approximately 200 ptL, filling up to the
1 mL mark with hexane, reducing the volume to a few hundred microliters, then
repeating the process one more time until the sample volume is reduced to approximately
150 jtL.
ExperimentalProcedure
6.2.4.4 SiO2 Gel Column Chromatography
The silica gel columns described previously were used to separate the samples into three
fractions (F). The following eluants were collected:
Fi: 6 mL hexane
F2 : 44 mL hexane
F3 : 30 mL 10% DCM/hexane
F3 , the PAH-containing sample, was re-concentrated using Kuderna-Danish concentration
and N2 blowdown procedures in preparation for the gas chromatography.
It is worth mentioning that the lab SPMDs were expected to be relatively "clean", i.e.
containing 8 compounds only (dio-acenaphthene, djo-phenathrene, three recovery
standards, 2-methynaphthalene, pyrene, and benzo(a)pyrene), therefore it was assumed
that fractionation would not be necessary.
The pre-silica gel column chromatography
samples turned out to be complex mixtures of various indeterminate groups so
fractionation was also performed on them. The source(s) of the contamination have not
been clearly identified. Contamination from brief exposures to the ambient air may be
partially responsible.
6.2.4.5 Gas Chromatography
The gas chromatograph used in this experiment was equipped with a flame ionization
detector (GC-FID). It is a Carlo Erba, HRGC 5160 mega series with an on-column
injector, 30 m DB5 column, and hydrogen as the carrier gas. A Hewlett-Packard model
3396 series II integrator was used for data collection and peak area determinations.
The temperature program for the gas chromatograph was as follows: initial temperature
of 700 C with a hold time of 1 min; increase temperature at 20 0C/min until 180 0 C then
ExperimentalProcedure
increase temperature at 60C/min until 300 0 C. For the lab samples, the temperature was
held at 300NC for 5 minutes. The hold time was increased to 15 minutes for the field
samples to ensure complete elution of high-molecular weight compounds between runs.
F3 sample volumes were reduced to approximately 150 tiL then 50 tiL, of n-C 24 was
added as an internal standard for volume calculations.
Each injection contained
approximately 1 pL of each sample.
ExperimentalProcedure
55
7.0 RESULTS AND DISCUSSION
7.1 Laboratory Experiment: Dissipation of Compounds from SPMDs
For half of the samples, the front and end regions of the gas chromatogram were difficult to
analyze because of interfering peaks from contamination and a baseline drift. It has not been
determined what the source(s) of contamination was (were). The sample recoveries for d8 naphthalene were very low and ranged from 0-17%, most likely because of its loss through
volatilization.
Because of the poor recovery of its corresponding
methylnaphthalene could not be quantified with confidence.
standard, 2-
On the other end, sample
recoveries for d12-perylene were erratic and unreasonable, ranging from 7% to 180% for the
various samples. This may indicate that the dl2-perylene peak was not correctly identified or
the integrator may not have been able to resolve it well. Chromatography runs through the
silica column may have also been erratic.
As a result, benzo(a)pyrene was also not
quantified. The two compounds, dio-acenaphthaneand djo-phenanthrene,which were spiked
into the SPMDs by EST Technology, were also difficult to identify in the gas
chromatograms.
Except for one of the samples (day 16), the recoveries for p-terphenyl were reasonably
consistent, although somewhat low, and both p-terphenyl and pyrene were identifiable from
the chromatograms. The sample recoveries ranged from 31% to 52% (42% ± 10%). Pyrene
concentrations decreased smoothly over the 16-day test period (Figure 7.1).
Results and Discussion
Dissipation of Pyrene from the SPMDs in the Lab Experiment
I;n
500
Mass of
t)
450
Pyrene (ng) 400
per SPMD
350
300
250
0.00
2.00
4.00
6.00
8.00
10.00
12.00
14.00
16.00
Time of Exposure (days)
Figure 7.1. Dissipation of pyrene from the SPMDs in the laboratory experiment. For the
actual data, see Appendix A. Note that the SPMDs were originally spiked with 500 ng of
pyrene prior to dissipation. Error bars on the two points are related to injection imprecision.
Day 4 (n=4 injections), day 8 (n=2), day 12 (n=l). Day 16 was not quantified because the
recovery was unreasonably high at an average of 160% for three injections.
The data for pyrene dissipation from SPMDs over time were fit into both exponential and
linear models. As the graph demonstrates, the exponential dissipation kinetics of SPMDs for
pyrene was closely approximated by a linear relationship over this time interval, in
agreement with Huckins' model. Assuming that the uptake and dissipation behavior of the
SPMDs were the same, the results suggested that, in the case of uptake, the following
relationship could be used to relate pyrene concentration in the triolein, Cs, to its
concentration in the water, Cw, over this time interval: Cs = RwsCw (see section 5.3, Eqn. 8),
where Rws is a function of time.
Results andDiscussion
Recent work by Huckins et al. (1998)
gave the equivalent relationship:
Cw =
(CSPMDVSPMD)/Rst, where CSPMDVSPMD is the mass of the analyte extracted from the
membrane and the triolein in the dialysis procedure, and Rs = keKSPMDVSPMD with KSPMD =
CSPMD/Cw. In this case, Rs is a variable that is independent of time but is a function of the
membrane design and the compound of interest. The ke can be derived from dissipation
studies such as this one by using the following relationship between the initial concentration
in the SPMDs, CSPMDo, and concentrations over time, CSPMD: CSPMD = CSPMDoexp(-ket). The
ke for pyrene in this controlled laboratory experiment was found to be 0.028/day + .006 /day
at an average temperature of approximately 180 C. The errors associated with the fitting
parameters (including ke) were calculated using Sigma Plot TM. This value is slightly higher
than what can be expected from the tabulated values in the Hucks Table (Huckins et al.,
1998) of 0.024/day at 26 0 C and 0.015/day at 180C and 100C, but it is in reasonable agreement
(Table 7.1). Using pyrene's KSPMD value of 62,100 tabulated in the same table and a total
SPMD volume, VSPMD, of 0.0057 L (volume of triolein = ImL and volume of membrane =
4.7 mL), Rs was calculated to be 9.2 L/day. Huckins et al. (1998) calculated Rs to be 7.9
L/day at 26 0 C, 5.2 L/day at 180C, and 5.1 L/day at 100 C. Given the error associated with ke,
the value of Rs calculated here is in reasonable agreement with the value given by Huckins et
al. (1998).
Results and Discussion
phenanthrene
29,600
fluoranthene
pyrene
48,000
62,100
benz(a)anthracene
chrysene
210,800
209,300
3.9 (6)
4.3" (-)
0.024
3.4 (9)
5.1(10)
0.016
0.015
ab
4.6 (-)
5.2 (12)
3.6 (9)
4 (11)
0.003
0.004
3.6 (9)
5.1 (7)
0.021
4.6 (5)
0.029
0.018
0.015
b
7.2 (8)
7.9 (9)
0.028
0.024
0.003
0.004
5.5 (8)
7.4 (9)
0.005
0.006
Table 7.1. Rs or sampling rates (relative standard deviation in % ), KspMD, and ke or
dissipation rates at different temperatures. The relative standard deviation is the standard
deviation divided by the mean multiplied by 100%. These values were taken from
unpublished data in the Hucks Table. an< 2, bRecovery from SPMDs based on average of
anthracene and pyrene values because of interfering peaks (only in recovery studies). The Rs
values in the Hucks Table were derived from 14-day controlled laboratory exposures of
SPMDs to water containing 100 ng/L of the target compound (Huckins et al., 1998).
Results and Discussion
7.2 Field Measurements
The hydrophobic, and generally nonpolar, structure of triolein makes it able to dissolve many
organic compounds (e.g., PAHs, PCBs, etc.) found in the aquatic environment.
Upon
exposure in Boston Harbor, the triolein then became a complex "soup" of organic
compounds. The extract from the field samples remained a complex mixture of compounds,
even after clean-up with silica gel column chromatography. There appeared to be unresolved
mixtures of compounds in the front ends of the chromatograms and interfering peaks at the
back ends which obscured the PAH signal pattern (Figure 7.2). Lebo et al. (1992) speculated
that, in addition to other contaminants in the water
(e.g., chlorinated hydrocarbons),
interferences may result from trace oligomers from the polyethylene that remained even after
extraction of the tubings, co-dialyzed triolein, and various biogenic materials from the small
amount of aufwuchs (biofilm) found on the membrane surface. As noted in Section 6.2.4,
the outside surfaces of the SPMDs were wiped down with lint-free wipes but did not undergo
any other extensive clean-up prior to dialysis. It is possible that the silica gel column became
saturated with the various compounds and was not able to fractionate the various compounds
effectively.
A second run through a silica gel column was performed on one of the samples, and it
removed some of the signals at the back end, but it was unable to clean up the unresolved
mixture at the front end. However, a combination of a second run through a silica gel
column, attenuation of the integrator output, and a dilution of the sample to approximately
340 ýtL (most samples were concentrated down to about 100 tiL or less prior to injection into
the GC) did yield a chromatogram that clearly showed a PAH pattern in the samples similar
to that of a PAH chromatogram of Charles River sediment (Figure 7.3). The removal of
some of the end peaks after a second run through a silica gel column indicated that the
interferences in the back end were most likely not PAHs.
Results and Discussion
It,
Figure 7.2. PAH chromatogram of the Logan Airport - Buoy #12 (LA) sample after first run through the silica column. For reference,
pyrene is marked with an asterisk (*).
(a)
(b)
Figure 7.3. (a) GC-FID chromatogram of the Tobin Bridge-surface PAH sample fraction and
(b) High resolution gas chromatogram of PAHs in Charles River sediment (Laflamme and
Hites, 1978). For reference, pyrene is marked with an asterisk (*).
Results and Discussion
The use of a mass spectrometer in conjunction with a gas chromatogram (GC-MS) would
have greatly simplified the process of compound identification since a GC-MS can be
programmed to scan only certain pre-selected masses in the selected ion-monitoring mode
(SIM). Unfortunately, a GC-MS was unavailable for use in this study. Further experiments
should examine the improvements afforded by the use of this detection method.
A standard containing various PAHs commonly found in environmental samples was
injected into the GC-FID in order to obtain a reference chromatogram. Under the same
conditions (i.e., temperature settings, injection method, column length, etc.), compounds can
be expected to travel through the column in the same manner. Therefore, this reference
chromatogram can provide the expected retention times of the individual PAHs in the
column and the means of identification of PAHs in the field samples (Figure 7.4). In
addition, the reference chromatogram can be used to calculate relative retention times which
are also useful tools for confirming the identity of a compound. For example, knowing that
the retention time of pyrene in the column is usually 1.06 times greater than that of
fluoranthene provides an alternative means of identifying fluoranthene when pyrene can be
identified with confidence.
As with the laboratory samples, the two recovery standards d8-naphthalene and d 12 -perylene
were difficult to identify from the gas chromatograms, and only p-terphenyl yielded
recoveries that could be applied.
The recovery for p-terphenyl was 63% ± 18%.
The
recoveries ranged from 26% to 99%, which is quite a big range. This suggests problems with
the reproducibility of the data. This perhaps could improve as the person doing the analysis
becomes more familiarized and experienced with the analytical procedure.
For most of the samples, the following compounds were identified: p-terphenyl, d1 ophenanthrene, phenanthrene, methyl-phenanthrene's (methyl groups in the 1, 2, 3, 4, and 9
positions; see Figure 2.1), fluoranthene, pyrene, benz(a)anthracene, and chrysene.
Results and Discussion
F
7
7~
(b)
Figure 7.4. (a) Gas chromatogram of a PAH standard and (b) gas chromatogram of the Tobin
Bridge-bottom PAH fraction sample. For reference, pyrene is marked with an asterisk (*).
Results and Discussion
In deducing the water column concentrations of these compounds from their concentrations
in the SPMDs, the following assumptions were made:
(1) the recovery ofp-terphenyl is representative of the recoveries for the range of
compounds identified and analyzed;
(2) the uptake kinetics for all compounds are in the linear region over 14 days;
(3) the sampling rates at 100 C tabulated in the Hucks Table are valid for this field
sampling procedure; and
(4) biofouling did not significantly impede uptake into the SPMDs.
The use of p-terphenyl as the recovery standard for all compounds was obviously not ideal
but was necessitated by the lack of data for any other recovery standards.
However, p-
terphenyl can be expected to be a reasonable representative for the bigger compounds
(fluoranthene, benz(a)anthracene, chrysene, and pyrene).
Previous experiments (data not
shown) have demonstrated that the behavior of p-terphenyl throughout the analytical
procedure used here mimics those of pyrene and structurally- and chemically-similar
compounds (as exhibited by Kow's, column retention times, etc.) (MacFarlane, 1998). The
greater vapor pressures of the smaller compounds relative to the heavier p-terphenyl possibly
leads to greater loss through volatilization. In the past, experiments employing the same
procedure used here have shown that p-terphenyl recoveries were usually greater by 10-20%
compared to a smaller standard like d8-naphthalene (MacFarlane, 1998).
The linearity of the uptake kinetics for all compounds identified is a justifiable assumption.
Studies by Huckins et al. (1998) have shown that dissipation from the SPMDs is a linear
function of time, or closely approximated by such, over 14 days for the range of compounds
in this study (Huckins, 1998). In order to use data derived from homogeneous experimental
procedures, all values used for Rs, including that of pyrene, were taken from the Hucks Table
(Table 7.1). These values were derived from flow-through exposures of the SPMDs to 100
ng/L concentrations (kept constant over the duration of the experiment). Because only a
Results andDiscussion
value for phenanthrene was available in the Hucks Table, this Rs value was assumed to be a
good approximation for d1 o-phenanthrene and the methylated phenanthrene's.
The field sampling procedure deviated from the laboratory procedure of Huckins et al.
(1998) in several important aspects. The fact that the Rs values used were derived for 100 C
temperatures and the average water temperature in the harbor during the SPMD exposure
was approximately 30 C may have resulted in an underestimation of the water column
concentrations since sampling rates can be expected to decrease with lower temperatures. It
should also be noted that the laboratory experiments were conducted under relatively
quiescent flow conditions with velocities in the range of a few cm/s. Field velocities were
greater, with velocities ranging from 0 to 30 cm/s.
Uptake into the membrane can be
expected to increase with increasing flow velocities because of the thinning of the water film
layer, thus decreasing the water film resistance. An experiment by Huckins et al. (1993)
showed that the amount of a PCB ([ 14C]-2,2',5,5'-TCB) associated with the membrane under
quiescent conditions versus turbulent conditions was greater by 26% .
The Rs values in the Hucks Table were derived from laboratory exposures, so biofouling was
not taken into account. Assuming that biofouling did not significantly impede the uptake
into the SPMDs over the 14-day field exposure may have resulted in an underestimation of
extrapolated water concentrations since biofouling lowers sampling rates. According to a
study by Huckins et al. (1998), biofouling impedance to PAH uptake increased with
compound Kow and ranged from a 20 to 70% decrease in uptake. However, as was noted
earlier, the biofouling on the membranes did not appear extensive (probably because of the
low water temperatures) and was assumed to have had a negligible impact on uptake rates. It
is possible that the temperature, velocity, and biofouling effects could have partially offset
each other.
The field measurements ranged from 0.61 to 90 ng/L for the various PAHs (Tables 7.2-7.4)
Blank concentrations, which ranged from 0.0-0.64 ng/L, have been subtracted from the
concentrations measured in each sample. The data appear reasonable in that the signal-to-
Results and Discussion
noise ratio is good. On the average, the blank concentrations were only 2% of sample
concentrations.
Results and Discussion
Blank
BTB-b
BTB-s
MCR
LA
0.64
3.4
39
N.La
25
0.51
5.6
90
45
55
0.80
1.7
2.3
N.L a
2.2
0.42
6.4
78
30
48
0.24
5.7
58
28
29
0.59
0.90
0.75
0.96
0.61
nd
1.3
15
5.0
7.1
nd
0.7
8.7
2.6
3.3
Table 7.2.
Blank
BTB-b
BTB-s
MCR
LA
Table 7.3.
Blank
BTB-b
BTB-s
MCR
LA
----
~
Table 7.4.
Tables 7.2-7.4. The extrapolated concentrations in the water column of the various PAHs
identified in the gas chromatograms. a N.I.- could not be identified in the chromatograms.
Base of Tobin Bridge - bottom (BTB-b), base of Tobin Bridge - surface (BTB-s), Mouth of
Chelsea River (MCR), and Logan Airport (LA). See Figure 6.2 for the location of the
sampling sites. For an explanation of the conversion from SPMD concentrations to water
concentrations, see the text. All concentrations shown are in nanograms per liter. See
Appendix B for the raw data and notes on uncertainties and assumptions.
Results and Discussion
For all compounds measured, the highest concentrations were found in the Tobin Bridgesurface sample (Figure 7.5). There was a difference in the bottom (8 m from the surface) and
surface (4 m from the surface) PAH concentrations in the Tobin Bridge site.
The
concentrations in the upper layer (BTB-s, MCR, and LA) versus the lower layer (BTB-b)
were greater by approximately a factor of 10. All concentrations were found to be in the
order of parts per trillion. For the three samples located in approximately the same depth (4
m below the surface), the magnitudes of the various PAH concentrations were quite
comparable, varying within a factor of 3.
The ratios of methyphenanthrene's-to-phenanthrene
and pyrene-to-fluoranthene
were
calculated as possible indicators of source. In all three sites, methylated phenanthrene levels
were found to be about twice that of phenanthrene levels. This may indicate that one of the
main sources of phenanthrene and related compounds into these sections of the harbor are of
petrogenic origin. The abundance of small analytes in the samples (Figure 7.3a) suggest a
low-molecular weight petroleum source such as diesel, creosote, and/or coal tar.
However, the ratios of pyrene-to-fluoranthene suggest another type of origin. The ratios
were found to be less than one for all sites with a mean of 0.81 and a standard deviation of
0.16. Pyrene-to-fluoranthene ratios in the environment vary according to the primary sources
(Table 7.5). Based on the pyrene-to-fluoranthene ratios obtained here, the potential major
primary sources in the study areas are Boston air, street dust, and creosote.
Although creosote is expected to be present in areas of the harbor which have creosotecoated pilings, it seems unlikely that it was the major source. Combustion effluents have
been found to be the major source of PAHs in diverse aquatic settings (Laflamme and Hites,
1977), suggesting that the Boston air is a likely source in this group. This does not
necessarily mean that the main mechanism of PAH input into the Inner Harbor is direct
deposition from the atmosphere or diffusive air-water exchange. Other means of input
(CSOs, runoffs, and river discharges) into the harbor could also carry with them previouslyairborne PAHs.
Results and Discussion
PAH Concentrations at the Different Sampling Sites
Logan Airport
Ochrysene
E benz(a)anthracene
1 pyrene
Sfluoranthene
III methylphenanthrene's
6 phenanthrene
Mouth of
Chelsea River
Base of Tobin
<_'.:.:.:.']
Bridge - surface
IIIIIIIIIIIIIIIIIIII~
lllllllllllllllllllllIIIIIIIIIIIIIIIIIIIIIIIIIIIIIII
IIIIIIIIIIIIIIIIIII
Base of Tobin
Bridge -
bottom
Blank
0.00
20.00
40.00
60.00
80.00
Concentration (ng/L or pptr)
Figure 7.5. Summary of PAH concentrations at the different sampling sites.
Results andDiscussion
100.00
Boston air a
0.77
Gasoline exhaustb
1.67
Street dustc
0.98
Creosoted
0.68
No.2 fuel oile
1.11
SRC II coal liquid f
5.08
Coal synthoil Cg
> 18.4
Table 7.5. Pyrene-to-fluoranthene concentration ratios of common sources of PAHs in the
environment. aGschwend and Hites, 1981. bGiger and Schaffner, 1978. cTakada et al.,
1990. dCarey and Farrington, 1989. ePancirov and Brown, 1975. rNishioka et al., 1988.
gGuerin et al., 1978.
The pyrene-to-fluoranthene ratios found in this study agree well with those calculated for the
rivers, CSOs, and stormwater drains that empty into the Inner Harbor (Table 7.6) (MenzieCura and Associates, 1995; Metcalf and Eddy, 1994; USGS, 1992). As illustrated in the
table, the Charles River is a major source of pyrene and fluoranthene into the Inner Harbor.
Performing a rigorous error analysis on the use of SPMDs to deduce water concentrations
was seriously hampered by the lack of sample duplicates. However, one can evaluate known
errors associated with the calculations here in order estimate a minimum error. The errors
(relative standard deviation = [standard deviation /mean] x 100%) associated with the
injections and the p-terphenyl recoveries were 4% and 28%, respectively. The relative
standard deviation associated with the Rs value of pyrene was 10%. Given these values, the
combined error is approximately 42%.
In the case of pyrene concentration at the base of Tobin Bridge-surface, for example, a total
error of 42% yields a result of 58 ng/L + 24 ng/L. Even with the associated errors, the
concentration can still be expected to be well within an order of magnitude of the reported
value of 58 ng/L.
Results and Discussion
One could also account for possible biofouling effects. Previous studies found a 20-70%
impedance in uptake as a result of biofouling (Huckins et al., 1998). It is reasonable to
assume that any impedance by biofouling in the measurements here would be in the low end
of the range. Assuming a maximum impedance of 30%, which corresponds to a reduction in
the sampling rate by 30%, the range then becomes 49-117 ng/L; again, this range is still in
reasonable agreement with the reported value of 58 ng/L.
It is therefore safe to assume that the values reported from the measurements are correct
within a factor of two or three.
Charles River
Mystic River
Chelsea River
Total from Rivers
73
7.6
0.4
81
120
11
0.6
132
CSOs + stormwater
9
14
(a)
0.61
0.64
Rivers
CSOs + Stormwater
(b)
Table 7.6. (a) Pyrene and fluoranthene loadings from rivers and CSOs and stormwater drains
into Boston Harbor. (b) Pyrene-to-fluoranthene ratios in rivers and CSOs and stormwater.
These loadings were calculated by multiplying averaged concentrations of pyrene and
fluoranthene which were measured by Menzie-Cura and Associates in 3/25/92, 4/30/92, and
10/15/92 (1995) with average annual flow rates. The Charles River flow rates were taken
from USGS measurements over the years 1931-1992 (USGS, 1992) and the Mystic and
Chelsea River flow rates were scaled relative to those of the Charles River (Chan, 1993).
The flow rates from the CSOs and stormwater drains were calculated from data collected by
Metcalf and Eddy (1994).
Results and Discussion
7.3 Comparison of Measurements to a Mass Balance (Box) Model and a 3-D
Hydrodynamic Model
A mass balance or box model quantified the various sources and sinks of pyrene in the Inner
Harbor (Petroni and Israelsson, 1998). In this model, the Inner Harbor was assumed to be a
well-mixed box with various sources and sinks (Table 7.7). Petroni and Israelsson (1998) did
not include boat traffic and pilings for lack of available data and they assumed that chemical
transformations,
insignificant.
biological
transformations,
and
indirect
photodegradation
were
This model was intended to give only an order-of-magnitude estimate of
pyrene concentration in the study area.
Nsealment-water
exchange
River loadings
CSO loadings
Stormwater loading
Atmospheric deposition
& Air-water exchange
transformations
Biological
Boat traffic,
pilings
transformations
Direct
photodegradation
Indirect
photodegradation
Table 7.7. Sources and sinks of PAHs in the Inner Harbor. Note that some of these terms
were not included in the mass balance calculations (Petroni and Israelsson, 1998).
A more complex 3-D hydrodynamic computer model called ECOMsi was used to identify
the spatial distribution of pyrene inside the Inner Harbor. In addition to spatially-distributed
sources and sinks, the 3-D hydrodynamic model also included a representation of the
hydrodynamics of the study site.
It is important to note that the models were based on winter conditions in the Harbor and did
not make a distinction between sorbed and "truly-dissolved" concentrations. For more
Results and Discussion
detailed information on the model assumptions and parameters, see Petroni and Israelsson
(1998).
The measured pyrene values from the SPMDs had the same order of magnitude as the values
predicted by both models (Table 7.8). In both the 3-D hydrodynamic model and the
measurements, the concentrations of pyrene increased as the distance from the mouth of the
Inner Harbor increased. Furthermore, the vertical concentration distribution obtained with
the 3-D model in the confluence of the Chelsea and Mystic Rivers was comparable to the
surface and bottom SPMD measurements at the base of Tobin Bridge (Figure 7.6). The
agreement among the three sets of values obtained using different techniques further supports
the validity of the results obtained here.
bottom
(3.3-8.1)
Base of Tobin Bridge -
58
surface
(34-82)
Mouth of the Chelsea
28
River
(16-40)
Logan Airport
29
(Buoy #12)
(17-41)
40
21
40
21
6
21
Table 7.8. SPMD-derived concentrations (accounting for + 42% error), predicted steadystate concentration from mass balance or box model, and predicted concentrations from 3-D
hydrodynamic model.
Results and Discussion
Vertical Distribution
Pyrene - Inner Confluence
U
-1
-2
-3
-4
-J
-5
-6
anding
-7
iding
-8
-9
-10
--
--
Concentration (ng/I)
Figure 7.6. Vertical profile of pyrene concentrations at the confluence of the Chelsea and
Mystic Rivers predicted by 3-D hydrodynamic model and data from SPMD measurements at
the Base of Tobin Bridge (BTB; see Figure 6.2) and box model- or mass balance-predicted
steady-state concentration (Petroni and Israelsson, 1998).
Results and Discussion
7.4 Comparison of measured water column concentrations to sediment concentrations
Shiaris and Jambard-Sweet measured sediment concentrations at several locations within
Boston Harbor in 1986. Although more recent data sets do exist, the Shiaris data set was
used in this study because of the geographic distribution of the sampling stations. Six of the
twenty-four sampling stations were within the Inner Harbor (Figure 7.7). If each sediment
quality data point was taken as representative of a portion of the Inner Harbor, the Inner
Harbor can be divided into six "sediment quality regions" and a weighted average of
sediment concentrations can be calculated (Table 7.9). For a more detailed explanation of
this calculation, see Petroni and Israelsson (1998).
Chelsea River Region
Mystic-Chelsea
Confluence Region
1
Charles River
Mouth Region
Fort Point Channel
Fort Point Channel Region
Reserved Channel Region
Figure 7.7. Sampling locations in the PAH sediment concentration study by Shiaris and
Jambard-Sweet 1986) and the breakdown of the Inner Harbor into "sediment quality
regions".
Results and Discussion
1) Chelsea River
2) Mystic-Chelsea
Confluence
3) Charles River Mouth
4) Fort Point Channel
Mouth
5) Fort Point Channel
6) Reserved Channel
Ii..
0.03
0.25
8900
50000
0.14
4400
0.35
3200
0.02
0.21
6700
1600
1.00
0
Table 7.9. Weighted sediment pyrene concentrations (Petroni and Israelsson, 1998).
With a sediment concentration, Cs, of 17,000 ng/g or 8.4 x 10-5 mol pyrene/kg soil and a KI
of 27,000 L/kg (Petroni and Israelsson, 1998), Cw is expected to be 600 ng/L. This is about
an order of magnitude greater than the average value (30 ng/L) obtained from the SPMD
measurements. This implies that the sediments are behaving as a source of pyrene in the
water column and that the two media are not in equilibrium.
This is supported by the
findings of the SPMD measurements and the 3-D hydrodynamic model which revealed that
the Inner Harbor is not well-mixed vertically because the residence time of water in the Inner
Harbor does not allow sufficient time for complete mixing.
Results and Discussion
7.5 Comparison of loadings
With an average dissolved pyrene concentration of 30 ng/L derived from the SPMD
measurements, one can calculate a rough estimate of the inventory of pyrene in the Inner
Harbor at a given point in time. The average volume of the Inner Harbor is about 7x1010 L,
so the total mass of dissolved pyrene in the entire Inner Harbor is approximately 2 kg.
The distribution coefficient, Kd, between water and suspended solids (ss) can be derived from
the following equations:
log mol / kgo
Lmol / Lwater
log K mol / Locanol
d
mol / Lwater
Kd = fom* Kom
The log Kow for pyrene is 5 (Schwarzenbach et al., 1993) and the coefficients c and d for
polycyclic aromatic hydrocarbons are 1.01 and -0.72, respectively (Schwarzenbach et al.,
1993). Assuming that the suspended solids in the water column contain 20% organic matter
(fom = 0.2), the Kd is then approximately 6000 Lwater/kgss. Assuming a total suspended solids
(TSS) concentration of 5 mg/L or, equivalently, a rsw of 5x10-6 kg ss/Lwater, and neglecting
soot, the fraction of the total pyrene that is dissolved in the water, fw, can be calculated using
the following relationship:
1
1+ rswKd
This calculation yields a dissolved fraction of pyrene in the water of 97%, so the total pyrene
in the Inner Harbor is approximately 2 kg. Given a residence time of 3 days under winter
conditions (Petroni and Israelsson, 1998), the loading of pyrene into the Inner Harbor is then
about 2 kg over 3 days or 240 kg/yr.
Results and Discussion
A study of PAH loadings into the Inner Harbor (Petroni and Israelsson, 1998) using data
from various sources (Menzie-Cura and Associates, 1995; Golomb et al., 1996; Shiaris and
Jambard-Sweet, 1986) estimated total pyrene loading to be in the order of 270 kg/yr (Table
7.10), in very reasonable agreement with the loading calculated above.
rivers: 2Lriverstrivers
13U
CSOs: EQCSOSCCSOS
.5
stormwater: MQstormCstorm
8.5
atmospheric deposition: FatmA
.8
air-water exchange: CavtotA/KH'
7.3
sediments: CsDwA/SwKd
100
Total Loading
270
Table 7.10. Loadings of pyrene into the Inner Harbor (Petroni and Israelsson, 1998).
Results and Discussion
8. CONCLUSIONS
The concentrations of PAHs (phenanthrene, methyl-phenanthrene's, fluoranthene, pyrene,
benz(a)anthracene, and chrysene) in the Boston Inner Harbor are in the order of parts per
trillion, ranging from 0.61 to 90 ng/L. There is a vertical gradient in PAH concentrations at
the base of Tobin Bridge, the concentration in the upper layer (4 m from the surface) being
ten times greater than the lower layer (8 m from the surface). The concentrations of the
PAHs examined here increase with distance from the mouth of the Inner Harbor. The
sediments are a source of pyrene and they are generally not in equilibrium with the water
column pyrene concentrations.
The main source of low molecular weight PAHs in the study areas appears to be of
petrogenic origin, possibly diesel, creosote, and/or coal tar. Heavier PAHs in the Inner
Harbor, such as pyrene and fluoranthene, probably originate from airborne combustion
products delivered directly through deposition from the atmosphere and diffusive air-water
exchange, and indirectly through CSOs, runoffs, and river discharges which also carry with
them previously-airborne PAHs. Pyrene loading into the Inner Harbor is in the order of 200300 kg/yr.
SPMDs appear to be a useful tool for quantifying PAHs in the field. The measurements
obtained in this study were in good agreement with the results of a box model and a 3-D
hydrodynamic model of PAH concentrations in the Inner Harbor. The number of compounds
that can be quantified using SPMDs could be significantly increased through the use of a
mass spectrometer. The analytical technique used here should be improved so that higher
recoveries could be attained and a wider range of compounds could be quantified.
Conclusion
REFERENCES
Alber M., Chan A.B. 1994. Sources of contaminants to Boston Harbor: revised loading
estimates. Tech. Report 94-1. Environmental Quality Dept., Massachusetts Water Resources
Authority, Boston, MA.
Bjorseth A. (ed.). 1983. Handbook of Polycyclic Aromatic Hydrocarbons. New York, NY.
Bjorseth A., Ramdahl T., Dekker M. (ed.). 1985. Handbook of Polycyclic Aromatic
Hydrocarbons Vol. 2. New York, NY.
Borneff J., Kunte H. 1969. Carcinogenic substances in water and soil. Part XVI: Evidence of
polycyclic aromatic hydrocarbons in water samples through direct extraction. Arch. Hyg.
(Berlin) 148, 585-597 (German). As cited in Neff (1979).
Bridboard K., Finklea J.F., Wagoner J.K., Moran J.B. and Caplan P. 1976. Human exposure to
polynuclear aromatic hydrocarbons. Pp. 319-324. In: Freudenthal R., Jones P.W. (editors).
Carcinogenesis - A comprehensive survey. Vol. I. Polynuclear aromatic hydrocarbons.
Chemistry, Metabolism, and carcinogenesis. New York. Raven Press. As cited in Neff (1979).
Budavari S. (ed.). 1996. The Merck Index. Merck & CO., Inc. Whitehouse Station, NJ.
Bumpus D.F., Butcher W.S., Athern W.D., Day C.G. 1953. Inshore survey project, Boston final
harbor report. Ref 53-20, Woods Hole Oceanographic Institution, Woods Hole, MA. As cited in
Chan-Hilton et al. (1998).
Byrne, G.A., Aylott, R.I. 1980. British Patent 1566253. Concentrator for Removing Organic
Materials from Aqueous Systems. As cited in Huckins et al. (1990).
California Environmental Protection Agency. 1994. http://www.calepa.cahwnet.gov/oehha/
docs/fish/memerc.htm.
Carey D., Farrington J.W. 1989. Estuarine and CoastalShelfScience. 29, 97-113. As cited in
McGroddy et al. (1996).
Chan A.B. 1995. A numerical investigation of the effects of freshwater inflow on the flushing in
Boston's Inner Harbor. Master of Science thesis. Massachusetts Institute of Technology,
Cambridge, MA.
Chan-Hilton A.B., McGillivary D.L., Adams E.E. 1998. The residence time of freshwater in
Boston's Inner Harbor. Journalof Waterway Port Coastaland Ocean EngineeringASCE. In
press.
References
Chiou, C.T. 1985. Partition coefficients of organic compounds in lipid-water systems and
correlations with fish bioconcentration factors. EnvironmentalScience and Technology. 19(1):
57-62.
Cleveland L., Little EE, Petty JD, Johnson BT, Lebo JA, Orazio CE, Dionne J., Crockett A.
1997. Toxicological and chemical screening of Antarctica sediments: Use of whole sediment
toxicity tests, Microtox, Mutatox and semipermeable membrane devices (SPMDs). Marine
Pollution Bulletin. 34(3), 194-202.
Crunkilton R.L., DeVita W.M. 1997. Determination of aqueous concentrations of polycyclic
aromatic hydrocarbons (PAHs) in an Urban Stream. Chemosphere. 35(7), 1447-1463.
Cura J., Studer M. 1996. Measurement of PAH loadings to Massachusetts Bay from various
waterborne sources. Proceedings of the Water Environment Federation. 6 9 th Annual Conference
and Exposition. Dallas, TX.
Ellis G.S., Huckins J.N., Rostad C.E., Schmitt C.J., Petty J.D., MacCarthy P. 1995. Evaluation
of lipid-containing semipermeable membrane devices for monitoring organochlorine
contaminants in the Upper Mississippi River. EnvironmentalToxicology and Chemistry. 14(11),
1875-1884.
Environmental Protection Agency (EPA). 1998a. http://www.epa.gov/OGWDW/wot/appa.html.
Environmental Protection Agency (EPA). 1998b. http://www.epa.gov/ reg3hwmd/ brownfld/analytic.htm#svoc.
Environmental Protection Agency (EPA). 1998c. http://www.epa.gov/ ogwdw000/dwh/csoc/benzopyr.html.
Giger W., Schaffner C. 1978. Analytical Chemistry. 50, 243-249. As cited in McGroddy et al.
(1996).
Golomb D., Ryan D., Eby N., Underhill J., Wade M., Zemba S. 1996. Atmospheric deposition
of contaminants onto Massachusetts and Cape Cod Bays. MBP-95-07, Massachusetts Bays
Program. Boston, MA.
Gray M.A., Spacie A.1991. 12 th Meeting of the Society of Environmental Toxicology and
Chemistry, EMAP Session. Poster #272. As cited in Huckins et al. (1993).
Gschwend, P.M. Hites R.A. 1981. Fluxes of polycyclic aromatic hydrocarbons to marine and
lacustrine environments in the northeastern United States. Geochimica et CosmochimicaActa.
45, 2359-2367.
Guerin M.R., Epler J.L, Griest W.H., Clark B.R., Rao T.K., Jones P.W.(ed), Freudenthal
R.I.(ed). 1978. Carcinogenesis: Polynuclear aromatic hydrocarbons, Vol. 3. Raven Press. New
York. 21-33. As cited in McGroddy et al. (1996).
References
Gustafson K.E., Dickhut R.M. 1997. Distribution of polycyclic aromatic hydrocarbons in
Southern Chesapeake Bay surface water: Evaluation of three methods for determining freelydissolved water concentrations. EnvironmentalToxicology and Chemistry. 16(3), 452-461.
Gustaffson O., Haghseta F., Chan C., MacFarlane J., Gschwend P.M. 1997. Quantification of
the dilute sedimentary soot phase: Implications for PAH speciation and bioavailability.
EnvironmentalScience and Technology. 31(1), 203-209.
Harvey R.G. 1991. Polycyclic Aromatic Hydrocarbons: Chemistry and Carcinogenicity.
Cambridge University Press: Cambridge, U.K. Chapter 4.
Harvey R.G. 1997. Polycyclic Aromatic Hydrocarbons. Wiley-VCH, New York, NY.
Hassett J.P., Force M., Song H. Abstracts of Papers. 1989. 19 8th Meeting of the American
Chemical Society, Miami Beach, FL. American Chemical Society: Washington D.C.
Hellmann, von H. 1974. Occurrence and origin of so-called carcinogens and other polycyclic
hydrocarbons in water. Deut. Gewasserkund. Mitteil. 18, 155-157 (German). As cited in Neff
(1979).
Herve S., Prest H.F., Heinonen P., Hyotylainen T., Koistinen J., Paasivirta J. 1995. Lipid-filled
semipermeable membranes and mussels as samplers of organochlorine compounds in lake water.
Envi. Sci. Pollut. Res. Int. 2(1), 24-30. As cited in Prest et al. (1995a).
Hofelt C.S., Shea D. 1997. Accumulation of organochlorine pesticides and PCBs by
semipermeable membrane devices and Mytilus edulis in New Bedford Harbor. Environmental
Science and Technology. 31(1), 154-159.
Huckins, J.N. 1998. Personal Communication. Environmental and Contaminants Research
Center, U.S. Geological Survey. Columbia, MO.
Huckins J.N., Manuweera G.K., Petty J.D., MacKay D., Lebo J.A. 1993. Lipid-containing
semipermeable membrane devices for monitoring organic contaminants in water. Environmental
Science and Technology. 27(12), 2489-2496.
Huckins J.N., Petty J.D., Orazio C.E., Lebo J.A., Clark R.C., Gibson V.L., Gala W.R., and
Kaiser E.M. 1998. Draft: Calibration of semipermeable membrane devices (SPMDs) for
estimation of polycyclic aromatic hydrocarbon (PAH) concentrations in water. Unpublished as
of 5/98.
Huckins J.N., Petty J.D., Prest H.F., Lebo J.A., Orazio C.E., Gale R.D., Clark R.C. 1997.
Important considerations in semipermeable membrane devices (SPMDs): Design, application,
performance, and data comparability. 18 th Annual Society of Environmental Toxicology and
Chemistry (SETAC) Meeting. San Francisco, CA.
References
Huckins J.N., Petty J.D., Lebo J.A., Orazio C.E., Prest D.E. 1997. Accumulation of
organochlorine pesticides and PCBs by semipermeable membrane devices and Mytilus edulis in
New Bedford Harbor - Comment. EnvironmentalScience and Technology. 31(12), 3732-3733.
Huckins J.N., Petty J.D., Lebo J.A., Orazio C.E., Clark R.C., Gibson V.L. 1997. SPMD
technology - a tutorial. Midwest Science Center, Biological Resources Division, US Geological
Survey. 573-875-5399.
Huckins J.N., Tubergen M.W., Lebo J.A., Gale R.W., Schwartz T.R. 1990. Polymeric film
dialysis in organic solvent media for cleanup of organic contaminants. Journalof the
Association of Official Analytical Chemists. 73(2), 290-293.
International Agency for Research on Cancer. 1973. Monograph on the evaluation of
carcinogenic risk of the chemical to man: Certain polycyclic aromatic hydrocarbons and
heterocyclic compounds.. Vol. 3. Geneva, Switzerland: World Health Organization. As cited in
Neff (1979).
Johnson G.D. 1991. Hexane-filled Dialysis Bags for Monitoring Organic Contaminants in Water.
EnvironmentalScience and Technology. 25(11), 1897-1903.
Laflamme R.E., Hites R.A. 1977. The global distribution of polycyclic aromatic hydrocarbons
in recent sediments. Geochimica et CosmochimicaActa. 42, 289-303.
Lebo J.A., Zajicek J.L., Huckins J.N., Petty J.D., Peterman P.H. 1992. Use of semipermeablemembrane devices for in-situ monitoring of polycyclic aromatic-hydrocarbons in aquatic
environments. Chemosphere. 25(5), 697-718.
MacFarlane, J. 1998. Personal Communication. Ralph M. Parsons Laboratory, MIT.
Cambridge, MA.
Massachusetts Water Resources Authority (MWRA). 1990. The State of Boston Harbor. Boston,
MA.
Massachusetts Water Resources Authority (MWRA). 1991. The State of Boston Harbor: 1991.
Technical Report No. 92-3. Boston, MA.
Massachusetts Water Resources Authority (MWRA). 1993. The State of Boston Harbor. Boston,
MA.
Massachusetts Water Resources Authority (MWRA). 1996. History: Boston Harbor. Boston,
MA.
McGroddy S.E., Farrington J.W., Gschwend P.M. 1996. Comparison of in situ and desorption
sediment-water partitioning of polycyclic aromatic hydrocarbons and polychlorinated biphenyls.
Environmental Science and Technology. 30 (1), 172-177.
References
Meadows J., Tillitt D., Huckins J., Schroeder D. 1993. Large-scale dialysis of sample lipids
using a semipermeable-membrane device. 1993. Chemosphere. 26(11), 1993-2006.
Menzie-Cura and Associates. 1995. Measurements and loadings of polycyclic aromatic
hydrocarbons (PAH) in stormwater, combined sewer overflows, rivers, and publicly-owned
treatment works (POTWs) discharging to Massachusetts Bays. Submitted to the Massachusetts
Bays Program. MBP-95-06.
Metcalf & Eddy. 1994. Master Planning and CSO Facility Planning: System Master Plan
Baseline Assessment.
Miere J.P., Mappes G.W., Tucker E.S., Dietrich M.W., Keith L.H. (editor). 1977. Identification
and Analysis of Organic Pollutants in Water. Ann Arbor Science Publishers, Inc. Ann Arbor,
MI. Pp. 113-133. As cited in Huckins et al. (1990).
Moring J.B., Rose D.R. 1997. Occurrence and concentrations of polycyclic aromatic
hydrocarbons in semipermeable membrane devices and clams in three urban streams of the
Dallas-Fort Worth Metropolitan Area, Texas. Chemosphere. 34(3), 551-566.
National Academy of Sciences, Committee on Biological Effects of Atmospheric Pollutants.
1972. Particulate Polycyclic Organic Matter. Washington, D.C. As cited in Neff (1979).
Neff J.M. 1979. Polycyclic Aromatic Hydrocarbons in the Aquatic Environment: Sources, Fates
and Biological Effects. Applied Science Publishers, London, U.K.
Neff J.M. 1985. Fundamentals of Aquatic Toxicology. Hemisphere Publishers Corporation.
Nishioka M., Lee M.L., Ebert L.B. (ed). 1988. Polynuclear Aromatic Compounds. American
Chemical Society. Washington D.C. 235-269. As cited in McGroddy et al.(1996).
Pancirov R.J., Brown R.A. 1975. Proc. Conf Prev. Control Oil Pollut. 103-113. As cited in
McGroddy et al. (1996).
Petroni R., Israelsson P. 1998. Mass Balance and 3D Model of PAHs in Boston's Inner Harbor.
Master of Engineering Thesis.
Petty J.D., Huckins J.N., Zajicek J. 1993. Application of semipermeable membrane devices
(SPMDs) as passive air samplers. Chemosphere. 27(9): 1609-1624.
Peven C.S., Uhler A.D., Querzoli F.J. 1996. Caged mussels and semipermeable membrane
devices as indicators of organic contaminant uptake in Dorchester and Duxbury Bays,
Massachusetts. Environmental Toxicology and Chemistry. 15(2), 144-149.
References
Prest H.F., Huckins J.N., Petty J.D., Herve S., Paasivirta J., Heinonen P. 1995a. A survey of
recent results in passive sampling of water and air by semipermeable membrane devices. Marine
Pollution Bulletin. 31(4-12), 306-312.
Prest H.F., Jacobson L.A., Huckins J.N. Passive sampling of water and coastal air via
semipermeable membrane devices. 1990. Chemosphere. 30(7), 1351-1361. As cited in Prest et
al. (1995a).
Prest H.F., Jacobson L.A., Wilson M. 1997. Passive water sampling for polynuclear aromatic
hydrocarbons using lipid-containing semipermeable membrane devices (SPMDs):
Application to contaminant residence times. Chemosphere. 35(12), 3047-3063.
Prest H.F., Jarman W.M., Burns S.A., Weismiller T., Martin M., Huckins J.N. 1992.
Chemosphere. 25, 1811-1823.
Prest H.F., Richardson B., Jacobson L.A., Vedder J., and Martin M. 1995b. Monitoring
Organochlorines with SPMDs and Mussels (Mytilus edulis) in Corio Bay, Victoria, Australia.
Marine Pollution Bulletin. 30(5): 543-554.
Rex A., Connor M.S. 1996. The State of Boston Harbor. Technical Report No. 97-5.
Massachusetts Water Resources Authority (MWRA). Boston, MA.
Santodonato J., Howard P., Basu D. 1981. Health and Ecological Assessment of Polynuclear
Aromatic Hydrocarbons. Center for Chemical Hazard Assessment, Syracuse Research
Corporation. Pathotox Publishers, Inc. Park Forest South, Illinois.
Schwarzenbach R.P., Gschwend P.M., Imboden D.M. 1993. Environmental Organic Chemistry.
John Wiley & Sons, Inc. New York, NY.
Sea Grant Program. 1996. http://www.whoi.edu/seagrant/Exhibits/Background.html
Shiaris M.P., Jambard-Sweet D. 1986. Polycyclic aromatic hydrocarbons in surficial sediments
of Boston Harbour, Massachusetts, USA. Marine PollutionBulletin. 17(10): 469-472.
Shiaris M.P. 1989. Seasonal biotransformation of naphthalene, phenanthrene, and
benzo(a)pyrene in surficial estuarine sediments. Applied EnvironmentalMicrobiology.
1391-1399.
Sodergren A. 1987. Solvent-filled dialysis membranes simulate uptake of pollutants by aquatic
organisms. EnvironmentalScience and Technology. 21(9), 855-859.
Speers G.C., Whitehead E.V. 1969. Crude Petroleum in Organic Geochemistry: Methods and
Results (eds G. Eglinton and M.R.J. Murphy). Pp. 638-675). Springer-Verlag.
References
Stivers C.E., Sullivan R. 1994. Restoration and Capping of Contaminated Sediments.
Proceedings of the Second International Conference on Dredging and Dredged Material
Placement. American Society of Civil Engineers. Lake Buena Vista, Florida.
Stolzenbach, K.D., Adams E.E. 1998. Contaminated Sediments in Boston Harbor. Marine
Center for Coastal Processes, MIT Sea Grant College Program.
Strandberg B., Wagman N., Bergqvist P-A., Haglund P., Rappe C. 1997. Semipermeable
membrane devices as passive samplers to determine organochlorine pollutants in compost.
EnvironmentalScience and Technology. 31(10), 2960-2965.
Suess M.J. 1976. The environmental load and cycle of polycyclic aromatic hydrocarbons. Sci.
Total Environ. 6, 239-250. As cited in Neff (1979).
Suffet I.H., Jafvert C.T., Kukkonen J., Servos M.R., Spacie A., Williams L., and Noblet J.A.
1994. Bioavailability: Physical, Chemical, and Biological Interactions - Influences of particulate
and dissolved material on the bioavailability of organic compounds. Pp. 93-108. As cited in
Crunkilton et al. (1994).
Supelco Catalog: Chromatography Products. 1996.
Takada H., Onda T., Ogura N. 1990. EnvironmentalScience and Technology. 24, 1179-1186.
As cited in McGroddy et al. (1996).
United States Army Corps Engineers (USACOE), New England Division. Draft 1988.
Navigation Improvement Study: Feasibility Study and Environmental Assessment, Boston
Harbor, Massachusetts: Mystic River, Chelsea River, and Reserved Channel.
United States Geological Survey (USGS). 1992. Water Resources Data, Massachusetts and
Rhode Island, Water Year 1992, U.S. Geological Survey Water-Data Report MA-RI-92-1. As
cited in Chan-Hilton et al. (1998).
Warshawsky D., Cody T., Radike M., Reilman R., Schumann B., LaDow K., Schneider J. 1995.
Biotransformation of benzo(a)pyrene and other polycyclic aromatic hydrocarbons and
heterocyclic analogs by several green algae and other algal species under gold and white light.
Chemico-BiologicalInteractions.97: 131-148.
Yasuda H.J. 1967. Polymer Science. 5 (Part A-1), 2952.
Youngblood W.W., Blumer M. 1975. Polycyclic aromatic hydrocarbons in the environment:
homologous series in soils and recent marine sediments. Geochimica et CosmochimicaActa.
39, 1303-1314.
References
APPENDIX A
Laboratory Experiment Data and Calculations
Appendix A
SPMD LAB EXPERIMENT CALCULATIONS
W
41*.0---
Date
Run #
Retention
Area Units (AU)
Volume
AU/Vol
(Vol)
Time
d8-naphthalene
3/6/98
3.632
83515
d8-naphthalene
3/23/98
3.652
68900
0.9
76556
d8-naphthalene
3/23/98
3.665
65659
0.9
72954
d8-naphthalene
3/26/98
3.649
61698
0.975
63280
83515
74076
p-terphenyl
3/6/98
13.6
28765
1
28765
p-terphenyl
3/23/98
13.722
25178
0.9
27976
p-terphenyl
3/23/98
13.775
20642
0.9
22936
p-terphenyl
3/26/98
13.727
22241
0.975
22811
d12-perylene
3/6/98
22.513
81227
d12-perylene
3/23/98
22.85
58656
d12-perylene
3/23/98
22.895
d12-perylene
3/26/98
22.804
81227
0.9
65173
56821
0.9
63134
64103
0.975
65747
68820
Date
Run #
Retention
Area Units (AU)
Time
Volume
Area Units TOT
(Vol)
3/6/98
116
16.635
100245
1
5012250
3/6/98
115
16.64
98714
0.9
5484111
3/23/98
187
16.675
101393
0.9
5632944
3/26/98
196
16.669
105701
0.95
5563211
Area Units TOT =(AUNol)*50 microliters
5423129
,.... n
" ....
Date
Run #
Retention Time
Area Units (AU)
Volume
AUNol
Nanograms/AU
4.4546E-05
(Vol)
2-methylnaphthalene
3/26/98
197
4.483
150610
150610
2-methylnaphthalene
3/27/98
205
4.498
166306
151187
4.4375E-05
150899
4.4460E-05
average
Benzo(a)pyrene
3/26/98
197
22.541
151320
1
151320
4.4337E-05
Benzo(a)pyrene
3/27/98
205
22.526
168978
1.1
153616
4.3674E-05
average
152468
4.4005E-05
Pyrene
3/26/98
197
12.813
164191
1
164191
4.0861E-05
Pyrene
3/27/98
205
12.802
185603
1.1
168730
3.9762E-05
average
166461
4.0311E-05
std dev
7.7725E-07
% error
1.9%
Pyrene: 6.709 ngluL in the combined solution
nC24
3/26/98
199
16.65
85611
0.9
95123
57.01
nC24
3/27/98
206
16.697
117007
1
117007
46.35
d8-naphthalene
3/26/98
199
3.653
19698
21887
57.01
129932
d8-naphthalene
3/27/98
206
3.622, 3.674
5553, 26143
5553, 26143
46.35
159824
p-terphenyl
3/26/98
199
13.704
20071
22301
57.01
44942
49.62
p-terphenyl
3/27/98
206
13.732
29501
29501
46.35
55281
53.37
d12-perylene
3/26/98
199
22.782 (low?)
250830
278700
57.01
120713
d12-perylene
3/27/98
206
22.818
352685
352685
46.35
148484
2-methylnaphthalene
3/26/98
199
nd - (4.330)
0
57.01
2-methylnaphthalene
3/27/98
206
nd (4.773)
0
46.35
pyrene
3/26/98
199
nd
0
57.01
pyrene
3/27/98
206
nd (12.533)
0
46.35
benzo(a)pyrene
3/26/98
199
nd - (22.486)
149686
166318
57.01
benzo(a)pyrene
3/27/98
206
22.521 !!!
370506
370506
46.35
Sample Volume = Ave AU TOT *(Volume)/AU
Expected Spike = [Ave AuNol (spike)]*(100 uUSample Volume)
Percent Recovery = [AuNol (sample)/Expected Spike] * 100%
nC24
3/23/98
190
16.684
113702
0.95
119686
45.31
nC24
3/23/98
191
16.701
140183
0.95
147561
36.75
nC24
3/23/98
192
16.701
154823
0.9
172026
31.53
nC24
3/27/98
207
16.701
243543
0.9
270603
20.04
d8-naphthalene
3/23/98
190
3.65
5184
0.95
5457
45.31
163483
3.34
d8-naphthalene
3/23/98
191
0.95
0
36.75
201558
0.00
d8-naphthalene
3/23/98
192
0.9
0
31.53
234975
0.00
d8-naphthalene
3/27/98
207
3.653
6416
0.9
7129
20.04
369626
1.93
p-terphenyl
3/23/98
190
13.75
17415
0.95
18332
45.31
56546
32.42
p-terphenyl
3/23/98
191
13.77
20579
0.95
21662
36.75
69716
31.07
p-terphenyl
3/23/98
192
13.762
20387
0.9
22652
31.53
81274
27.87
p-terphenyl
3/27/98
207
13.736
39196
0.9
43551
20.04
127848
34.06
d12-perylene
3/23/98
190
22.630 (low, hybrid?)
149949
0.95
157841
45.31
151884
103.92
d12-perylene
3/23/98
191
22.778 (22.626?)
23664
0.95
24909
36.75
187257
13.30
d12-perylene
3/23/98
192
22.782
25492
0.9
28324
31.53
218303
12.97
dl2-perylene
3/27/98
207
22.895
21211
0.9
23568
20.04
343400
6.86
2-methylnaphthalene
3/23/98
190
0.95
0
45.31
2-methylnaphthalene
3/23/98
191
0.95
0
36.75
3/23/98
192
0
31.53
6174
20.04
70935
45.31
2-methyInaphthalene
2-methyInaphthalene
3/27/98
207
4.513
(high?)
5557
pyrene
3/23/98
190
12.884
67388
0.95
pyrene
3/23/98
191
12.901 (high?)
80191
0.95
84412
36.75
pyrene
3/23/98
192
12.898
93410
0.9
103789
31.53
pyrene
3/27/98
207
12.868
140010
0.9
155567
20.04
benzo(a)pyrene
3/23/98
190
4.340 (low?)
40915
0.95
43068
45.31
benzo(a)pyrene
3/23/98
191
22.626(high?)
29851
0.95
31422
36.75
benzo(a)pyrene
3/23/98
192
22.638
32410
0.9
36011
31.53
benzo(a)pyrene
3/27/98
207
22.572
51540
0.9
57267
20.04
nC24
3/26/98
200
16.667
73812
1
73812
73.47
nC24
3/27/98
208
16.658
85207
1
85207
63.65
nC24
4/5/98
225
16.478
162070
1
162070
33.46
d8-naphthalene
3/26/98
200
3.65
13238
1
13238
73.47
100822
13.13
d8-naphthalene
3/27/98
208
3.63
16613
1
16613
63.65
116387
14.27
d8-naphthalene
4/5/98
225
p-terphenyl
3/26/98
200
13.717
16598
1
16598
73.47
34873
47.60
p-terphenyl
3/27/98
208
13.691
20280
1
20280
63.65
40256
50.38
p-terphenyl
4/5/98
225
13.494
38743
1
38743
33.46
76571
50.60
d12-perylene
3/26/98
200
22.787(low?)
158974
1
158974
73.47
93669
169.72
d12-perylene
3/27/98
208
22.905(high?)
40551
1
40551
63.65
108129
37.50
d12-perylene
4/5/98
225
2-methylnaphthalene
3/26/98
200
4.508(?)
10550
1
10550
73.47
2-methylnaphthalene
3/27/98
208
4.488
12089
1
12089
63.65
2-methylnaphthalene
4/5/98
225
pyrene
3/26/98
200
12.845
62806
1
62806
73.47
pyrene
3/27/98
208
12.811
70851
1
70851
63.65
pyrene
4/5/98
225
12.61
151856
1
151856
33.46
benzo(a)pyrene
3/26/98
200
22.544
153618
1
153618
73.47
benzo(a)pyrene
3/27/98
208
22.512
66072
1
66072
63.65
benzo(a)pyrene
4/5/98
225
nC24
3/26/98
201
16.654
112510
1
112510
48.20
d8-naphthalene
3/26/98
201
3.633
14952
1
14952
48.20
153681
9.73
p-terphenyl
3/26/98
201
13.692
22347
1
22347
48.20
53156
42.04
d12-perylene
3/26/98
201
22.758(?)
30638
1
30638
48.20
142777
21.46
2-methylnaphthalene
3/26/98
201
4.494
7618
1
7618
48.20
pyrene
3/26/98
201
12.826
77872
1
77872
48.20
benzo(a)pyrene
3/26/98
201
22.545
37911
1
37911
48.20
nC24
3/26/98
203
16.63
94892
0.95
99886
54.29
nC24
4/3/98
212
16.67
123845
1
123845
43.79
nC24
4/3/98
214
16.681
126247
0.9
140274
38.66
d8-naphthalene
3/26/98
203
3.64
21458
0.95
22587
54.29
136438
d8-naphthalene
4/3/98
212
3.664
14858
1
14858
43.79
169164
8.78
d8-naphthalene
4/3/98
214
3.665
18087
0.9
20097
38.66
191605
10.49
p-terphenyl
3/26/98
203
13.674 (low?)
72619
0.95
76441
54.29
47192
161.98
p-terphenyl
4/3/98
212
13.692
93942
1
93942
43.79
58511
160.55
p-terphenyl
4/3/98
214
13.692
93078
0.9
103420
38.66
66273
156.05
d12-perylene
3/26/98
203
22.9 (high?)
158479
0.95
166820
54.29
126757
131.61
d12-perylene
4/3/98
212
22.897
198904
1
198904
43.79
157161
126.56
d12-perylene
4/3/98
214
22.720 (low?)
286319
0.9
318132
38.66
178010
178.72
2-methylnaphthalene
3/26/98
203
4.485
6983
0.95
7351
54.29
2-methylnaphthalene
4/3/98
212
4.509
8960
1
8960
43.79
2-methylnaphthalene
4/3/98
214
4.508
8667
0.9
9630
38.66
16.56
pyrene
3/26/98
203
12.798 (low?)
87286
0.95
91880
54.29
pyrene
4/3/98
212
12.813
104333
1
104333
43.79
pyrene
4/3/98
214
12.821
108545
0.9
120606
38.66
benzo(a)pyrene
3/26/98
22.504 (low?)
268687
0.95
282828
54.29
benzo(a)pyrene
4/3/98
22.491 (low?)
348667
1
348667
43.79
benzo(a)pyrene
4/3/98
22.491
378989
0.9
421099
38.66
Pyrene Concentration Calculations
BLANK
Average recovery
Uncorrected Recovery
Corrected Recovery
Nanogramsl
Volume
Nanograms Pyrene
of p-terphenyl
of Pyrene (AUluL)
of Pyrene (AUluL)
AU Pyrene
(uL)
in Each Sample
49.62
0.00
0 00
4 0311E-05
57 0116
0.0000
53.37
0.00
0 00
4 0311E-05
46.3488
0.0000
0.0000
DAY 4
DAY 8
3242
70934.74
21880846
4 0311E-05
453112
399.6657
31 07
84411.58
27166496
4 0311E-05
36.7518
402.4752
27 87
103788 89
372386 16
4 0311E-05
31 5251
473 2361
34 06
155566 67
456678 92
4.0311E-05
20.0409
368.9394
average
411 0791
std dev
44 1344
error
10.74
47 60
62806.00
131957 13
4.0311E-05
73.4722
390.8257
50 38
70851.00
140641.61
4.0311E-05
63.6465
360.8410
50 60
151856 00
300124 78
4 0311E-05
33.4616
404 8336
average
385 5001
stddev
22.4746
5.83
error
DAY 12
42 04
ave recovery:
41.90
std dev:
9.6
77872.00
185231 03
4 0311E-05
48.2013
359.9150
F
359.9150
Assuming average recovery of pyrene (based on average p-terphenyl recoveries of other samples):
DAY 16
Co
40.94
91880.00
224445 25
4 0311E-05
54.2930
491.2264
40 94
104333 00
254865.54
4 0311E-05
43 7896
449 8937
40.94
120605 56
294616 28
4.0311E-05
38.6608
459 1509
% error
APPENDIX B
Field Experiment Data and Calculations
Appendix B
SPMD FIELD SAMPLING CALCULATIONS
rec Notel
ltldrds e ..
'
i2-pe..
.
.
.
Date
Run #
Retention Time
d8-naphthalene
4/5/98
227
3.586
87012
96680
d8-naphthalene
4/10/98
240
3.581
105602
96002
d8-naphthalene
4/11/98
243
3.579
96114
96114
d8-naphthalene
4/11/98
244
3.58
93105
84641
ave
3.5815
std dev
0.0031
% error
0.1%
Area Units (AU)
Volume (uLI)
AU/uL
p-terphenyl
4/5/98
227
13.505
36771
40857
p-terphenyl
4/10/98
240
13.462
41409
37645
p-terphenyl
4/11/98
243
13.436
36232
36232
p-terphenyl
4/11/98
244
ave
13.436
13.4598
33121
30110
std dev
0.0326
% error
0.2%
dl2-perylene
4/5/98
227
22.45
93553
103948
d12-perylene
4/10/98
240
22.393
115534
105031
d12-perylene
4/11/98
243
22.351
99642
99642
dl2-perylene
4/11/98
244
ave
22.354
22.3870
97921
RSA~nA
std dev
0.0462
% error
0.2%
done on the day of spiking
done on the day of spiking
done on the day of spiking
V
;qe••
Caation 74,1W
Date
Run #
Retention Time
Area Units (AU)
Volume (uL)
Area Units TOT
4/11/98
242
16.399
150428
1
7521400
4/11/98
249
16.384
172582
1
8629100
4/12/98
251
16.342
170459
1
8522950
ave
16.375
std dev
0.0295
% error
0.2%
Area Units TOT =(AUNol)*50 microliters
Combine
i
p.ke o.2-methyinaph...ena'
r'ne
Date
Run #
Retention Time
Area Units (AU)
2-methylnaphthalene
4/13/98
257
4.397
2-methylnaphthalene
4/14/98
263
4.382
ave
4.3895
std dev
0.0106
% error
0.2%
Volume (uL)
AU/uL
Nanograms/AU
237446
237446
2.8255E-05
259688
216407
31in2F-05
Pyrene
4/13/98
257
12.512
260351
1
260351
2.5769E-05
Pyrene
4/14/98
263
12.487
288263
1.2
240219
2.7929E-05
ave
12.4995
std dev
0.0177
% error
0.1%
1
234648
2.8592E-05
217R81
3
Benzo(a)pyrene
4/13/98
257
22.079
234648
Benzo(a)pyrene
4/14/98
263
22.057
261193
ave
22.068
std dev
0.0156
% error
0.1%
1
0823F-n5
* Based on John MacFarlane's F3 Sample, C, 10/15/96
Date
Run #
Retention Time
Area Units (AU)
dl0-phenanthrene
4/15/98
272
8.390
dl 0-phenanthrene
4/15/98
273
8.400
phenanthrene
4/15/98
272
phenanthrene
4/15/98
273
Nanograms/AU
AU/uL
Conc (ng/uL)
45948
45948
1.20
2.6116E-05
44521
44521
1.20
2.6954E-05
8.440
63136
63136
1.33
2.1066E-05
8.450
61169
61169
1.33
2.1743E-05
Volume (uL)
averale210E0
2-methylphenanthrene
2-methylphenanthrene
4/15/98
4/15/98
272
273
9.770
9.781
62909
62909
1.26
59649
59649
1.26
2.0029E-05
2.1124E-05
mvrg,
2.066E-0
fluoranthene
4/15/98
272
11.749
60536
60536
1.33
2.1970E-05
fluoranthene
4/15/98
273
11.766
58078
58078
1.33
2.2900E-05
benz(a)anthacene
4/15/98
272
16.898
60915
60915
1.33
2.1834E-05
benz(a)anthacene
4/15/98
273
16.913
56881
56881
1.33
2.3382E-05
chrysene
4/15/98
272
17.021
64733
64733
1.33
2.0546E-05
chrysene
4/15/98
273
17.035
60328
60328
1.33
ave.a. e
2.2046E-05
.
1
2.,.IN
0
.
nC24
4/10/98
239
16.459
79920
0.95
84126
97.76
16.361
97909
1
97909
84.00
nC24
4/11/98
247
nC24
4/12/98
250
16.34
106739
1
106739
77.05
nC24
4/12/98
253
16.374
100635
1
100635
81.73
p-terphenyl
4/10/98
239
13.471
22737
0.95
23934
97.76
37039
64.62%
p-terphenyl
4/11/98
247
13.369
27670
1
27670
84.00
43107
64.19%
p-terphenyl
4/12/98
250
13.342
30796
1
30796
77.05
46995
65.53%
p-terphenyl
4/12/98
253
13.382
28303
1
28303
81.73
44308
63.88%
dlO-phenanthrene
4/10/98
239
8.493
53857
0.95
56692
97.76
dlO-phenanthrene
4/11/98
247
8.417
68038
1
68038
84.00
dlO-phenanthrene
4/12/98
250
8.39
75299
1
75299
77.05
dlO-phenanthrene
4/12/98
253
8.436
73286
1
73286
81.73
phenanthrene
4/10/98
239
8.565
10916
0.95
11491
97.76
phenanthrene
4/11/98
247
8.494
12438
1
12438
84.00
phenanthrene
4/12/98
250
8.468
13906
1
13906
77.05
phenanthrene
4/12/98
253
8.517
12075
1
12075
81.73
methylphenanthrene's
4/10/98
239
9.74
7583
0.95
7982
97.76
8371
84.00
methylphenanthrene's
4/11/98
247
9.656
8371
1
methylphenanthrene's
4/12/98
250
9.628
11485
1
11485
77.05
methylphenanthrene's
4/12/98
253
9.677
13847
1
13847
81.73
fluoranthene
4/10/98
239
11.905
7546
0.95
7943
97.76
fluoranthene
4/11/98
247
11.81
8024
1
8024
84.00
fluoranthene
4/12/98
250
11.785
9317
1
9317
77.05
8738
1
8738
81.73
fluoranthene
4/12/98
253
11.829
pyrene
4/10/98
239
nd
0
0.95
0
97.76
pyrene
4/11/98
247
12.489
6675
1
6675
84.00
pyrene
4/12/98
250
12.458
7204
1
7204
77.05
pyrene
4/12/98
253
12.5
6809
1
6809
81.73
benz(a)anthracene
4/10/98
0.95
97.76
benz(a)anthracene
4/11/98
1
84.00
benz(a)anthracene
4/12/98
1
77.05
benz(a)anthracene
4/12/98
1
81.73
chrysene
4/10/98
0.95
97.76
chrysene
4/11/98
1
84.00
chrysene
4/12/98
1
77.05
chrysene
4/12/98
1
81.73
Sample Volume = Ave AU TOT *(Volume)/AU
Expected Spike = [Ave AuNol (spike)]*(100 uL/Sample Volume)
Percent Recovery = [AuNol (sample)/Expected Spike] * 100%
nC24
4/10/98
234
16.44
131401
1
131401
62.59
nC24
4/11/98
245
16.393
142094
0.95
149573
54.99
nC24
4/11/98
248
16.403
212131
1
212131
38.77
nC24
4/12/98
255
16.347
300004
1
300004
27.41
p-terphenyl
4/10/98
234
13.454
34474
1
34474
62.59
57853
59.59%
p-terphenyl
4/11/98
245
13.415
45585
0.95
47984
54.99
65854
72.86%
p-terphenyl
4/11/98
248
13.41
64677
1
64677
38.77
93397
69.25%
p-terphenyl
4/12/98
255
13.35
86057
1
86057
27.41
132086
65.15%
dlO-phenanthrene
4/10/98
234
8.47
42137
1
42137
62.59
dl0-phenanthrene
4/11/98
245
8.462
43420
0.95
45705
54.99
dlO-phenanthrene
4/11/98
248
8.449
77068
1
77068
38.77
dl0-phenanthrene
4/12/98
255
8.409
98043
1
98043
27.41
phenanthrene
4/10/98
234
8.525
142171
1
142171
62.59
phenanthrene
4/11/98
245
8.514
153546
0.95
161627
54.99
phenanthrene
4/11/98
248
8.499
251629
1
251629
38.77
phenanthrene
4/12/98
255
8.46
343550
1
343550
27.41
methylphenanthrene's
4/10/98
234
9.800-10.152
258228
1
258228
62.59
methylphenanthrene's
4/11/98
245
9.782-10.135
306003
0.95
322108
54.99
methylphenanthrene's
4/11/98
248
9.770-10.050
348645
1
348645
38.77
methylphenanthrene's
4/12/98
255
9.792-10.000
434281
1
434281
27.41
fluoranthene
4/10/98
234
11.875
248372
1
248372
62.59
fluoranthene
4/11/98
245
11.843
291133
0.95
306456
54.99
fluoranthene
4/11/98
248
11.84
427101
1
427101
38.77
fluoranthene
4/12/98
255
11.789
617303
1
617303
27.41
pyrene
4/10/98
234
12.568
218378
1
218378
62.59
pyrene
4/11/98
245
12.535
255347
0.95
268786
54.99
pyrene
4/11/98
248
12.532
372070
1
372070
38.77
pyrene
4/12/98
255
12.475
539108
1
539108
27.41
benz(a)anthracene
benz(a)anthracene
benz(a)anthracene
benz(a)anthracene
4/10/98
4/11/98
4/11/98
4/12/98
234
245
248
255
17.051
16.988
16.991
16.93
22542
22883
36429
56323
1
0.95
1
1
22542
24087
36429
56323
62.59
54.99
38.77
27.41
chrysene
chrysene
chrysene
chrysene
4/10/98
4/11/98
4/11/98
4/12/98
234
245
248
255
17.171
17.103
17.11
17.045
51557
58035
87428
125846
1
0.95
1
1
51557
61089
87428
125846
62.59
54.99
38.77
27.41
nC24
4/13/98
259
16.353
91028
82753
99.39
nC24
4/13/98
261
16.346
69541
69541
118.27
nC24
4/14/98
264
16.4
73866
73866
111.34
nC24
4/14/98
265
16.384
93625
78021
105.41
nC24
4/14/98
267
16.338
93482
84984
96.78
p-terphenyl
4/13/98
259
13.52
16437
14943
99.39
36434
41.01%
p-terphenyl
4/13/98
261
13.514
14978
14978
118.27
30618
48.92%
p-terphenyl
4/14/98
264
13.571
17750
17750
111.34
32522
54.58%
p-terphenyl
4/14/98
265
13.548
21534
17945
105.41
34351
52.24%
p-terphenyl
4/14/98
267
13.505
24889
22626
96.78
37417
60.47%
dlO-phenanthrene
4/13/98
259
8.418
55338
50307
99.39
d10-phenanthrene
4/13/98
261
8.409
56163
56163
118.27
dl0-phenanthrene
4/14/98
264
8.448
54663
54663
111.34
dl0-phenanthrene
4/14/98
265
8.439
71166
59305
105.41
dl0-phenanthrene
4/14/98
267
8.454
445628
405116
96.78
phenanthrene
4/13/98
259
8.469
292726
266115
99.39
phenanthrene
4/13/98
261
8.461
280448
280448
118.27
phenanthrene
4/14/98
264
8.5
305299
305299
111.34
phenanthrene
4/14/98
265
8.49
376587
313823
105.41
phenanthrene
4/14/98
267
8.571
147278
133889
96.78
methylphenanthrene's
4/13/98
259
9.733-10.083
680922
619020
99.39
methylphenanthrene's
4/13/98
261
9.723-10.075
614921
614921
118.27
methylphenanthrene's
4/14/98
264
9.770-10.122
699249
699249
111.34
methylphenanthrene's
4/14/98
265
9.755-10.106
866146
721788
105.41
methylphenanthrene's
4/14/98
267
9.860-10.117
539485
490441
96.78
1.1
not well-resolved
not well-resolved
not well-resolved
fluoranthene
4/13/98
259
11.792
492763
1.1
447966
99.39
fluoranthene
4/13/98
261
11.782
474613
1
474613
118.27
fluoranthene
4/14/98
264
11.831
508975
1
508975
111.34
fluoranthene
4/14/98
265
11.816
630559
1.2
525466
105.41
fluoranthene
4/14/98
267
11.775
629265
1.1
572059
96.78
pyrene
4/13/98
259
12.478
367348
1.1
333953
99.39
pyrene
4/13/98
261
12.47
351770
1
351770
118.27
pyrene
4/14/98
264
12.521
376782
1
376782
111.34
pyrene
4/14/98
265
12.5
467880
1.2
389900
105.41
pyrene
4/14/98
267
12.459
473752
1.1
430684
96.78
benz(a)anthracene
4/13/98
259
16.93
34209
1.1
31099
99.39
benz(a)anthracene
4/13/98
261
16.925
33323
1
33323
118.27
benz(a)anthracene
4/14/98
264
16.972
34577
1
34577
111.34
benz(a)anthracene
4/14/98
265
16.954
45092
1.2
37577
105.41
benz(a)anthracene
4/14/98
267
16.905
43289
1.1
39354
96.78
chrysene
4/13/98
259
17.042
92945
1.1
84495
99.39
chrysene
4/13/98
261
17.04
89241
1
89241
118.27
chrysene
4/14/98
264
17.089
94628
1
94628
111.34
chrysene
4/14/98
265
17.074
118885
1.2
99071
105.41
chrysene
4/14/98
267
17.021
115089
1.1
104626
96.78
nC24
4/13/98
258
16.348
129804
1
129804
63.36
nC24
4/13/98
260
16.389
141167
1.1
128334
64.09
p-terphenyl
4/13/98
258
13.515
56393
56393
63.36
57150
98.7%
p-terphenyl
4/13/98
260
13.562
59310
53918
64.09
56503
95.4%
dl0-phenanthrene
4/13/98
258
8.463
911189
911189
63.36
not well-resolved
dl0-phenanthrene
4/13/98
260
64.09
not well-resolved
phenanthrene
4/13/98
258
63.36
not well-resolved
phenanthrene
4/13/98
260
64.09
not well-resolved
methylphenanthrene's
4/13/98
258
9.725-10.078
2025480
2025480
63.36
methylphenanthrene's
4/13/98
260
9.839-10.120
1783597
1621452
64.09
8.58
406821
406821
fluoranthene
4/13/98
258
11.798
1229966
1229966
63.36
fluoranthene
4/13/98
260
11.845
1343230
1221118
64.09
pyrene
4/13/98
258
12.482
1160083
1160083
63.36
pyrene
4/13/98
260
12.526
1266166
1151060
64.09
benz(a)anthracene
4/13/98
258
16.925
89513
89513
63.36
benz(a)anthracene
4/13/98
260
16.965
98922
89929
64.09
chrysene
4/13/98
258
17.046
213740
213740
63.36
chrysene
4/13/98
260
17.079
237521
215928
64.09
nC24
4/12/98
254
16.36
247212
1
247212
nC24
4/15/98
269
16.375
141057
1
141057
58.31
nC24
4/17/98
275
16.342
31081
1
31081
264.61
p-terphenyl
4/12/98
254
p-terphenyl
4/15/98
269
13.455
16055
58.31
62105
p-terphenyl
4/17/98
275
264.61
not well-resolved
dl0-phenanthrene
4/12/98
254
33.27
not well-resolved
d10-phenanthrene
4/15/98
269
8.434
112259
112259
58.31
not well-resolved
dlO-phenanthrene
4/17/98
275
8.391
16282
16282
264.61
phenanthrene
4/12/98
254
phenanthrene
4/15/98
269
8.489
471682
phenanthrene
4/17/98
275
8.441
91543
methylphenanthrene's
4/12/98
254
9.746-10.014
methylphenanthrene's
4/15/98
269
9.752-10.025
methylphenanthrene's
4/17/98
275
fluoranthene
4/12/98
fluoranthene
4/15/98
fluoranthene
33.27
33.27
16055
not well-resolved
33.27
not well-resolved
471682
58.31
not well-resolved
91543
264.61
1841903
1841903
33.27
not well-resolved
1010929
1010929
58.31
not well-resolved
9.702-10.055
246781
246781
264.61
254
11.825
1613227
1613227
33.27
269
11.82
961314
961314
58.31
4/17/98
275
11.756
200320
200320
264.61
pyrene
4/12/98
254
12.504
1257150
1257150
33.27
pyrene
4/15/98
269
12.5
686613
686613
58.31
pyrene
4/17/98
275
12.445
145741
145741
264.61
benz(a)anthracene
benz(a)anthracene
benz(a)anthracene
4/12/98
4/15/98
4/17/98
254
269
275
16.947
16.939
16.905
151166
91201
17784
151166
91201
17784
33.27
58.31
264.61
1
25.9%
chrysene
chrysene
4/12/98
4/15/98
254
269
17.068
17.057
312772
203427
1
1
312772
203427
33.27
58.31
chrysene
4/17/98
275
17.015
41253
1
41253
264.61
Pyrene Concentration Calculations
Average recovery
of p-terphenyl
BLANK SPMD
Tobin Bridge
Bottom
Buoy #12
Buoy #16
Tobin Bridge Surface*
Uncorrected Recovery
of Pyrene (AUluL)
Corrected Recovery
of Pyrene (AU/uL)
Nanograms/
AU Pyrene
Volume
(uL)
2.6849E-05
2.6849E-05
2.6849E-05
2.6849E-05
97.7635
84.0013
77.0523
R1 79R lR
Nanograms Pyrene
in Each SPMD
or per mL triolein
Concentration
in the water (nglL or pptr)
(Rs = 5.1 LUd@ 10OoC)
0
23
23
0.00
0.33
0.32
64.62%
64.19%
65.53%
63.88%
0.00
6675.00
7204.00
6809.00
0.00
10399.06
10993.40
10659.32
59.59%
72.86%
69.25%
65.15%
218378.00
268786.32
372070.00
539108.00
366475.89
368884.55
537289.63
827458.37
2.6849E-05
2.6849E-05
2.6849E-05
2.6849E-05
45.3112
36.7518
31.5251
6.24
5.10
6.37
in nAnQ
A 'A
41.01%
48.92%
54.58%
52.24%
60.47%
333952.73
351770.00
376782.00
389900.00
430683.64
814267.26
719077.06
690344.55
746362.63
712210.63
2.6849E-05
2.6849E-05
2.6849E-05
2.6849E-05
2.6849E-05
99.3863
118.2681
111.3433
105.4139
96.7773
2173
2283
2064
2112
1851
30.43
98.70%
95.43%
1160083
1151060
1175362.72
1206237.30
2.6849E-05
2.6849E-05
63.36
64.09
1999
2076
28.00
29.07
25.9%
1257150.00
686613.00
145741
4862962.99
2655986.64
563761.75
2.6849E-05
2.6849E-05
2.6849E-05
33.27
58.31
964 R1
4344
4158
60.8
58.2
A4RI
Ar
25.9%
25.9%
* Recoveries could not be calculated for the first and last injection.
Recovery from second run was assumed to be representative.
n A-
31.98
28.90
29.59
25.92
1
dl0-Phenanthrene Concentration Calculations
Note that 400 ng dl0-phenanthrene were spiked into the SPMDs before deployment.
Average recovery
of p-terphenyl
Nanograms/
Uncorrected Recovery
Corrected Recovery
of dl0-phenanthrene (AUIuL) of d10-phenanthrene (AUluL) AU d10-phenanthrene
Volume
(uL)
BLANK SPMD
64.62%
64.19%
65.53%
63.88%
56691.58
68038.00
75299.00
73286.00
87734.50
105997.23
114907.28
114727.39
2.6535E-05
2.6535E-05
2.6535E-05
2.6535E-05
97.7635
84.0013
77.0523
81 7259
Tobin Bridge
Bottom
59.59%
72.86%
69.25%
65.15%
42137
45705
77068
98043
70713.14
62726.28
111290.45
150482.84
2.6535E-05
2.6535E-05
2.6535E-05
2.6535E-05
45.3112
36.7518
31.5251
Buoy #12
41.01%
48.92%
54.58%
52.24%
50307
56163
54663
59305
122662.77
114806.62
100154.21
113524.07
2.6535E-05
2.6535E-05
2.6535E-05
2.6535E-05
99.3863
118.2681
111.3433
105.4139
Buoy #16
98.70%
95.43%
0.00
0.00
2.6535E-05
2.6535E-05
63.36
64.09
0.00
434245.21
62982.75
2.6535E-05
2.6535E-05
2.6535E-05
33.27
58.31
264.61
Tobin Bridge Surface*
25.9%
25.9%
25.9%
112259.00
16282
Nanograms dl0-phenanthrene
In Each SPMD
or per mL triolein
228
236
235
249
gn nAnQ
TOO HIGH!!!
* Recoveries could not be calculated for the first and last injection.
Recovery from second run was assumed to be representative.
Phenanthrene Concentration Calculations
Average recovery
of p-terphenyl
Uncorrected Recovery
of Phenanthrene (AUIuL)
Corrected Recovery
of Phenanthrene (AUluL)
Nanogramsl
AU Phenanthrene
Volume
(uL)
BLANK SPMD
64.62%
64.19%
65.53%
63.88%
11490.53
12438.00
13906.00
12075.00
17782.46
19377.31
21220.74
18903.11
2.1404E-05
2.1404E-05
2.1404E-05
2.1404E-05
97.7635
84.0013
77.0523
81.7259
Tobin Bridge
Bottom
59.59%
72.86%
69.25%
65.15%
142171
161627
251629
343550
238587.42
221818.73
363366.17
527303.10
2.1404E-05
2.1404E-05
2.1404E-05
2.1404E-05
45.3112
36.7518
31.5251
20.0409
Buoy #12
41.01%
48.92%
54.58%
52.24%
266115
280448
305299
313823
648859.38
573282.89
559372.53
600731.95
2.1404E-05
2.1404E-05
2.1404E-05
2.1404E-05
99.3863
118.2681
111.3433
105.4139
Buoy #16
98.70%
95.43%
0.00
0.00
2.1404E-05
2.1404E-05
63.36
64.09
1824581.08
354110.66
2.1404E-05
2.1404E-05
2.1404E-05
33.27
58.31
264.61
Tobin Bridge Surface*
25.9%
25.9%
25.9%
471682.00
91543.00
* Recoveries could not be calculated for the first and last injection.
Recovery from second run was assumed to be representative.
Nanograms Phenanthrene
in Each SPMD
or per mL triolein
37
35
35
33
Concentration
In the water (nglL or pptr)
(Rs =3.9 Lid @ 10oC)
0.68
0.64
0.64
0.61
4.24
3.20
4.49
4.14
1380
1451
1333
1355
25.28
26.58
24.42
24.82
2277
2006
41.70
36.73
Methylphenanthrene's Concentration Calculations
Average recovery
of p-terphenyl
Uncorrected Recovery
of Methyphenan.'s(AUluL)
Corrected Recovery
of Methyphenan.'s (AUIuL)
Nanogramsl
AU Methylphenan.'s
Volume
(uL)
BLANK SPMD
64.62%
64.19%
65.53%
63.88%
7982.11
8371.00
11485.00
13847.00
12352.91
13041.28
17526.26
21677.13
2.0576E-05
2.0576E-05
2.0576E-05
2.0576E-05
97.7635
84.0013
77.0523
81.7259
Tobin Bridge
Bottom
59.59%
72.86%
69.25%
65.15%
258228
322108
348645
434281
433351.05
442064.25
503462.64
666563.00
2.0576E-05
2.0576E-05
2.0576E-05
2.0576E-05
45.3112
36.7518
31.5251
20.0409
Buoy #12
41.01%
48.92%
54.58%
52.24%
619020
614921
699249
721788
1509338.53
1257001.98
1281172.50
1381676.95
2.0576E-05
2.0576E-05
2.0576E-05
2.0576E-05
99.3863
118.2681
111.3433
1ns A411
3087
3059
2935
,9g7
56.53
56.02
53.76
5A oR
Buoy #16
98.70%
95.43%
2025480
1621452
2052158.05
1699177.86
2.0576E-05
2.0576E-05
63.36
64.09
2675
2241
49.00
41.04
25.9%
25.9%
25.9%
1841903
1010929.00
246781.00
7124930.29
3910520.07
954609.13
2.0576E-05
2.0576E-05
2.0576E-05
33.27
58.31
26461
4877
4692
5198
89.33
85.93
9519
Tobin Bridge Surface
* Recoveries could not be calculated for the first and last injection.
Recovery from second run was assumed to be representative.
Nanograms Methylphenan's
in Each SPMD
or per mL triolein
25
23
28
36
Concentration
In the water (nglL or pptr)
(Assume Rs =3.9 Ud @ 10OoC)
0.46
0.41
0.51
0.67
7.40
6.12
5.98
5.03
Fluoranthene's Concentration Calculations
Average recovery
of p-terphenyl
Uncorrected Recovery
of Fluoranthene's(AUluL)
Corrected Recovery
of Fluoranthene's (AUluL)
Nanogramsl
AU Fluoranthene's
Volume
(uL)
BLANK SPMD
64.62%
64.19%
65.53%
63.88%
7943
8024
9317
8738
12292.64
12500.69
14217.87
13679.12
2.2435E-05
2.2435E-05
2.2435E-05
2.2435E-05
97.7635
84.0013
77.0523
81.7259
Nanograms Fluoranthene
In Each SPMD
or per mL triolein
27
24
25
25
Tobin Bridge
Bottom
59.59%
72.86%
69.25%
65.15%
248372
306456
427101
617303
416810.99
420582.45
616757.43
947477.19
2.2435E-05
2.2435E-05
2.2435E-05
2.2435E-05
45.3112
36.7518
31.5251
20?0409
424
347
436
426
7.04
5.76
7.25
7 nA
Buoy #12
41.01%
48.92%
54.58%
52.24%
60.47%
554173
585979
623139
649573
649573
1351223.30
1197839.66
1141722.84
1243438.42
1074181.60
2.2435E-05
2.2435E-05
2.2435E-05
2.2435E-05
2.2435E-05
99.3863
118.2681
111.3433
105.4139
10iI413
3013
3178
2852
2941
50.05
52.80
47.38
48.85
98.70%
95.43%
1229966
1221118
1246166.16
1279653.80
2.2435E-05
2.2435E-05
63.36
64.09
1771
1840
29.43
30.56
25.9%
25.9%
25.9%
1613227.00
961314.00
200320
6228675.68
3718597.14
774886.64
2.2435E-05
2.2435E-05
2.2435E-05
33.27
58.31
264.61
4649
4864
4600
77.23
80.80
76.42
Buoy #16
Tobin Bridge Surface*
* Recoveries could not be calculated for the first and last injection.
Recovery from second run was assumed to be representative.
A540
Concentration
in the water (nglL or pptr)
(Rs =4.3 Lid @ 10oC)
0.45
0.39
0.41
042
A9 9n
Benz(a)anthracene's Concentration Calculations
Average recovery
of p-terphenyl
Uncorrected Recovery
of Benz(a)anthra's(AUluL)
Corrected Recovery
of Benz(a)anthra's (AUIuL)
Nanogramsl
AU Benz(a)anthra
Volume
(uL)
64.62%
0.00
0.00
0.00
0.00
2.2608E-05
2.2608E-05
2.2608E-05
2.2608E-05
97.7635
64.19%
65.53%
63.88%
0
0
0
0
Nanograms Benz(a)anthra
in Each SPMD
or per mL triolein
0
84.0013
77.0523
81.7259
0
0
0
Tobin Bridge
Bottom
59.59%
72.86%
69.25%
65.15%
22542
24087
36429
56323
37829.36
33057.70
52605.49
86448.24
2.2608E-05
2.2608E-05
2.2608E-05
2.2608E-05
45.3112
36.7518
31.5251
20.0409
Buoy #12
41.01%
75828.01
68117.82
63352.40
71930.80
65078.11
2.2608E-05
2.2608E-05
2.2608E-05
2.2608E-05
2.2608E-05
99.3863
118.2681
111.3433
105.4139
3.38
3.61
3.16
60.47%
31099
33323
34577
37577
39354
1inl A1VQ
Ann
98.70%
95.43%
89513
89929
90692.00
94239.94
2.2608E-05
2.2608E-05
63.36
2.58
Ad no
9 71
25.9%
25.9%
25.9%
151166.00
91201.00
17784
583652.51
352787.72
68792.85
2.2608E-05
2.2608E-05
2.2608E-05
33.27
58.31
264.61
BLANK SPMD
48.92%
54.58%
52.24%
Buoy #16
Tobin Bridge Surface
* Recoveries could not be calculated for the first and last injection.
Recovery from second run was assumed to be representative.
Concentration
in the water (nglL or pptr)
(Rs =3.6 L/d @ 10oC)
0.00
0.00
0.00
Sn00
3.40
439
465
412
8.71
9.23
A 17
Chrysene's Concentration Calculations
Average recovery
of p-terphenyl
Uncorrected Recovery
of Chrysene's(AUluL)
Corrected Recovery
of Chrysene's (AUIuL)
Nanogramsl
AU Chrysene's
Volume
(uL)
Nanograms Chrysene
in Each SPMD
or per mL triolein
0
0
0
0
Concentration
in the water (nglL or pptr)
(Rs =4.0 Lid @ 10oC)
0.00
0.00
0.00
0.00
BLANK SPMD
64.62%
64.19%
65.53%
63.88%
0
0
0
0
0.00
0.00
0.00
0.00
2.1296E-05
2.1296E-05
2.1296E-05
2.1296E-05
97.7635
84.0013
77.0523
81.7259
Tobin Bridge
Bottom
59.59%
72.86%
69.25%
65.15%
51557
61089
87428
125846
86521.52
83839.70
126250.86
193156.71
2.1296E-05
2.1296E-05
2.1296E-05
2.1296E-05
45.3112
36.7518
31.5251
on nArQ
Buoy #12
41.01%
48.92%
54.58%
52.24%
60.47%
84495
89241
94628
99071
104626
206022.82
182423.62
173378.57
189645.47
173017.97
2.1296E-05
2.1296E-05
2.1296E-05
2.1296E-05
2.1296E-05
99.3863
118.2681
111.3433
105.4139
7.27
7.66
6.85
7.10
inr
A A7
98.70%
95.43%
213740
215928
216555.22
226278.93
2.1296E-05
2.1296E-05
63.36
25.9%
25.9%
25.9%
312772.00
203427.00
41253
1207613.90
786905.28
159576.67
2.1296E-05
2.1296E-05
2.1296E-05
33.27
58.31
264.61
Buoy #16
Tobin Bridge Surface*
* Recoveries could not be calculated for the first and last injection.
Recovery from second run was assumed to be representative.
AlA
AA no
292
Ano
4.87
A 1
14.26
16.28
14.99
Summary of Results
phenanthrene
0.64
4.0
39.2
methylphenanthrene's
0.51
6.1
90.1
45.0
55.3
fluoranthene
0.42
6.8
78.1
30.0
48.3
pyrene
0.24
6.0
58.4
28.5
29.4
benz(a)anthracene
0.00
0.7
8.7
2.6
chrysene
0.00
1.3
15.2
5.0
methylphenanthrene's
0.51
6.1
90.1
45.0
55.3
fluoranthene
0.42
6.8
78.1
30.0
48.3
pyrene
0.24
6.0
58.4
28.5
29.4
25.3
dj&pqennthrene
Blank
Tobin Bridge - bottom
Tobin Bridge - surface
237
80
557
Buoy #16
Buoy #12
324
phenanthrene
0.64
4.0
39.2
25.3
benz(a)anthracene
0.00
0.7
8.7
2.6
3.3
chrysene
0.00
1.3
15.2
5.0
7.1